Stability of Commercially Available Macular Carotenoid Supplements in Oil and Powder Formulations
Abstract
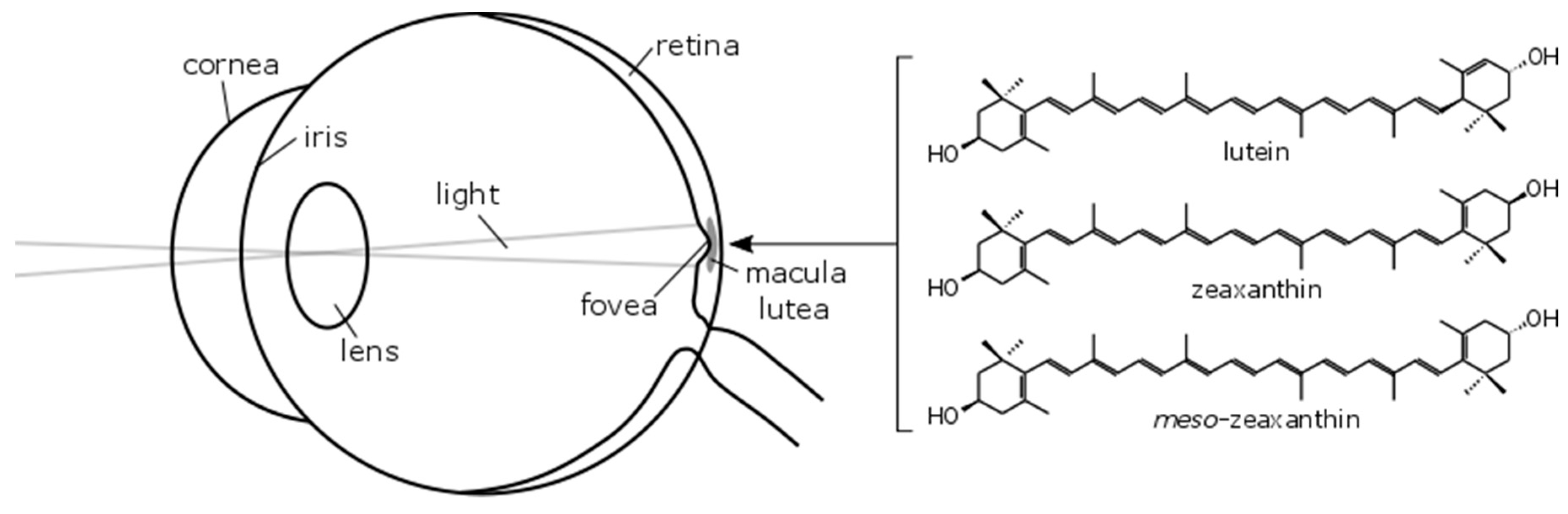
:1. Introduction
2. Materials and Methods
2.1. Supplements Analysed
2.2. Macular Carotenoid Standards and Solvents
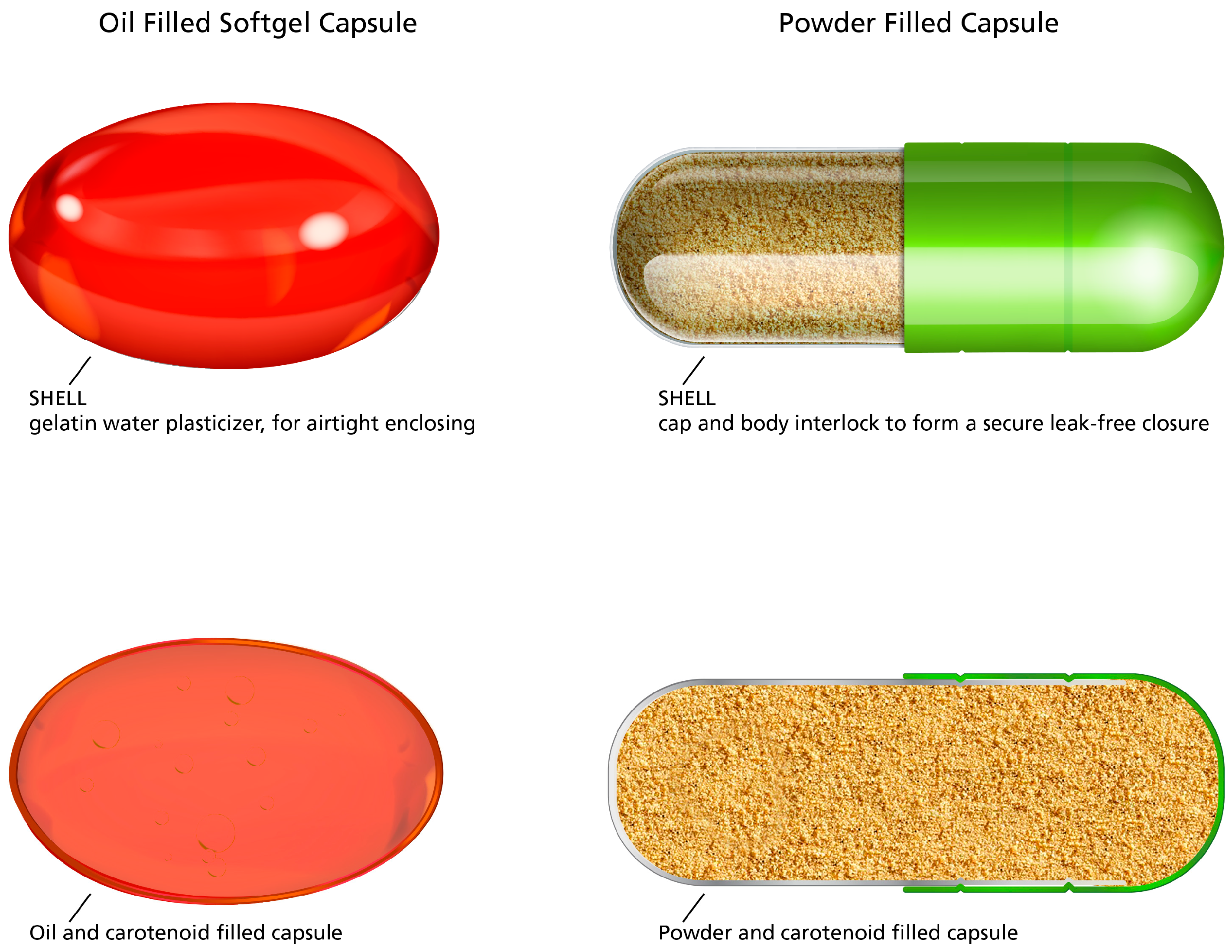
2.3. Supplement Extraction Method 1: Oil Filled Soft Gel Capsules and Powder Filled Capsules
2.4. Supplement Extraction Method 2: Beadlet Analysis
2.5. High Performance Liquid Chromatography
3. Results
3.1. Oil Filled Soft Gel Capsules
3.2. Powder Filled Capsules
3.3. Powder Filled Beadlet Capsules
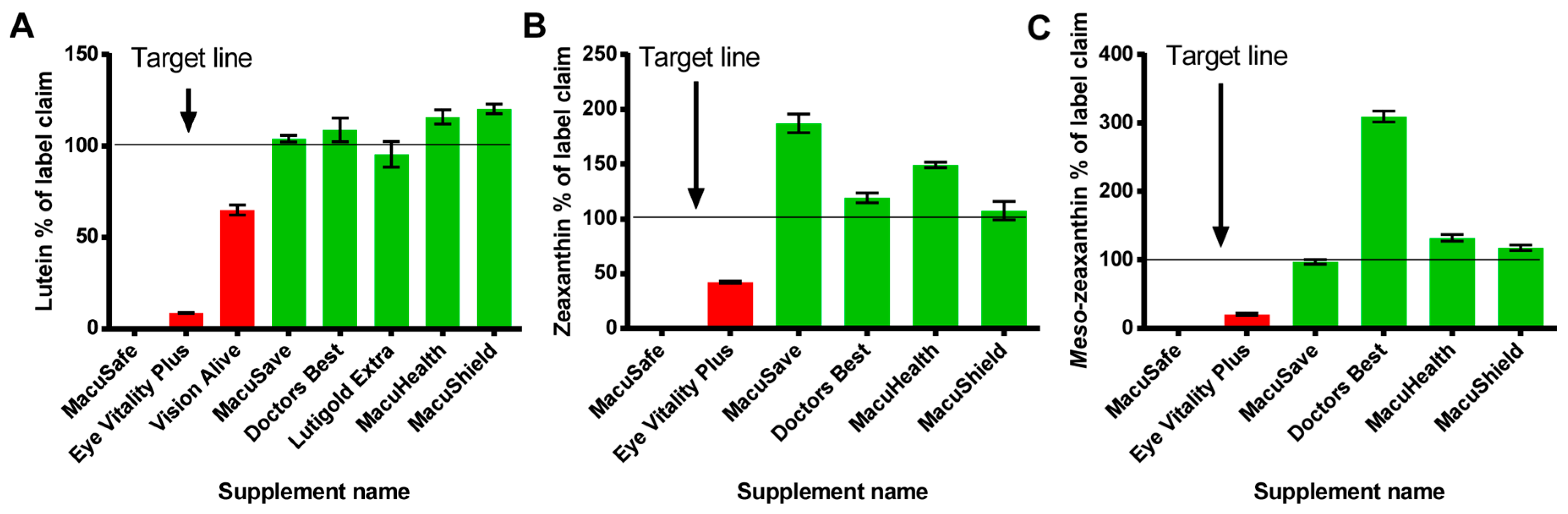
3.4. Stability of Carotenoid Supplements
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hammond, B.R., Jr.; Johnson, E.J.; Russell, R.M.; Krinsky, N.I.; Yeum, K.J.; Edwards, R.B.; Snodderly, D.M. Dietary modification of human macular pigment density. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1795–1801. [Google Scholar]
- Hirsch, J.; Curcio, C.A. The spatial resolution capacity of human foveal retina. Vis. Res. 1989, 29, 1095–1101. [Google Scholar] [CrossRef]
- Naguib, Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Snodderly, D.M. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am. J. Clin. Nutr. 1995, 62, 1448s–1461s. [Google Scholar] [PubMed]
- Snodderly, D.M.; Auran, J.D.; Delori, F.C. The macular pigment. II. Spatial distribution in primate retinas. Investig. Ophthalmol. Vis. Sci. 1984, 25, 674–685. [Google Scholar]
- Bone, R.A.; Landrum, J.T.; Hime, G.W.; Cains, A.; Zamor, J. Stereochemistry of the human macular carotenoids. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2033–2040. [Google Scholar]
