Micronutrient Dietary Intake in Latina Pregnant Adolescents and Its Association with Level of Depression, Stress, and Social Support
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Procedures
2.3. Measures
2.3.1. General stress
2.3.2. Pregnancy-Specific Stress
2.3.3. Depressive Symptoms
2.3.4. Social Support
2.3.5. Dietary Intake
2.4. Statistical Analysis
3. Results
3.1. Demographic and Participant Characteristics
3.2. Nutrient Summary and Comparisons with U.S. Requirements
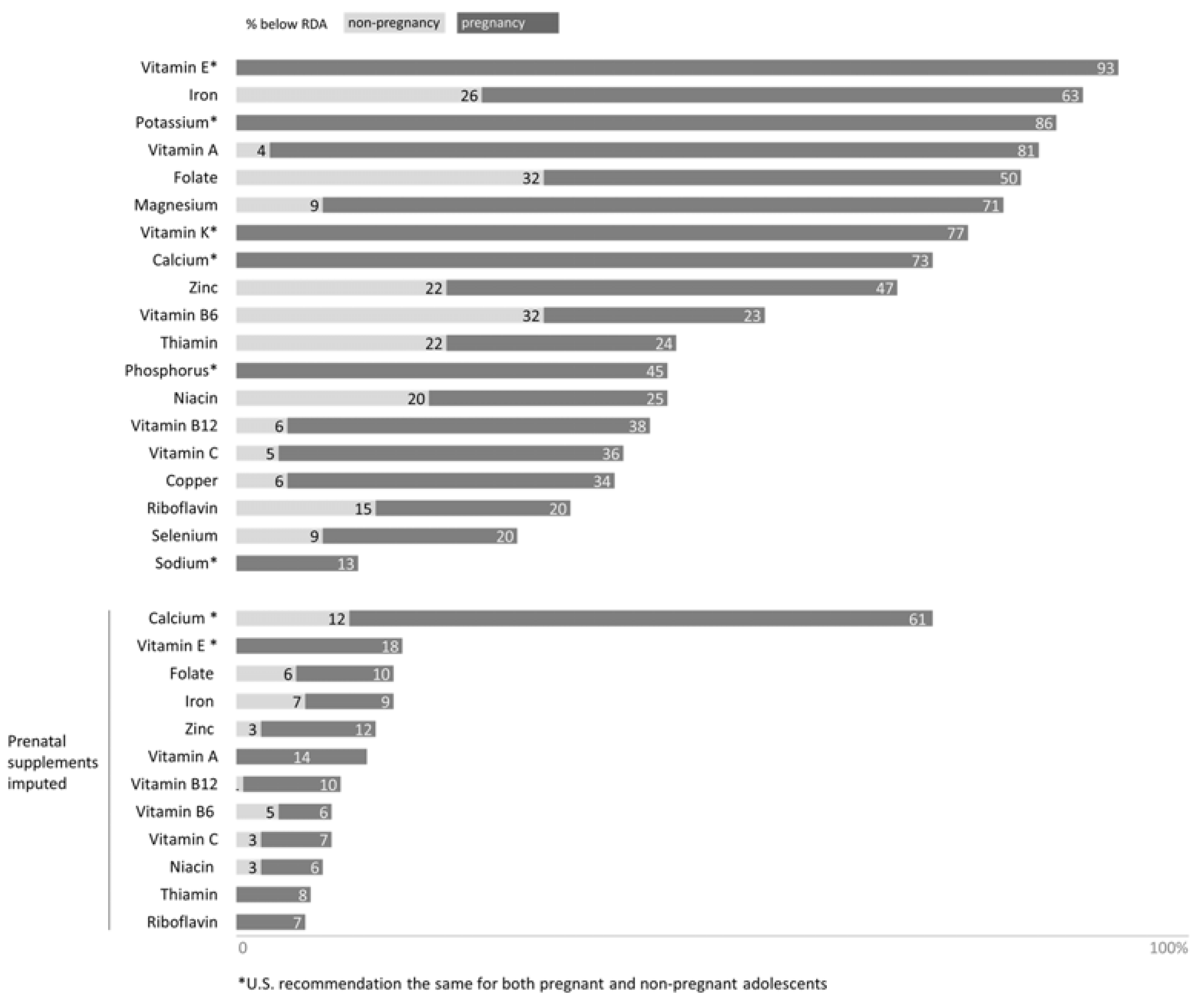
3.2.1. Micronutrient Status in Pregnant Adolescents Provided by Food Consumption
3.2.2. Micronutrient Status in Pregnant Adolescents Provided by Food Consumption and Dietary Supplement Intake
3.3. Nutrient Intake and Psychosocial Risk Factors
4. Discussion
4.1. Micronutrient Status in Pregnant Adolescents Provided by Food Consumption
4.2. Micronutrient Status in Pregnant Adolescents Provided by Food Consumption and Prenatal Supplement Intake
4.3. Psychosocial Factors and Nutrient Intake
Strengths and Limitations of the Study
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Piernas, C.; Popkin, B.M. Increased portion sizes from energy-dense foods affect total energy intake at eating occasions in us children and adolescents: Patterns and trends by age group and sociodemographic characteristics, 1977–2006. Am. J. Clin. Nutr. 2011, 94, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Reedy, J.; Krebs-Smith, S.M. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J. Am. Diet. Assoc. 2010, 110, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.L.; Singer, M.R.; Qureshi, M.M.; Bradlee, M.L.; Daniels, S.R. Food group intake and micronutrient adequacy in adolescent girls. Nutrients 2012, 4, 1692–1708. [Google Scholar] [CrossRef] [PubMed]
- Bienz, D.; Cori, H.; Hornig, D. Adequate dosing of micronutrients for different age groups in the life cycle. Food Nutr. Bull. 2003, 24, S7–S15. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Laoreti, A.; Cetin, I. Multiple micronutrient needs in pregnancy in industrialized countries. Ann. Nutr. Metab. 2014, 65, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Frisancho, A.R.; Matos, J.; Flegel, P. Maternal nutritional status and adolescent pregnancy outcome. Am. J. Clin. Nutr. 1983, 38, 739–746. [Google Scholar] [PubMed]
- Baker, P.N.; Wheeler, S.J.; Sanders, T.A.; Thomas, J.E.; Hutchinson, C.J.; Clarke, K.; Berry, J.L.; Jones, R.L.; Seed, P.T.; Poston, L. A prospective study of micronutrient status in adolescent pregnancy. Am. J. Clin. Nutr. 2009, 89, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, Y.M. Cultural factors affecting diet and pregnancy outcome of mexican american adolescents. J. Adolesc. Health 1999, 25, 227–237. [Google Scholar] [CrossRef]
- Skinner, J.; Carruth, B.; Pope, J.; Varner, L.; Goldberg, D. Food and nutrient intake of white, pregnant adolescents. J. Am. Diet. Assoc. USA 1992, 92, 1127–1129. [Google Scholar]
