Effect of Altering Dietary n-6:n-3 Polyunsaturated Fatty Acid Ratio with Plant and Marine-Based Supplement on Biomarkers of Bone Turnover in Healthy Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Study Diets
2.4. Data Collection and Analyses
2.5. Statistical Analyses
3. Results
3.1. Nutrient Analyses and Dietary Compliance
3.2. N-3 Fatty Acids and Bone Markers
3.3. Correlation between Bone Markers, n-3 Fatty Acids, and Age
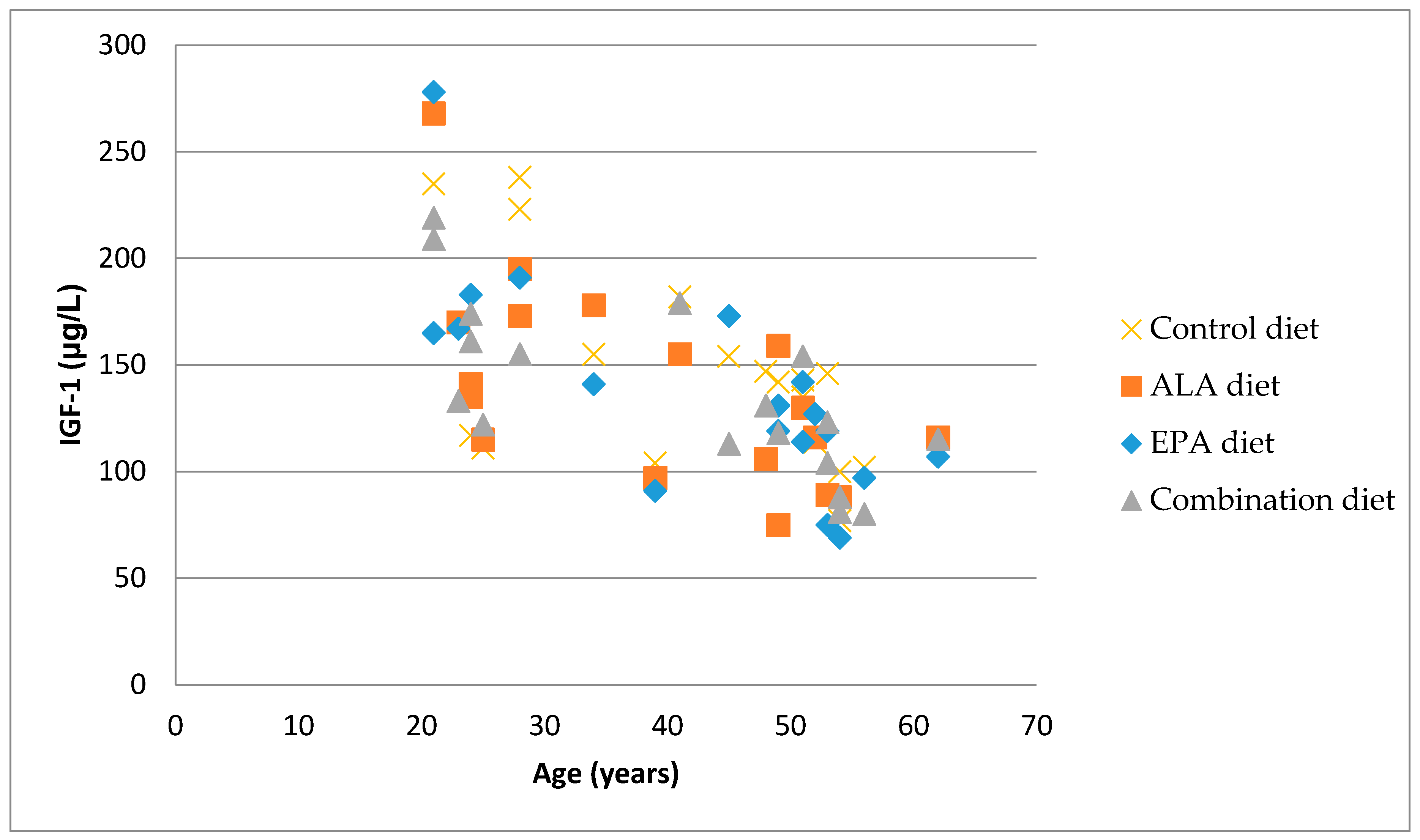
3.4. Correlation between Bone Markers and IGF-1
3.5. Gene Expression
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: A science advisory from the American heart association. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef]
- Chen, C.; Yu, X.; Shao, S. Effects of omega-3 fatty acid supplementation on glucose control and lipid levels in type 2 diabetes: A meta-analysis. PLoS ONE 2015, 10, e0139565. [Google Scholar] [CrossRef]
- Sanders, T.A. Protective effects of dietary PUFA against chronic disease: Evidence from epidemiological studies and intervention trials. Proc. Nutr. Soc. 2014, 73, 73–79. [Google Scholar] [CrossRef]
- Griel, A.E.; Kris-Etherton, P.M.; Hilpert, K.F.; Zhao, G.; West, S.G.; Corwin, R.L. An increase in dietary n-3 fatty acids decreases a marker of bone resorption in humans. Nutr. J. 2007, 6, 2. [Google Scholar] [CrossRef]
- Weiss, L.A.; Barrett-Connor, E.; von Muhlen, D. Ratio of n-6 to n-3 fatty acids and bone mineral density in older adults: The Rancho Bernardo Study. Am. J. Clin. Nutr. 2005, 81, 934–938. [Google Scholar]
- Sun, D.; Krishnan, A.; Zaman, K.; Lawrence, R.; Bhattacharya, A.; Fernandes, G. Dietary n-3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J. Bone Miner. Res. 2003, 18, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, H.; Barrios, J.A.; Shea, J.E.; Miller, S.C. Dietary fish oil results in a greater bone mass and bone formation indices in aged ovariectomized rats. J. Bone Miner. Metab. 2008, 26, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Pauneascu, A.C.; Ayotte, P.; Dewailly, E.; Dodin, S.; Pedersen, H.S.; Mulvad, G.; Côté, S. Polyunsaturated fatty acids and calcaneal ultrasound parameters among Inuit women from Nuuk (Greenland): A longitudinal study. Int. J. Circumpolar Health 2013, 72, 20988. [Google Scholar] [CrossRef] [PubMed]
- Salari, P.; Rezaie, A.; Larijani, B.; Abdollahi, M. A systematic review of the impact of n-3 fatty acids in bone health and osteoporosis. Med. Sci. Monit. 2008, 14, RA37–RA44. [Google Scholar] [PubMed]
- Watkins, B.A.; Li, Y.; Lippman, H.E.; Feng, S. Modulatory effect of omega-3 polyunsaturated fatty acids on osteoblast function and bone metabolism. Prostaglandins Leukot. Essent. Fatty Acids 2003, 68, 387–398. [Google Scholar] [CrossRef]