- Hammond, B.R., Jr.; Wooten, B.R.; Snodderly, D.M. Preservation of visual sensitivity of older subjects: Association with macular pigment density. Investig. Ophthalmol. Vis. Sci. 1998, 39, 397–406. [Google Scholar]
- Howarth, P.A.; Bradley, A. The longitudinal chromatic aberration of the human eye, and its correction. Vision Res. 1986, 26, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ahmed, F.; Bernstein, P.S. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Arch. Biochem. Biophys. 2010, 504, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Maras, J.E.; Rasmussen, H.M.; Tucker, K.L. Intake of lutein and zeaxanthin differ with age, sex, and ethnicity. J. Am. Diet. Assoc. 2010, 110, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Nebeling, L.C.; Forman, M.R.; Graubard, B.I.; Snyder, R.A. Changes in carotenoid intake in the United States: The 1987 and 1992 national health interview surveys. J. Am. Diet. Assoc. 1997, 97, 991–996. [Google Scholar] [CrossRef]
- Maoka, T.; Arai, A.; Shimizu, M.; Matsuno, T. The first isolation of enantiomeric and meso-zeaxanthin in nature. Comp. Biochem. Physiol. B 1986, 83, 121–124. [Google Scholar] [CrossRef]
- Khachik, F.; de Moura, F.F.; Zhao, D.Y.; Aebischer, C.P.; Bernstein, P.S. Transformations of selected carotenoids in plasma, liver, and ocular tissues of humans and in nonprimate animal models. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3383–3392. [Google Scholar]
- Nolan, J.M.; Beatty, S.; Meagher, K.A.; Howard, A.N.; Kelly, D.; Thurnham, D.I. Verification of Meso-zeaxanthin in fish. J. Food Process. Technol. 2014, 5, 335. [Google Scholar] [CrossRef] [PubMed]