- Lederman, S.A. Nutritional support for the pregnant adolescent. Ann. N. Y. Acad. Sci. 1997, 817, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Giddens, J.B.; Krug, S.K.; Tsang, R.C.; Guo, S.; Miodovnik, M.; Prada, J.A. Pregnant adolescent and adult women have similarly low intakes of selected nutrients. J. Am. Diet. Assoc. 2000, 100, 1334–1340. [Google Scholar] [CrossRef]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.L.; Hendricks, M.K.; Perez, E.M.; Murray-Kolb, L.E.; Berg, A.; Vernon-Feagans, L.; Irlam, J.; Isaacs, W.; Sive, A.; Tomlinson, M. Maternal iron deficiency anemia affects postpartum emotions and cognition. J. Nutr. 2005, 135, 267–272. [Google Scholar] [PubMed]
- Wójcik, J.; Dudek, D.; Schlegel-Zawadzka, M.G.; Grabowska, M.; Marcinek, A.; Florek, E.; Piekoszewski, W.; Nowak, R.J.; Opoka, W.O.; Nowak, G. Antepartum/postpartum depressive symptoms and serum zinc and magnesium levels. Pharmacol. Rep. 2006, 58, 571. [Google Scholar] [PubMed]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Yokoyama, T.; Ohya, Y.; Fukushima, W.; Saito, K.; Ohfuji, S.; Kiyohara, C.; Hirota, Y. Dietary folate and vitamins B 12, B 6, and B 2 intake and the risk of postpartum depression in Japan: The Osaka maternal and child health study. J. Affect. Disord. 2006, 96, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Harrison-Hohner, J.; Coste, S.; Dorato, V.; Curet, L.; McCarron, D.; Hatton, D. Prenatal calcium supplementation and postpartum depression: An ancillary study to a randomized trial of calcium for prevention of preeclampsia. Arch. Women Ment. Health 2001, 3, 141–146. [Google Scholar] [CrossRef]
- Andrew, R.; Tiggemann, M.; Clark, L. Predictors of intuitive eating in adolescent girls. J. Adolesc. Health 2015, 56, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Fowles, E.R.; Fowles, S.L. Healthy eating during pregnancy: Determinants and supportive strategies. J. Community Health Nurs. 2008, 25, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Hurley, K.M.; Caulfield, L.E.; Sacco, L.M.; Costigan, K.A.; Dipietro, J.A. Psychosocial influences in dietary patterns during pregnancy. J. Am. Diet. Assoc. 2005, 105, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Monk, C.; Georgieff, M.K.; Osterholm, E.A. Research review: Maternal prenatal distress and poor nutrition—mutually influencing risk factors affecting infant neurocognitive development. J. Child Psychol. Psychiatry 2013, 54, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Pearson, R.M.; Goodman, S.H.; Rapa, E.; Rahman, A.; McCallum, M.; Howard, L.M.; Pariante, C.M. Effects of perinatal mental disorders on the fetus and child. Lancet 2014, 384, 1800–1819. [Google Scholar] [CrossRef]
- Hamilton, B.; Martin, J.; Osterman, M.; Curtin, S. Births: Final Data for 2014; National Center for Health Statistics: Hyattsville, MD, USA, 2015.
- Doyle, C.; Werner, E.; Feng, T.; Lee, S.; Altemus, M.; Isler, J.R.; Monk, C. Pregnancy distress gets under fetal skin: Maternal ambulatory assessment & sex differences in prenatal development. Dev. Psychobiol. 2015, 57, 607–625. [Google Scholar] [PubMed]