- Albertazzi, P.; Coupland, K. Polyunsaturated fatty acids: Is there a role in postmenopausal osteoporosis prevention. Maturitas 2002, 42, 13–22. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Conversion of α-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipids Res. 2016, 64, 30–56. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.C.; Coetzer, H.; de Winter, R.; Gericke, G.; van Papendorp, D.H. Calcium, gamma-linolenic acid and eicosapentaenoic acid supplementation in senile osteoporosis. Aging Clin. Exp. Res. 1998, 10, 385–394. [Google Scholar] [CrossRef]
- Van Papendrop, D.H.; Coetzer, H.; Kruger, M.G. Biochemical profile of osteoporotic patients on essential fatty acids supplementation. Nutr. Res. 1995, 15, 325–334. [Google Scholar] [CrossRef]
- Martin-Bautista, E.; Muñoz-Torres, M.; Fonolla, J.; Quesada, M.; Poyatos, A.; Lopez-Huertas, E. Improvement of bone formation biomarkers after 1-year consumption with milk fortified with eicosapentaenoic acid, docosahexaenoic acid, oleic acid, and selected vitamins. Nutr. Res. 2010, 30, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Fonolla-Joya, J.; Reyes-Garcia, R.; Garcia-Martin, A.; Lopez-Huertas, E.; Munoz-Torres, M. Daily intake of milk enriched with n-3 fatty acids, oleic acid and calcium improves metabolic and bone biomarkers in postmenopausal women. J. Am. Coll. Nutr. 2016, 35, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Wien, M.; Rajaram, S.; Oda, K.; Sabaté, J. Decreasing the linoleic acid to alpha-linolenic acid diet ratio increases eicosapentaenoic acid in erythrocytes in adults. Lipids 2010, 45, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Redmond, J.; Fulford, A.J.; Jarjou, L.; Zhou, B.; Prentice, A.; Schoenmakers, I. Diurnal rhythms of bone turnover markers in three ethnic groups. J. Clin. Endocrinol. Metab. 2016, 101, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Haddad, E.H.; Mejia, A.; Sabate, J. Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: A randomized controlled study. Am. J. Clin. Nutr. 2009, 89, 1657S–1663S. [Google Scholar] [CrossRef] [PubMed]
- Hogstrom, M.; Nordstrom, P.; Nordstrom, A. N-3 Fatty acids are positively associated with peak bone mineral density and bone accrual in healthy men: The NO2 Study. Am. J. Clin. Nutr. 2007, 85, 803–807. [Google Scholar] [PubMed]
- Farina, E.K.; Kiel, D.P.; Roubenhoff, R.; Schaefer, E.J.; Cupples, L.A.; Tucker, K.L. Dietary intakes of arachidonic acid and α-linolenic acid are associated with reduced risk of hip fracture in older adults. J. Nutr. 2011, 141, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Weiler, H.A.; Fitzpatrick-Wong, S.C. Modulation of essential (n-6):(n-3) fatty acid ratios alters fatty acid status but not bone mass in piglets. J. Nutr. 2002, 132, 2667–2672. [Google Scholar] [PubMed]