- Prado-Cabrero, A.; Beatty, S.; Howard, A.; Stack, J.; Bettin, P.; Nolan, J.M. Assessment of lutein, zeaxanthin and Meso-zeaxanthin concentrations in dietary supplements by chiral high-performance liquid chromatography. Eur. Food Res. Technol. 2016, 242, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.; Meagher, K.; Kashani, S.; Beatty, S. What is meso-zeaxanthin, and where does it come from? Eye 2013, 27, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.M.; Power, R.; Stringham, J.; Dennison, J.; Stack, J.; Kelly, D.; Moran, R.; Akuffo, K.O.; Corcoran, L.; Beatty, S. Enrichment of macular pigment enhances contrast sensitivity in subjects free of retinal disease: Central retinal enrichment supplementation trials—Report 1. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3429–3439. [Google Scholar] [CrossRef] [PubMed]
- Akuffo, K.; Nolan, J.; Howard, A.; Moran, R.; Stack, J.; Klein, R.; Klein, B.; Meuer, S.; Sabour-Pickett, S.; Thurnham, D.; et al. Sustained supplementation and monitored response with differing carotenoid formulations in early age-related macular degeneration. Eye 2015, 29, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.E.; Beatty, S.; Loughman, J.; Howard, A.N.; Louw, M.S.; Nolan, J.M. Supplementation with all three macular carotenoids: Response, stability, and safety. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9207–9217. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.E.; Beatty, S.; Thurnham, D.I.; Loughman, J.; Howard, A.N.; Stack, J.; Nolan, J.M. Augmentation of macular pigment following supplementation with all three macular carotenoids: An exploratory study. Curr. Eye Res. 2010, 35, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, R.; Du, J.H.; Liu, T.; Wu, S.S.; Liu, X.H. Lutein, zeaxanthin and meso-zeaxanthin supplementation associated with macular pigment optical density. Nutrients 2016, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Meagher, K.A.; Thurnham, D.I.; Beatty, S.; Howard, A.N.; Connolly, E.; Cummins, W.; Nolan, J.M. Serum response to supplemental macular carotenoids in subjects with and without age-related macular degeneration. Br. J. Nutr. 2013, 110, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.M.; Stringham, J.M.; Beatty, S.; Snodderly, D.M. Spatial profile of macular pigment and its relationship to foveal architecture. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- Thurnham, D.; Nolan, J.; Howard, A.; Beatty, S. Macular response to supplementation with differing xanthophyll formulations in subjects with and without age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Crosby-Nwaobi, R.; Hykin, P.; Peto, T.; Sivaprasad, S. An exploratory study evaluating the effects of macular carotenoid supplementation in various retinal diseases. Clin. Ophthalmol. 2016, 10, 835–844. [Google Scholar] [PubMed]
- Breithaupt, E.D.; Schlatterer, J. Lutein and Zeaxanthin in New Dietary Supplements—Analysis and Quantification. Eur. Food Res. Technol. 2005, 220, 648–652. [Google Scholar] [CrossRef]
- Guidance for Industry: Current Good Manufacturing Practice in Manufacturing, Packaging, Labeling, or Holding Operations for Dietary Supplements; Small Entity Compliance Guide. Available online: https://www.fda.gov/food/guidanceregulation/guidancedocumentsregulatoryinformation/dietarysupplements/ucm238182.htm (accessed on 31 August 2017).