- Matthews, A.; Haas, D.M.; O’Mathúna, D.P.; Dowswell, T. Interventions for nausea and vomiting in early pregnancy. In Cochrane Database of Systematic Reviews; Wiley-Blackwell: Hoboken, NJ, USA, 2015. [Google Scholar]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Yali, A.M.; Lobel, M. Coping and distress in pregnancy: An investigation of medically high risk women. J. Psychosom. Obstet. Gynecol. 1999, 20, 39–52. [Google Scholar] [CrossRef]
- Lobel, M.; Cannella, D.L.; Graham, J.E.; DeVincent, C.; Schneider, J.; Meyer, B.A. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008, 27, 604. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, W.M.; Mazza, J.J. Reliability and validity of the reynolds adolescent depression scale with young adolescents. J. School Psychol. 1998, 36, 295–312. [Google Scholar] [CrossRef]
- Sarason, I.G.; Levine, H.M.; Basham, R.B.; Sarason, B.R. Assessing social support: The social support questionnaire. J. Personal. Soc. Psychol. 1983, 44, 127. [Google Scholar] [CrossRef]
- Subar, A.F.; Kirkpatrick, S.I.; Mittl, B.; Zimmerman, T.P.; Thompson, F.E.; Bingley, C.; Willis, G.; Islam, N.G.; Baranowski, T.; McNutt, S. The automated self-administered 24 h dietary recall (ASA24): A resource for researchers, clinicians and educators from the national cancer institute. J. Acad. Nutr. Diet. 2012, 112, 1134. [Google Scholar] [CrossRef] [PubMed]
- Carriquiry, A.L. Assessing the prevalence of nutrient inadequacy. Public Health Nutr. 1999, 2, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Poos, M.I. Dietary reference intakes: Summary of applications in dietary assessment. Public Health Nutr. 2002, 5, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes its Panel on Folate, Other B Vitamins, and Choline. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- Council, N.R. Weight Gain During Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- DeSalvo, K.B.; Olson, R.; Casavale, K.O. Dietary guidelines for americans. JAMA 2016, 315, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Young, B.E.; Cooper, E.M.; Pressman, E.; Queenan, R.A.; Olson, C.M.; Guillet, R.; O’Brien, K.O. Nutrient inadequacy is prevalent in pregnant adolescents, and prenatal supplement use may not fully compensate for dietary deficiencies. ICAN Infant Child Adolesc. Nutr. 2014, 6, 152–159. [Google Scholar] [CrossRef]
- McGuire, S. US department of agriculture and US department of health and human services, dietary guidelines for americans, 2010. Washington, DC: US government printing office, January 2011. Adv. Nutr. Int. Rev. J. 2011, 2, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Phelan, S. Prenatal nutrition: Special considerations. Minerva Ginecol. 2009, 61, 373–400. [Google Scholar] [PubMed]
