Effects of a Short-Term High-Nitrate Diet on Exercise Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Experimental Overview
2.4. Exercise Tests
2.5. Measurements
2.6. Statistics
3. Results
3.1. Nitrate and Nitrite Plasma Levels
3.2. Moderate-Intensity Constant Work Rate Cycling Exercise
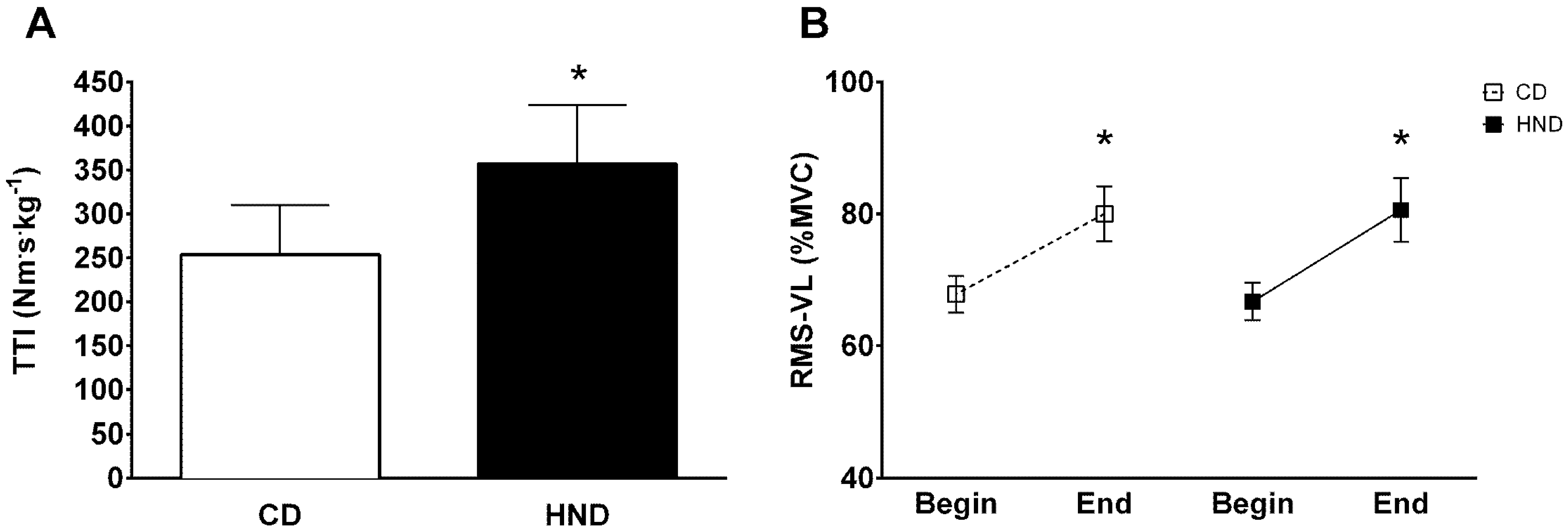
3.3. Isometric Knee Extension
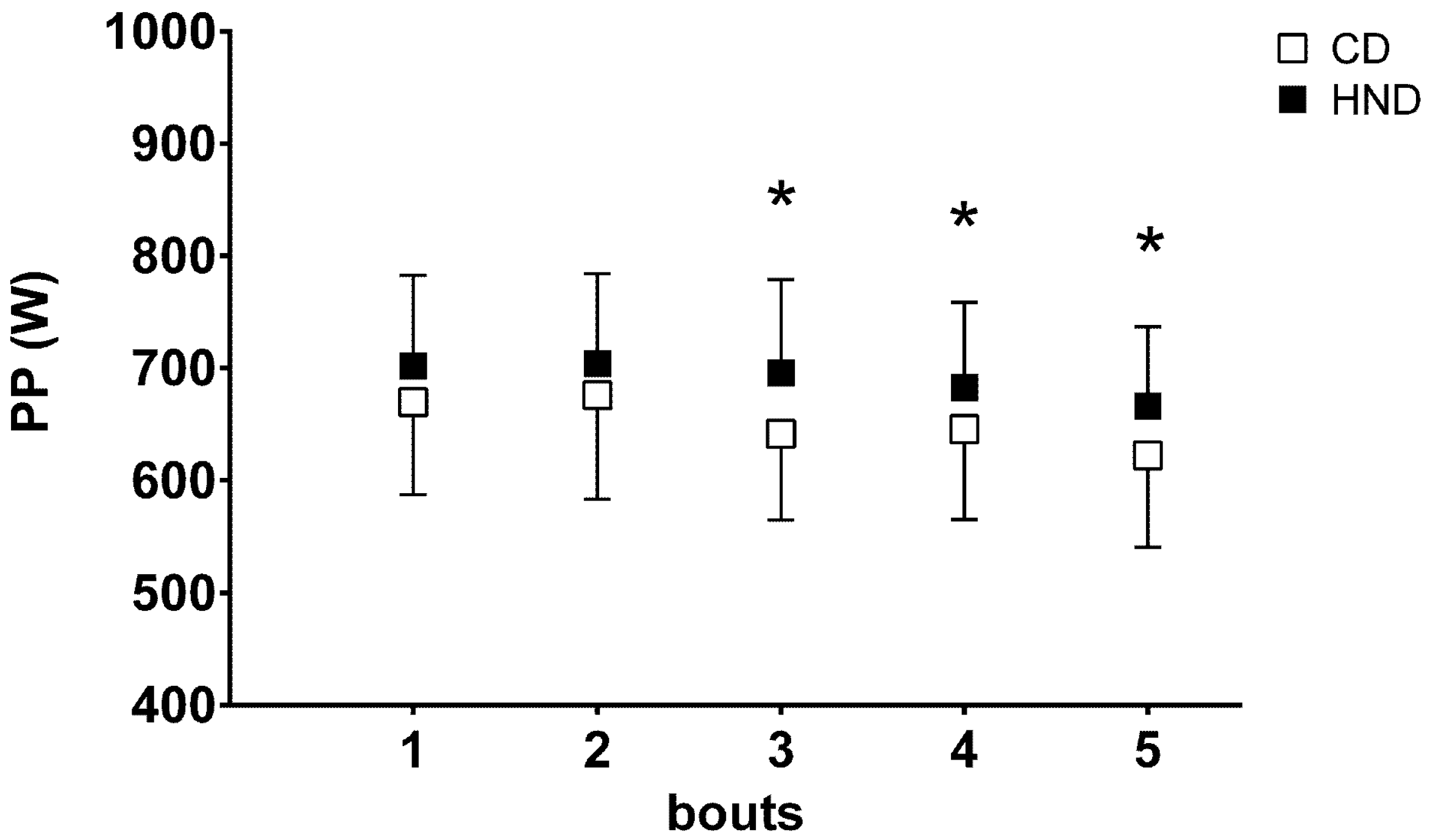
3.4. Repeated Sprint Ability (RSA)
4. Discussion
4.1. Plasma Nitrate/Nitrite Levels
4.2. Constant Work Rate Cycling Exercise
4.3. Intermittent High-Intensity Activities
4.4. Study Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stamler, J.S.; Meissner, G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 2001, 81, 209–237. [Google Scholar] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Cole, J.A.; Benjamin, N. Nitrate, bacteria and human health. Nat. Rev. Microbiol. 2004, 2, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic. Biol. Med. 2010, 48, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 2007, 191, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug. Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; Dimenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 4, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Fulford, J.; Vanhatalo, A.; Winyard, P.G.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol. 2010, 109, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; Dimenna, F.J.; Pavey, T.G.; Wilkerson, D.P.; Benjamin, N.; Winyard, P.G.; Jones, A.M. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1121–R1131. [Google Scholar] [CrossRef] [PubMed]
- Lansley, K.E.; Winyard, P.G.; Bailey, S.J.; Vanhatalo, A.; Wilkerson, D.P.; Blackwell, J.R.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Acute dietary nitrate supplementation improves cycling time trial performance. Med. Sci. Sports Exerc. 2011, 43, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, S.; Ramaglia, M.; Bellistri, G.; Pavei, G.; Pugliese, L.; Montorsi, M.; Rasica, L.; Marzorati, M. Aerobic Fitness Affects the Exercise Performance Responses to Nitrate Supplementation. Med. Sci. Sports Exerc. 2015, 47, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Hoon, M.W.; Johnson, N.A.; Chapman, P.G.; Burke, L.M. The effect of nitrate supplementation on exercise performance in healthy individuals: A systematic review and meta-analysis. Int. J. Sport. Nutr. Exerc. Metab. 2013, 23, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Modin, A.; Bjorne, H.; Herulf, M.; Alving, K.; Weitzberg, E.; Lundberg, J.O. Nitrite-derived nitric oxide: A possible mediator of ’acidic-metabolic’ vasodilation. Acta Physiol. Scand. 2001, 171, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.S.; Knight, D.R.; Poole, D.C.; Kurdak, S.S.; Hogan, M.C.; Grassi, B.; Wagner, P.D. Determinants of maximal exercise V’O2 during single leg knee-extensor exercise in humans. Am. J. Physiol. 1995, 268, H1453–H1461. [Google Scholar] [PubMed]