- Hu, M.; Jacobsen, C. Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Elsevier Science: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Gullapalli, R.P. Soft gelatin capsules (softgels). J. Pharm. Sci. 2010, 99, 4107–4148. [Google Scholar] [CrossRef] [PubMed]
- Shimidzu, N.; Goto, M.; Miki, W. Carotenoids as singlet oxygen quenchers in marine organisms. Fish. Sci. 1996, 62, 134–137. [Google Scholar] [CrossRef]
- Prado-Cabrero, A.; Beatty, S.; Stack, J.; Howard, A.; Nolan, J.M. Quantification of zeaxanthin stereoisomers and lutein in trout flesh using chiral high-performance liquid chromatography-diode array detection. J. Food Compost. Anal. 2016, 50, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Aman, R.; Bayha, S.; Carle, R.; Schieber, A. Determination of carotenoid stereoisomers in commercial dietary supplements by high-performance liquid chromatography. J. Agric. Food Chem. 2004, 52, 6086–6090. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [PubMed]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Deshpande, J. Stable Beadlets of Lipophilic Nutrients. U.S. Patent 2005/0095301 A1, 5 May 2005. [Google Scholar]
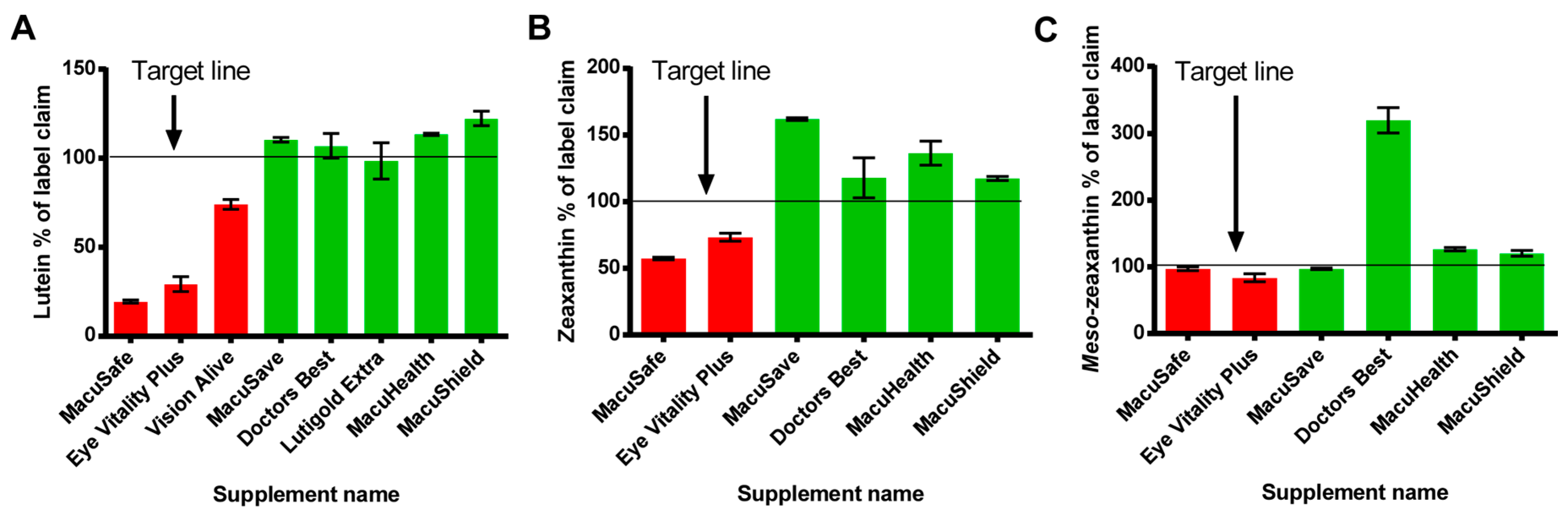
| Supplement Name | Type | Batch Number | Expiry | Time to Expiry at Time of Testing (Months) | Macular Carotenoid (mg/Capsule) | |
|---|---|---|---|---|---|---|
| Declared | Measured ± SD | |||||
| MacuSafe | 1 | 12603 | June 2019 | 28 | L→10 | 1.92 ± 0.09 |
| Z→2 | 1.14 ± 0.02 | |||||
| MZ→12 | 9.66 ± 0.28 | |||||
| Eye Vitality Plus | 1 | 13577 | August 2019 | 31 | L→15 | 4.35 ± 0.42 |
| Z→2 | 1.46 ± 0.06 | |||||
| MZ→10 | 8.32 ± 0.59 | |||||
| Vision Alive | 1 | 424951 | June 2018 | 14 | L→10 | 7.38 ± 0.27 |
| Z→nc | 1.24 ± 0.02 | |||||
| MZ→nc | 1.58 ± 0.04 | |||||
| MacuSave | 2 | 3001777 | August 2019 | 35 | L→10 | 11.01 ± 0.13 |
| Z→2 | 3.23 ± 0.02 | |||||
| MZ→10 | 9.67 ± 0.11 | |||||
| Doctors Best | 2 | 16052523A | December 2019 | 32 | L→20 | 21.33 ± 1.39 |
| Z→2 | 2.35 ± 0.30 | |||||
| MZ→1 | 3.19 ± 0.19 | |||||
| Lutigold Extra | 2 | 462536-02 | August 2019 | 27 | L→20 | 19.65 ± 1.02 |
| Z→nq | 2.14 ± 0.15 | |||||
| MZ→nq | 3.05 ± 0.11 | |||||
| MacuHealth | 2 | C1600284 | September 2019 | 25 | L→10 | 11.32 ± 0.06 |
| Z→2 | 2.72 ± 0.18 | |||||