- Buppasiri, P.; Lumbiganon, P.; Thinkhamrop, J.; Ngamjarus, C.; Laopaiboon, M. Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes. In Cochrane Database of Systematic Reviews; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Jacka, F.N.; Overland, S.; Stewart, R.; Tell, G.S.; Bjelland, I.; Mykletun, A. Association between magnesium intake and depression and anxiety in community-dwelling adults: The hordaland health study. Aust. N. Z. J. Psychiatry 2009, 43, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Akbaraly, T.N.; Brunner, E.J.; Ferrie, J.E.; Marmot, M.G.; Kivimaki, M.; Singh-Manoux, A. Dietary pattern and depressive symptoms in middle age. Br. J. Psychiatry 2009, 195, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, E.; Stronks, K.; Snijder, M.B.; Schene, A.H.; Lok, A.; de Vries, J.H.; Visser, M.; Brouwer, I.A.; Nicolaou, M. A combined high-sugar and high-saturated-fat dietary pattern is associated with more depressive symptoms in a multi-ethnic population: The helius (healthy life in an urban setting) study. Public Health Nutr. 2017, 20, 2374–2382. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villegas, A.; Verberne, L.; De Irala, J.; Ruíz-Canela, M.; Toledo, E.; Serra-Majem, L.; Martínez-González, M.A. Dietary fat intake and the risk of depression: The sun project. PLoS ONE 2011, 6, e16268. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villegas, A.; Toledo, E.; de Irala, J.; Ruiz-Canela, M.; Pla-Vidal, J.; Martínez-González, M.A. Fast-food and commercial baked goods consumption and the risk of depression. Public Health Nutr. 2012, 15, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Chocano-Bedoya, P.O.; O’reilly, E.J.; Lucas, M.; Mirzaei, F.; Okereke, O.I.; Fung, T.T.; Hu, F.B.; Ascherio, A. Prospective study on long-term dietary patterns and incident depression in middle-aged and older women. Am. J. Clin. Nutr. 2013, 98, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Baskin, R.; Hill, B.; Jacka, F.N.; O’Neil, A.; Skouteris, H. The association between diet quality and mental health during the perinatal period. A systematic review. Appetite 2015, 91, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Pina-Camacho, L.; Jensen, S.; Gaysina, D.; Barker, E. Maternal depression symptoms, unhealthy diet and child emotional–behavioural dysregulation. Psychol. Med. 2015, 45, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.H.; Kristal, A.R.; Neumark-Sztainer, D.; Rock, C.L.; Neuhouser, M.L. Psychological distress is associated with unhealthful dietary practices. J. Am. Diet. Assoc. 2002, 102, 699–703. [Google Scholar] [CrossRef]
- Jacka, F.N.; Ystrom, E.; Brantsaeter, A.L.; Karevold, E.; Roth, C.; Haugen, M.; Meltzer, H.M.; Schjolberg, S.; Berk, M. Maternal and early postnatal nutrition and mental health of offspring by age 5 years: A prospective cohort study. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Papier, K.; Ahmed, F.; Lee, P.; Wiseman, J. Stress and dietary behaviour among first-year university students in Australia: Sex differences. Nutrition 2015, 31, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Oliver, G.; Wardle, J.; Gibson, E.L. Stress and food choice: A laboratory study. Psychosom. Med. 2000, 62, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Zellner, D.A.; Loaiza, S.; Gonzalez, Z.; Pita, J.; Morales, J.; Pecora, D.; Wolf, A. Food selection changes under stress. Physiol. Behav. 2006, 87, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Unusan, N. Linkage between stress and fruit and vegetable intake among university students: An empirical analysis on Turkish students. Nutr. Res. 2006, 26, 385–390. [Google Scholar] [CrossRef]