- Hoon, M.W.; Fornusek, C.; Chapman, P.G.; Johnson, N.A. The effects of nitrate supplementation on muscle contraction in healthy adults. Eur. J. Sport. Sci. 2015, 15, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Haider, G.; Folland, J.P. Nitrate Supplementation Enhances the Contractile Properties of Human Skeletal Muscle. Med. Sci. Sports Exerc. 2014, 46, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Fulford, J.; Winyard, P.G.; Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; Jones, A.M. Influence of dietary nitrate supplementation on human skeletal muscle metabolism and force production during maximum voluntary contractions. Pflugers Arch. 2013, 465, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Bond, H.; Morton, L.; Braakhuis, A.J. Dietary nitrate supplementation improves rowing performance in well-trained rowers. Int. J. Sport Nutr. Exerc. Metab. 2012, 4, 251–256. [Google Scholar] [CrossRef]
- Wylie, L.J.; Mohr, M.; Krustrup, P.; Jackman, S.R.; Ermιdis, G.; Kelly, J.; Black, M.I.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur. J. Appl. Physiol. 2013, 113, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Aucouturier, J.; Boissière, J.; Pawlak-Chaouch, M.; Cuvelier, G.; Gamelin, F.X. Effect of dietary nitrate supplementation on tolerance to supramaximal intensity intermittent exercise. Nitric Oxide 2015, 49, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Wylie, L.J.; Fulford, J.; Kelly, J.; Black, M.I.; McDonagh, S.T.; Jeukendrup, A.E.; Vanhatalo, A.; Jones, A.M. Dietary nitrate improves sprint performance and cognitive function during prolonged intermittent exercise. Eur. J. Appl. Physiol. 2015, 115, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.M.; Nyberg, M.; Bangsbo, J. Influence of nitrate supplementation on O2 kinetics and endurance of elite cyclists. Scand. J. Med. Sci. Sports 2012, 23, e21–e31. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Smee, D.; Thompson, K.G.; Rattray, B. No improvement of repeated-sprint performance with dietary nitrate. Int. J. Sports Physiol. Perform. 2014, 9, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Wylie, L.J.; Bailey, S.J.; Kelly, J.; Blackwell, J.R.; Vanhatalo, A.; Jones, A.M. Influence of beetroot juice supplementation on intermittent exercise performance. Eur. J. Appl. Physiol. 2016, 116, 415–525. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.; Eliot, K.; Heuertz, R.M.; Weiss, E. Whole Beetroot Consumption Acutely Improves Running Performance. J. Acad. Nutr. Diet. 2012, 112, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Liu, A.H.; Croft, K.D.; Ward, N.C.; Yang, X.; Considine, M.J.; Puddey, I.B.; Woodman, R.J.; Hodgson, J.M. Short-term effects of nitrate-rich green leafy vegetables on blood pressure and arterial stiffness in individuals with high-normal blood pressure. Free Radic. Biol. Med. 2014, 77, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, A.; Mitchell, K.; Blackwell, J.R.; Vanhatalo, A.; Jones, A.M. High-nitrate vegetable diet increases plasma nitrate and nitrite concentrations and reduces blood pressure in healthy women. Public Health Nutr. 2015, 18, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food chain on a request from the European Commission to perform a scientific risk assessment on nitrate in vegetables. In The EFSA Journal; EFSA: Parma, Italy, 2008; pp. 1–79. [Google Scholar]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Govoni, M.; Jansson, E.A.; Weitzberg, E.; Lundberg, J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 2008, 19, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.; Spencer, M.; Duffield, R.; Lawrence, S. The validity of a repeated sprint ability test. J. Sci. Med. Sport 2001, 4, 19–29. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Russ, D.W.; Elliott, M.A.; Vandenborne, K.; Walter, G.A.; Binder-Macleod, S.A. Metabolic costs of isometric force generation and maintenance of human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E448–E457. [Google Scholar] [CrossRef] [PubMed]
- Mulder, E.R.; Kuebler, W.M.; Gerrits, K.H.; Rittweger, J.; Felsenberg, D.; Stegeman, D.F.; de Haan, A. Knee extensor fatigability after bedrest for 8 weeks with and without countermeasure. Muscle Nerve 2007, 36, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Sobko, T.; Marcus, C.; Govoni, M.; Kamiya, S. Dietary nitrate in Japanese traditional foods lowers diastolic blood pressure in healthy volunteers. Nitric Oxide 2010, 22, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Wylie, L.J.; Ortiz de Zevallos, J.; Isidore, T.; Nyman, L.; Vanhatalo, A.; Bailey, S.J.; Jones, A.M. Dose-dependent effects of dietary nitrate on the oxygen cost of moderate-intensity exercise: Acute vs. chronic supplementation. Nitric Oxide 2016, 57, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M. Dietary nitrate supplementation and exercise performance. Sports Med. 2014, 44, S35–S45. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.A.; Webb, A.J.; Lundberg, J.O.; Weitzberg, E. Therapeutic effects of inorganic nitrate and nitrite in cardiovascular and metabolic diseases. J. Intern. Med. 2016, 279, 315–336. [Google Scholar] [CrossRef] [PubMed]
