Accuracy of Continuous Glucose Monitoring (CGM) during Continuous and High-Intensity Interval Exercise in Patients with Type 1 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
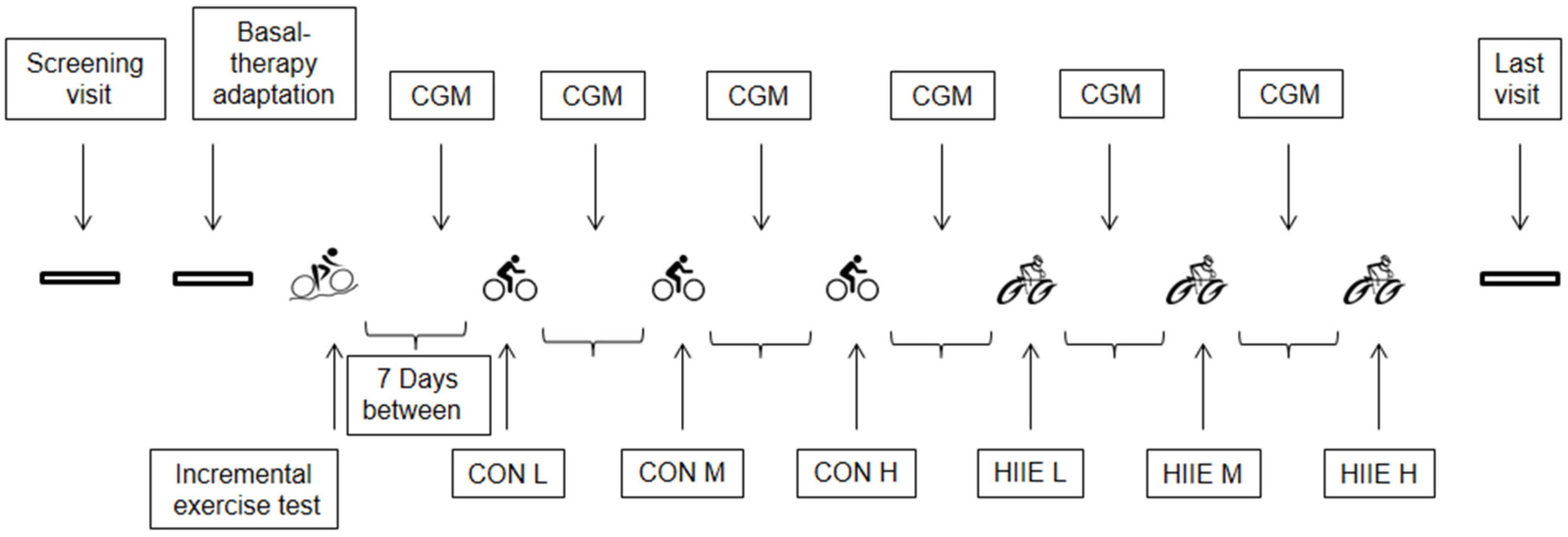
2.2. Experimental Design
2.2.1. Adaptation of Long-Acting Insulin Therapy and Dietary Recording
2.2.2. Incremental Exercise Testing (IET)
2.2.3. Continuous Exercise (CON)
2.2.4. High-Intensity Interval Exercise (HIIE)