- Singewald, N.; Sinner, C.; Hetzenauer, A.; Sartori, S.B.; Murck, H. Magnesium-deficient diet alters depression-and anxiety-related behavior in mice—Influence of desipramine and hypericum perforatum extract. Neuropharmacology 2004, 47, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.; Whittle, N.; Hetzenauer, A.; Singewald, N. Magnesium deficiency induces anxiety and HPA axis dysregulation: Modulation by therapeutic drug treatment. Neuropharmacology 2012, 62, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Laarakker, M.C.; van Lith, H.A.; Ohl, F. Behavioral characterization of A/J and C57BL/6J mice using a multidimensional test: Association between blood plasma and brain magnesium-ion concentration with anxiety. Physiol. Behav. 2011, 102, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Rajizadeh, A.; Mozaffari-Khosravi, H.; Yassini-Ardakani, M.; Dehghani, A. Serum magnesium status in patients subjects with depression in the city of Yazd in Iran 2013–2014. Biol. Trace Elem. Res. 2016, 171, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, E.K.; Littenberg, B. Magnesium intake and depression in adults. J. Am. Board Fam. Med. 2015, 28, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Yary, T.; Aazami, S.; Soleimannejad, K. Dietary intake of magnesium may modulate depression. Biol. Trace Elem. Res. 2013, 151, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Yary, T.; Lehto, S.M.; Tolmunen, T.; Tuomainen, T.P.; Kauhanen, J.; Voutilainen, S.; Ruusunen, A. Dietary magnesium intake and the incidence of depression: A 20-year follow-up study. J. Affect. Disord. 2016, 193, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Murck, H. Magnesium and affective disorders. Nutr. Neurosci. 2002, 5, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, G.; Cheng, L.A.; Mélo, E.N.; de Farias Júnior, J.C. Physical activity and social support in adolescents: A systematic review. Health Educ. Res. 2014, 29, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.K.; McLerran, D.; Turner-McGrievy, G.; Feng, Z.; Havas, S.; Sorensen, G.; Buller, D.; Beresford, S.A.; Nebeling, L. Mediation of adult fruit and vegetable consumption in the national 5 a day for better health community studies. Ann. Behav. Med. 2008, 35, 49. [Google Scholar] [CrossRef] [PubMed]
- Siega-Riz, A.M.; Hobel, C.J. Predictors of poor maternal weight gain from baseline anthropometric, psychpsocial, and demographic information in a hispanic population. J. Am. Diet. Assoc. 1997, 97, 1264–1268. [Google Scholar] [CrossRef]
- Sangi-Haghpeykar, H.; Lam, K.; Raine, S.P. Gestational weight gain among hispanic women. Matern. Child Health J. 2014, 18, 153–160. [Google Scholar] [CrossRef] [PubMed]
| Variable | N | % | Mean | SD | Min | Max |
|---|---|---|---|---|---|---|
| Age (years) | 18.0 | 1.2 | 14.0 | 20.0 | ||
| In relationship with father | ||||||