- Mangano, K.M.; Sahni, S.; Kerstetter, J.E.; Kenny, A.M.; Hannan, M.T. Polyunsaturated fatty acids and their relation to bone and muscle health in adults. Curr. Osteoporos. Rep. 2013, 11, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, M.; Haag, M.; Kruger, M.C. Effects of arachidonic acid, docosahexaenoic acid, prostaglandin E(2) and parathyroid hormone on osteoprotegerin and RANKL secretion by MC3T3-E1 osteoblast-like cells. J. Nutr. Biochem. 2007, 18, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Mollard, R.C.; Gillam, M.E.; Wood, T.M.; Taylor, C.G.; Weiler, H.A. (n-3) fatty acids reduce the release of prostaglandin E2 from bone but do not affect bone mass in obese (fa/fa) and lean Zucker rats. J. Nutr. 2005, 135, 499–504. [Google Scholar] [PubMed]
- Poulsen, R.C.; Moughan, P.J.; Kruger, M.C. Long-chain polyunsaturated fatty acids and the regulation of bone metabolism. Exp. Biol. Med. 2007, 232, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.A.; Li, Y.; Seifert, M.F. Dietary ratio of n-6/n-3 PUFAs and docosahexaenoic acid: Actions on bone mineral and serum biomarkers in ovariectomized rats. J. Nutr. Biochem. 2006, 17, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.Z.; Wu, X.P.; Liu, S.P.; Luo, X.H.; Cao, X.Z.; Xie, H.; Liao, E.Y. Age-related changes in bone biochemical markers and their relationship with bone mineral density in normal Chinese women. J. Bone Miner. Metab. 2006, 24, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P.; Delmas, P.D. Biochemical markers of bone turnover in men. Calcif. Tissue Int. 2001, 69, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Claudon, A.; Vergnaud, P.; Valverde, C.; Mayr, A.; Klause, U.; Garnero, P. New automated multiplex assay for bone turnover markers in osteoporosis. Clin. Chem. 2008, 54, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Baylin, D.J.; Mohan, S. Insulin-like growth factor binding proteins in serum and other biological fluids: Regulation and functions. Endocr. Rev. 1997, 18, 801–831. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, J.D.; Iranmanesh, A.; Bowers, C.Y. Joint mechanisms of impaired growth-hormone pulse renewal in aging men. J. Clin. Endocrinol. Metab. 2005, 90, 4177–4183. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Long-chain fatty acids and gene expression in inflammation and immunity. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Koehne, T.; Todter, K.; Reimer, R.; Muller, B.; Behler-Janbeck, F.; Heeren, J.; Scheja, L.; Niemeier, A. Quantification of bone fatty acid metabolism and its regulation by adipocyte lipoprotein lipase. Int. J. Mol. Sci. 2017, 18, 1264. [Google Scholar] [CrossRef] [PubMed]