- Hernàndez, A.; Schiffer, T.A.; Ivarsson, N.; Cheng, A.J.; Bruton, J.D.; Lundberg, J.O.; Weitzberg, E.; Westerblad, H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. J. Physiol. 2012, 590, 3575–3583. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.K.; Hirai, D.M.; Copp, S.W.; Holdsworth, C.T.; Allen, J.D.; Jones, A.M.; Musch, T.I.; Poole, D.C. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J. Physiol. 2013, 591, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, D.P.; Hayward, G.M.; Bailey, S.J.; Vanhatalo, A.; Blackwell, J.R.; Jones, A.M. Influence of acute dietary nitrate supplementation on 50 mile time trial performance in well-trained cyclists. Eur. J. Appl. Physiol. 2012, 112, 4127–4134. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.L.; Henry, T.; Guelfi, K.; Dawson, B.; McNaughton, L.R.; Wallman, K. Effects of sodium phosphate and beetroot juice supplementation on repeated-sprint ability in females. Eur. J. Appl. Physiol. 2015, 115, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Bescòs, R.; Surenda, A.; Tur, J.A.; Pons, A. The effect of nitric-oxide-related supplements on human performance. Sports Med. 2012, 42, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Webb, A.J.; Ahluwalia, A. Inorganic nitrate and the cardiovascular system. Heart 2010, 96, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
| CD | ||
| Food | Approximate Amount for Daily Servings | NO3− Content |
| salad mix | 180 g | 2.4 mmol |
| broccoli | 60 g | 0.4 mmol |
| orange | 150 g | 0.0 mmol |
| cranberry juice | 0.5 L | 0.1 mmol |
| HND | ||
| Food | Approximate Amount for Daily Servings | NO3− Content |
| raw spinach | 40 g | 4.8 mmol |
| cooked collard greens | 80 g | 3.2 mmol |
| banana | 130 g | 0.1 mmol |
| pomegranate juice | 0.5 L | 0.1 mmol |
| Work | R | [La]b | HR | |||||
|---|---|---|---|---|---|---|---|---|
| W | L·min−1 | mL·kg−1·min−1 | L·min−1 | L·min−1 | mM | b·min−1 | ||
| CD | 74 ± 5 | 1.269 ± 0.136 | 18.9 ± 1.6 | 1.127 ± 0.118 | 0.89 ± 0.05 | 34.5 ± 3.6 | 5.15 ± 2.18 | 116 ± 17 |
| HND | 74 ± 5 | 1.178 ± 0.141 * | 17.9 ± 2.8 * | 1.049 ± 0.137 * | 0.90 ± 0.05 | 33.0 ± 4.3 | 4.68 ± 1.84 | 112 ± 15 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcelli, S.; Pugliese, L.; Rejc, E.; Pavei, G.; Bonato, M.; Montorsi, M.; La Torre, A.; Rasica, L.; Marzorati, M. Effects of a Short-Term High-Nitrate Diet on Exercise Performance. Nutrients 2016, 8, 534. https://doi.org/10.3390/nu8090534
Porcelli S, Pugliese L, Rejc E, Pavei G, Bonato M, Montorsi M, La Torre A, Rasica L, Marzorati M. Effects of a Short-Term High-Nitrate Diet on Exercise Performance. Nutrients. 2016; 8(9):534. https://doi.org/10.3390/nu8090534
Chicago/Turabian StylePorcelli, Simone, Lorenzo Pugliese, Enrico Rejc, Gaspare Pavei, Matteo Bonato, Michela Montorsi, Antonio La Torre, Letizia Rasica, and Mauro Marzorati. 2016. "Effects of a Short-Term High-Nitrate Diet on Exercise Performance" Nutrients 8, no. 9: 534. https://doi.org/10.3390/nu8090534
APA StylePorcelli, S., Pugliese, L., Rejc, E., Pavei, G., Bonato, M., Montorsi, M., La Torre, A., Rasica, L., & Marzorati, M. (2016). Effects of a Short-Term High-Nitrate Diet on Exercise Performance. Nutrients, 8(9), 534. https://doi.org/10.3390/nu8090534