| Yes | 89 | 82% | ||||
| No | 17 | 16% | ||||
| missing | 2 | 2% | ||||
| Borough residence | ||||||
| Manhattan | 77 | 71% | ||||
| Bronx/Queens | 31 | 29% | ||||
| Overall family income | ||||||
| 0–$15,000 | 40 | 37% | ||||
| $16,000–$25,000 | 39 | 36% | ||||
| $26,000–$50,000 | 17 | 16% | ||||
| $51,000–$100,000 | 2 | 2% | ||||
| missing | 10 | 9% | ||||
| Employed | ||||||
| Yes | 21 | 20% | ||||
| No | 86 | 80% | ||||
| missing | 1 | 1% | ||||
| Level in school | ||||||
| 8th grade | 2 | 2% | ||||
| 9th grade | 9 | 8% | ||||
| 10th grade | 12 | 11% | ||||
| 11th grade | 23 | 22% | ||||
| 12th grade | 60 | 57% | ||||
| missing | 2 | 2% | ||||
| Pre-pregnancy BMI | 25.7 | 6.4 | 16.6 | 47.6 | ||
| missing | 1 | 1% | ||||
| Health Problems | ||||||
| None | 72 | 69% | ||||
| Asthma | 13 | 13% | ||||
| Other a | 19 | 18% | ||||
| missing | 4 | 4% | ||||
| Medication use | ||||||
| Prenatal vitamins | 88 | 81% | ||||
| Antibiotic | 16 | 15% | ||||
| Pain relief | 16 | 15% | ||||
| Asthma and allergy | 8 | 7% | ||||
| Other b | 15 | 14% | ||||
| Pregnancy History | ||||||
| Previous Pregnancy | 36 | 34% | ||||
| Previous Abortion | 20 | 19% | ||||
| Previous Miscarriage | 16 | 15% | ||||
| Perceived Stress c | 27.8 | 6.1 | 12.0 | 43.0 | ||
| missing | 1 | 1% | ||||
| Prenatal Distress d | 6.1 | 2.9 | 0.0 | 13.0 | ||
| missing | 2 | 2% | ||||
| Depressive Symptoms e | 63.6 | 14.5 | 34.0 | 110.0 | ||
| missing | 3 | 3% | ||||
| Social Support f | 5.5 | 0.7 | 2.9 | 6.0 | ||
| missing | 2 | 2% |
| Nutrient | EAR b | Mean | SD | Min–Max | Participant Below EAR for Pregnant Adolescents | |
|---|---|---|---|---|---|---|
| N | % | |||||
| Energy (kcal) | ND | 2133.0 | 1005.8 | 656.6–5831.6 | ||
| Carbohydrate (g) | 135 | 288.1 *** | 132.4 | 44.1–782.1 | 11 | 10% |
| Protein (g/(g/kg)) | 0.88 | 76.7/1.2 *** | 41.6/0.7 | 7.7/0.1–257.0/4.2 | 42 | 39% |
| Fat (g) | ND | 78.3 | 48.9 | 9.7–293.2 | ||
| Thiamin (µg) | 1.2 | 1.6 *** d | 0.9 | 0.1–5.3 | 36 | 33% |
| Thiamin (µg) | 1.2 | 3.1**** d | 1.2 | 0.5–7.1 | 8 | 7% |
| Riboflavin (mg) | 1.2 | 1.8 *** d | 1.0 | 0.2–6.3 | 29 | 27% |
| Riboflavin (mg) a | 1.2 | 3.2 **** d | 1.2 | 0.5–6.7 | 17 | 16% |
| Niacin (mg) | 14 | 21.0 *** | 10.2 | 1.0–60.3 | 27 | 25% |
| Niacin (mg) a | 14 | 37.29 **** d | 13.4 | 5.8–80.3 | 8 | 7% |
| Vitamin B6 (mg) | 1.6 | 1.9 ** | 0.9 | 0.2–5.4 | 45 | 42% |
| Vitamin B6 (mg) a | 1.6 | 4.0 **** d | 1.4 | 0.4–8.0 | 7 | 6% |
| Folate (µg) | 520 | 434.6 *** | 239.4 | 81.5–1566.6 | 78 | 72% |
| Folate (µg) a | 520 | 1086.5 **** d | 401.5 | 151.8–2366.6 | 15 | 14% |
| Vitamin B12 (mg) | 2.2 | 4.1 *** | 3.3 | 0.2–15.5 | 36 | 33% |
| Vitamin B12 (mg) a | 2.2 | 10.6 **** d | 4.6 | 0.2–23.5 | 10 | 9% |
| Vitamin C (mg) | 66/70 | 147.3 *** | 156.3 | 0.2–1040.6 | 39 | 36% |
| Vitamin C (mg) | 66/70 | 245.0 **** d | 164.9 | 10.5–1160.6 | 11 | 10% |
| Vitamin A (µg) | 530/550 | 543.5 ** | 632.0 | 51.0–5356.9 | 74 | 69% |
| Vitamin A (µg) a | 530/550 | 1522.1 **** d | 692.1 | 51.0–5356.91 | 12 | 11% |
| Vitamin E (mg) | 12 | 6.8 *** | 4.27 | 0.75–22.7 | 97 | 90% |