| Bone Markers | |||
|---|---|---|---|
| Diet | CTX (ng/mL) | PINP (µg/L) | OC (ng/mL) |
| Control (10:1) 2 | 0.538 (0.041) | 54.68 (2.96) | 18.46 (1.13) |
| EPA/DHA (10:1 + S) | 0.480 (0.041) | 51.44 (2.96) | 18.01 (1.13) |
| ALA (2:1) | 0.588 (0.041) | 50.10 (2.96) | 16.34 (1.13) |
| Combination (2:1 + S) | 0.583 (0.041) | 50.89 (2.96) | 16.91 (1.13) |
| Bone Markers | n-3 Fatty Acid | Estimate | p-Value |
|---|---|---|---|
| CTX | LA | −0.058 (0.023) | 0.0143 |
| ALA | −0.419 (0.208) | 0.0477 | |
| EPA | 0.068 (0.133) | NS | |
| DHA | 0.038 (0.018) | 0.0385 | |
| Age | −0.017 (0.00345) | <0.0001 | |
| P1NP | LA | 0.083 (1.50) | NS |
| ALA | 11.18 (13.13) | NS | |
| EPA | −11.46 (8.19) | NS | |
| DHA | −1.50 (1.13) | NS | |
| Age | −0.909 (0.225) | 0.0006 | |
| OC | LA | −0.903 (0.614) | NS |
| ALA | −2.98 (5.32) | NS | |
| EPA | −1.81 (3.36) | NS | |
| DHA | −0.102 (0.463) | NS | |
| Age | −0.429 (0.122) | 0.0019 |
| Diets | Fold Change ± SEM | p-Value |
|---|---|---|
| 10:1 versus 2:1 | 1.72 ± 0.26 | 0.19 |
| 10:1 versus 10:1 + S | 2.02 ± 0.32 | 0.11 |
| 10:1 versus 2:1 + S | 2.20 ± 0.55 | 0.38 |
| 2:1 versus 10:1 + S | 1.40 ± 0.22 | 0.55 |
| 2:1 versus 2:1 + S | 1.52 ± 0.38 | 0.85 |
| 10:1 + S versus 2:1 + S | 1.32 ± 0.33 | 0.55 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajaram, S.; Yip, E.L.; Reghunathan, R.; Mohan, S.; Sabaté, J. Effect of Altering Dietary n-6:n-3 Polyunsaturated Fatty Acid Ratio with Plant and Marine-Based Supplement on Biomarkers of Bone Turnover in Healthy Adults. Nutrients 2017, 9, 1162. https://doi.org/10.3390/nu9101162
Rajaram S, Yip EL, Reghunathan R, Mohan S, Sabaté J. Effect of Altering Dietary n-6:n-3 Polyunsaturated Fatty Acid Ratio with Plant and Marine-Based Supplement on Biomarkers of Bone Turnover in Healthy Adults. Nutrients. 2017; 9(10):1162. https://doi.org/10.3390/nu9101162
Chicago/Turabian StyleRajaram, Sujatha, Ellen Lan Yip, Rajneesh Reghunathan, Subburaman Mohan, and Joan Sabaté. 2017. "Effect of Altering Dietary n-6:n-3 Polyunsaturated Fatty Acid Ratio with Plant and Marine-Based Supplement on Biomarkers of Bone Turnover in Healthy Adults" Nutrients 9, no. 10: 1162. https://doi.org/10.3390/nu9101162
APA StyleRajaram, S., Yip, E. L., Reghunathan, R., Mohan, S., & Sabaté, J. (2017). Effect of Altering Dietary n-6:n-3 Polyunsaturated Fatty Acid Ratio with Plant and Marine-Based Supplement on Biomarkers of Bone Turnover in Healthy Adults. Nutrients, 9(10), 1162. https://doi.org/10.3390/nu9101162