| MZ→10 | 12.60 ± 0.26 | |||||
| MacuShield | 2 | 120480 | March 2017 | 4 | L→10 | 12.24 ± 0.41 |
| Z→2 | 11.98 ± 0.43 | |||||
| MZ→10 | 13.21 ± 0.48 | |||||
| Maxivision * | 3 | 33690818 | None | No expiry date on product | L→10 | 10.82 |
| Z→2 | 3.68 | |||||
| MZ→10 | 11.19 | |||||
| Supplement Name | Storage Time Months | Type | Batch Number | Expiry | Time to Expiry at Time of Testing (Months) | Macular Carotenoid (mg/Capsule) | |
|---|---|---|---|---|---|---|---|
| Declared | Measured ± SD | ||||||
| MacuSafe | 5 | 1 | 12603 | June 2019 | 23 | L→10 | 0.0 ± 0.0 |
| Z→2 | 0.0 ± 0.0 | ||||||
| MZ→12 | 0.0 ± 0.0 | ||||||
| Eye Vitality Plus | 6 | 1 | 13577 | August 2019 | 25 | L→15 | 0.84 ± 0.02 |
| Z→2 | 0.84 ± 0.02 | ||||||
| MZ→10 | 2.00 ± 0.19 | ||||||
| Vision Alive | 3 | 1 | 424951 | June 2018 | 11 | L→10 | 6.48 ± 0.27 |
| Z→nd | 1.14 ± 0.05 | ||||||
| MZ→nd | 1.52 ± 0.01 | ||||||
| MacuSave | 8 | 2 | 3001777 | August 2019 | 25 | L→10 | 10.39 ± 0.18 |
| Z→2 | 3.74 ± 0.17 | ||||||
| MZ→10 | 9.69 ± 0.33 | ||||||
| Doctors Best | 3 | 2 | 16052523A | December 2019 | 29 | L→20 | 21.74 ± 0.65 |
| Z→2 | 2.38 ± 0.09 | ||||||
| MZ→1 | 3.09 ± 0.08 | ||||||
| Lutigold Extra | 2 | 2 | 462536-02 | August 2019 | 25 | L→20 | 19.05 ± 0.70 |
| Z→nq | 1.99 ± 0.08 | ||||||
| MZ→nq | 2.97 ± 0.06 | ||||||
| MacuHealth | 5 | 2 | C1600284 | March 19 | 20 | L→10 | 11.58 ± 0.39 |
| Z→2 | 2.98 ± 0.05 | ||||||
| MZ→10 | 13.21 ± 0.48 | ||||||
| MacuShield | 3 | 2 | 120480 | March 2017 | 1 | L→10 | 11.75 ± 0.41 |
| Z→2 | 2.15 ± 0.17 | ||||||
| MZ→10 | 12.02 ± 0.27 | ||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phelan, D.; Prado-Cabrero, A.; Nolan, J.M. Stability of Commercially Available Macular Carotenoid Supplements in Oil and Powder Formulations. Nutrients 2017, 9, 1133. https://doi.org/10.3390/nu9101133
Phelan D, Prado-Cabrero A, Nolan JM. Stability of Commercially Available Macular Carotenoid Supplements in Oil and Powder Formulations. Nutrients. 2017; 9(10):1133. https://doi.org/10.3390/nu9101133
Chicago/Turabian StylePhelan, David, Alfonso Prado-Cabrero, and John M. Nolan. 2017. "Stability of Commercially Available Macular Carotenoid Supplements in Oil and Powder Formulations" Nutrients 9, no. 10: 1133. https://doi.org/10.3390/nu9101133