| Vitamin E (mg) | 12 | 31.2 **** | 12.86 | 0.75–52.7 | 20 | 19% |
| Iron (mg) | 23/22 | 16.0 *** | 9.28 | 1.58–47.8 | 87 | 81% |
| Iron (mg) a | 23/22 | 38.8 **** d | 14.15 | 5.27–75.8 | 17 | 16% |
| Zinc (mg) | 10.5/9.5 | 10.5 | 6.98 | 1.47–51.0 | 61 | 56% |
| Zinc (mg) a | 10.5/9.5 | 30.9 **** d | 12.95 | 2.43–76.0 | 7 | 13% |
| Calcium (mg) | 1000/800 | 854.5 * | 541.5 | 99.6–2483.1 | 72 | 67% |
| Calcium (mg) a | 1000/800 | 1017.5 **** d | 554.9 | 99.6–2683.1 | 66 | 61% |
| Magnesium (mg) | 335/290 | 282.1 ** | 146.1 | 75.4–914.6 | 74 | 69% |
| Phosphorus (mg) | 1055/580 | 1164.2 **** | 591.7 | 196.9–3097.5 | 77 | 71% |
| Copper (mg) | 0.79/0.80 | 1.3 **** | 0.7 | 0.2–4.3 | 27 | 25% |
| Selenium (µg) | 49 | 94.5 | 60.2 | 5.5–427.3 | 21 | 19% |
| Potassium (mg) | 4700 c | 2928.7 | 1528.0 | 288.0–8934.1 | ||
| Sodium (mg) | 1500 c | 3194.0 | 1660.0 | 891–8672.0 | ||
| Energy | Carbohydrates | Fats | Proteins | Thiamin | Thiamin a | Riboflavin | Riboflavin a | |
|---|---|---|---|---|---|---|---|---|
| Unadjusted coefficients | ||||||||
| Perceived Stress | 0.15 | 0.15 | 0.12 | 0.07 | 0.02 | −0.04 | −0.03 | 0.04 |
| Prenatal Distress | −0.10 | −0.09 | −0.11 | −0.11 | −0.19 | −0.11 | −0.14 | −0.09 |
| Depressive Symptoms | 0.25 ** | 0.24 * | 0.22 * | 0.14 | 0.11 | 0.17 | 0.12 | 0.19 |
| Social Support | 0.01 | 0.03 | 0.00 | −0.02 | 0.17 | 0.29** | 0.11 | 0.23 * |
| Energy adjusted coefficients | ||||||||
| Perceived Stress | 0.03 | 0.02 | −0.13 | −0.15 | −0.12 | −0.09 | −0.07 | |
| Prenatal Distress | 0.01 | −0.01 | −0.04 | −0.15 | −0.03 | −0.10 | −0.03 | |
| Depressive Symptoms | −0.02 | 0.06 | −0.10 | −0.12 | 0.04 | −0.07 | 0.03 | |
| Social Support | 0.05 | −0.03 | −0.05 | 0.26 | 0.19 * | 0.15 | 0.23 * | |
| Niacin | Niacin a | Vitamin B6 | Vitamin B6 a | Folate | Folate a | Vitamin B12 | Vitamin B12 a | |
| Unadjusted coefficients | ||||||||
| Perceived Stress | 0.04 | 0.08 | 0.01 | 0.04 | 0.00 | 0.04 | −0.13 | −0.04 |
| Prenatal Distress | −0.10 | −0.05 | −0.23 * | −0.14 | −0.15 | −0.09 | −0.14 | −0.07 |
| Depressive Symptoms | 0.17 | 0.24 * | 0.12 | 0.21 * | 0.07 | 0.17 | 0.04 | 0.15 |
| Social Support | 0.09 | 0.22 * | 0.09 | 0.29 ** | 0.07 | 0.29 ** | 0.03 | 0.21 * |
| Energy adjusted coefficients | ||||||||
| Perceived Stress | −0.12 | −0.03 | −0.07 | −0.06 | −0.11 | −0.04 | −0.09 | −0.12 |
| Prenatal Distress | 0.00 | 0.03 | −0.20 | −0.1 | −0.07 | −0.04 | −0.05 | −0.03 |
| Depressive Symptoms | 0.01 | 0.09 | −0.06 | 0.06 | −0.13 | 0.04 | −0.06 | 0.04 |
| Social Support | 0.15 | 0.24 * | 0.13 | 0.31 ** | 0.09 | 0.29 ** | 0.03 | 0.19 * |
| Vitamin C | Vitamin C a | Vitamin A | Vitamin A a | Vitamin E | Vitamin E a | Iron | Iron a | |
| Unadjusted coefficients | ||||||||
| Perceived Stress | −0.03 | −0.02 | 0.01 | 0.03 | 0.21 * | 0.17 | 0.00 | 0.03 |
| Prenatal Distress | −0.22 * | −0.22 * | −0.13 | −0.15 | −0.09 | −0.06 | −0.11 | −0.05 |
| Depressive Symptoms | −0.11 | −0.05 | 0.05 | 0.11 | 0.18 | 0.23 * | 0.11 | 0.21 * |
| Social Support | 0.16 | 0.31 * | 0.05 | 0.12 | −0.02 | 0.23 * | 0.06 | 0.25 ** |
| Energy adjusted coefficients | ||||||||
| Perceived Stress | −0.01 | −0.08 | 0.09 | −0.06 | 0.14 | 0.10 | −0.17 | −0.08 |
| Prenatal Distress | −0.22 * | −0.19 | −0.13 | −0.11 | 0.01 | 0.00 | −0.03 | 0.02 |
| Depressive Symptoms | −0.16 | −0.15 | −0.04 | −0.03 | 0.05 | 0.09 | −0.12 | 0.06 |
| Social Support | 0.16 | 0.30 ** | 0.05 | 0.09 | −0.04 | 0.22 * | 0.08 | 0.26 ** |
| Zinc | Zinc a | Calcium | Calcium a | Magnesium | Phosphorus | Copper | Selenium | |
| Unadjusted coefficients | ||||||||
| Perceived Stress | −0.01 | 0.00 | 0.02 | 0.03 | 0.06 | 0.09 | 0.15 | 0.14 |
| Prenatal Distress | −0.11 | −0.07 | −0.14 | −0.15 | −0.18 | −0.12 | −0.12 | −0.01 |
| Depressive Symptoms | 0.09 | 0.15 | 0.16 | 0.11 | 0.12 | 0.17 | 0.19 | 0.23 * |
| Social Support | −0.07 | 0.25 ** | 0.07 | 0.12 | 0.04 | 0.02 | 0.05 | 0.01 |
| Energy adjusted coefficients | ||||||||
| Perceived Stress | −0.19 | −0.12 | −0.03 | −0.06 | −0.16 | −0.02 | −0.07 | −0.03 |
| Prenatal Distress | −0.02 | −0.01 | −0.09 | −0.11 | −0.17 | −0.11 | −0.08 | 0.09 |
| Depressive Symptoms | −0.17 | −0.01 | 0.03 | −0.03 | −0.20 * | −0.07 | −0.09 | −0.02 |
| Social Support | −0.11 | 0.26 ** | 0.08 | 0.09 | 0.05 | 0.02 | 0.06 | 0.00 |
| Above EAR | Below EAR | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | F | p-Value | |
| Prenatal Distress | ||||||||
| Calcium | 35 | 5.1 | 2.5 | 71 | 6.6 | 2.9 | 3.2 | 0.0442 |
| Depressive Symptoms | ||||||||
| Zinc a | 94 | 64.6 | 14.5 | 11 | 54.8 | 11.0 | 3.3 | 0.0412 |
| Vitamin E a | 87 | 64.9 | 14.4 | 18 | 57.5 | 13.8 | 3.4 | 0.0359 |
| Social Support | ||||||||
| Folate a | 92 | 5.4’ | 0.9 | 14 | 4.2 | 1.9 | 7.6 | 0.0008 |
| Iron a | 90 | 5.4 | 0.9 | 16 | 4.3 | 1.8 | 6.6 | 0.0020 |
| Vitamin C a | 96 | 5.4 | 0.9 | 10 | 3.8 | 2.1 | 11.0 | <0.0001 |
| Riboflavin a | 90 | 5.4 | 0.9 | 16 | 4.3 | 1.8 | 6.6 | 0.0020 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Trumpff, C.; Genkinger, J.; Davis, A.; Spann, M.; Werner, E.; Monk, C. Micronutrient Dietary Intake in Latina Pregnant Adolescents and Its Association with Level of Depression, Stress, and Social Support. Nutrients 2017, 9, 1212. https://doi.org/10.3390/nu9111212
Singh A, Trumpff C, Genkinger J, Davis A, Spann M, Werner E, Monk C. Micronutrient Dietary Intake in Latina Pregnant Adolescents and Its Association with Level of Depression, Stress, and Social Support. Nutrients. 2017; 9(11):1212. https://doi.org/10.3390/nu9111212
Chicago/Turabian StyleSingh, Angelie, Caroline Trumpff, Jeanine Genkinger, Alida Davis, Marisa Spann, Elizabeth Werner, and Catherine Monk. 2017. "Micronutrient Dietary Intake in Latina Pregnant Adolescents and Its Association with Level of Depression, Stress, and Social Support" Nutrients 9, no. 11: 1212. https://doi.org/10.3390/nu9111212
APA StyleSingh, A., Trumpff, C., Genkinger, J., Davis, A., Spann, M., Werner, E., & Monk, C. (2017). Micronutrient Dietary Intake in Latina Pregnant Adolescents and Its Association with Level of Depression, Stress, and Social Support. Nutrients, 9(11), 1212. https://doi.org/10.3390/nu9111212