2.2.5. Continuous Glucose Monitoring (CGM)
2.2.6. Carbohydrate Intake and Short-Acting Insulin Reductions
2.3. Measurements
2.4. Statistical Analyses
3. Results
3.1. Subjects’ Characteristics and Performance Data
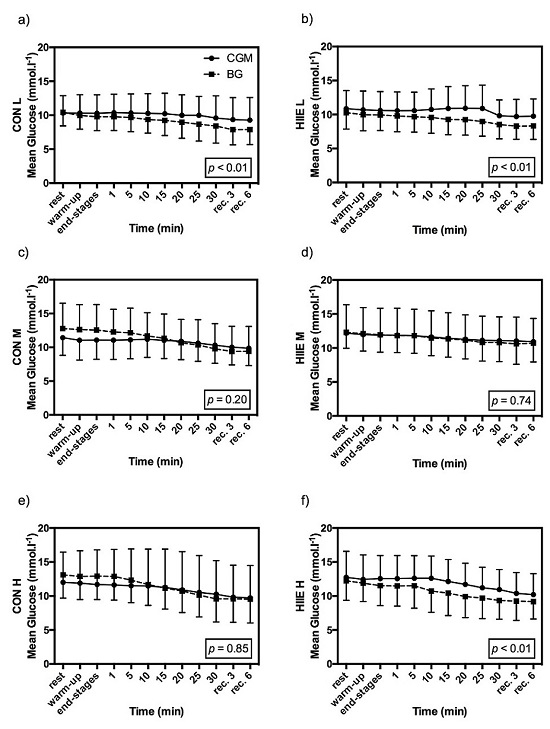
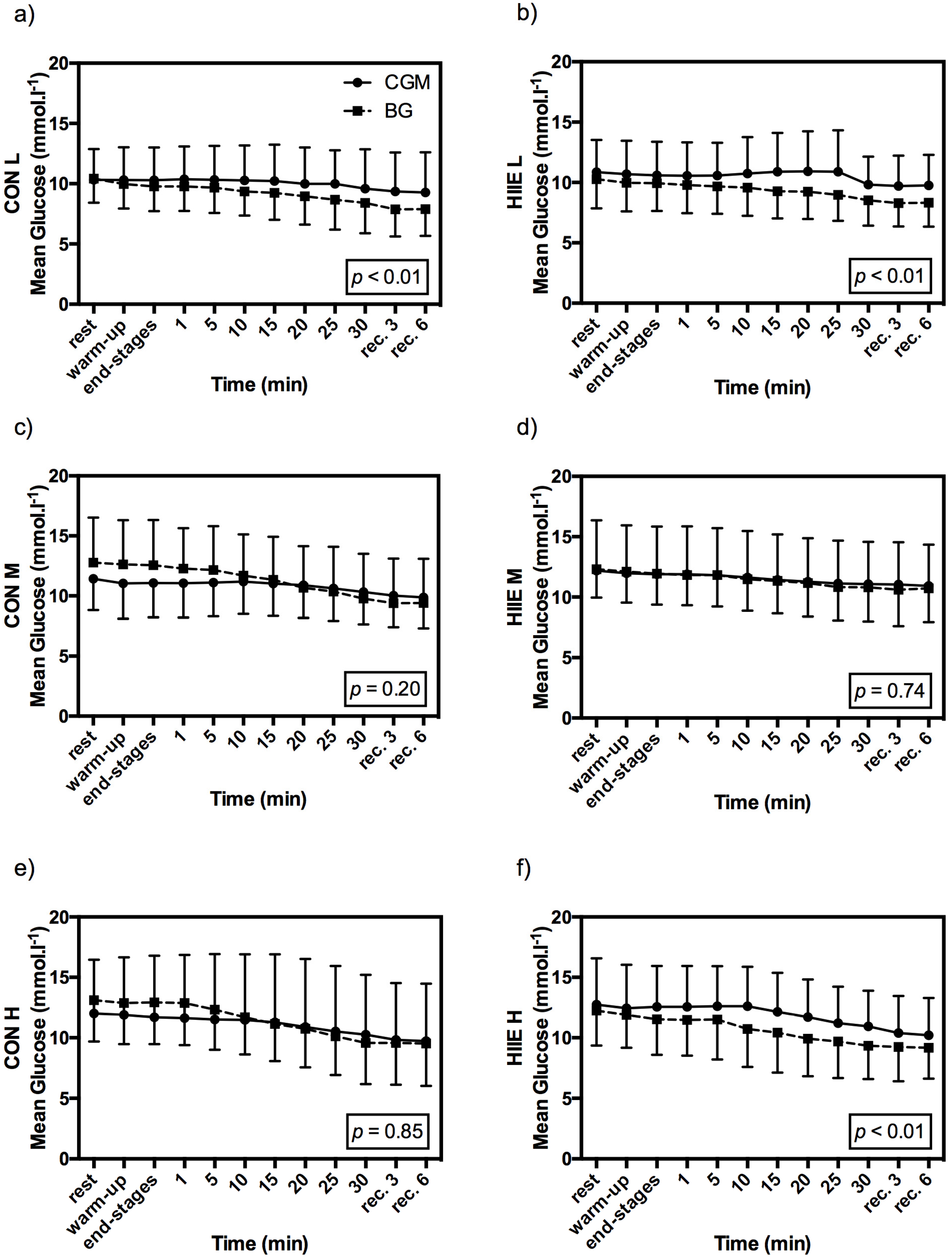
3.2. Comparison of Accuracy in CGM and BG
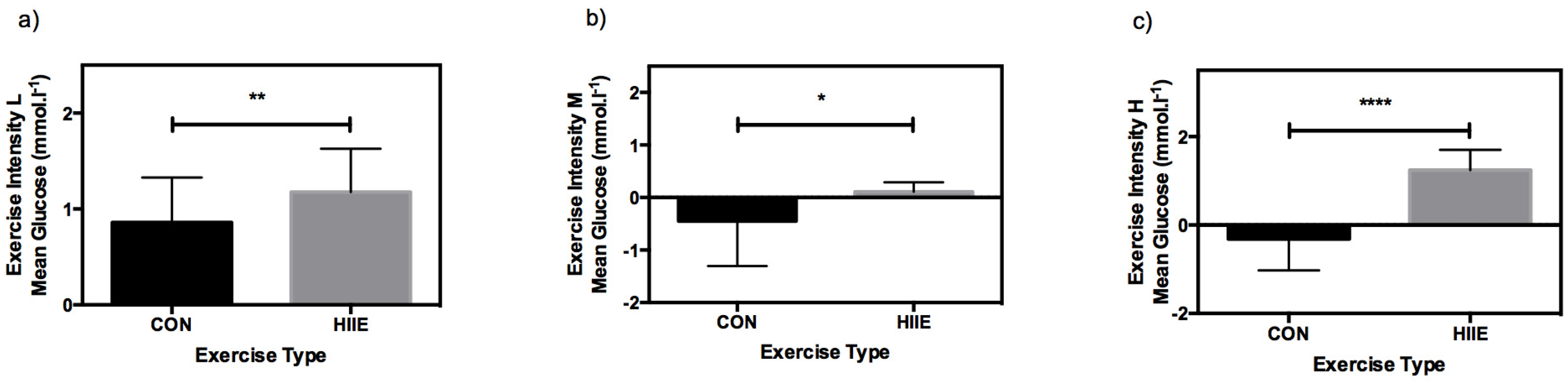
3.3. Influence of Exercise Type on CGM
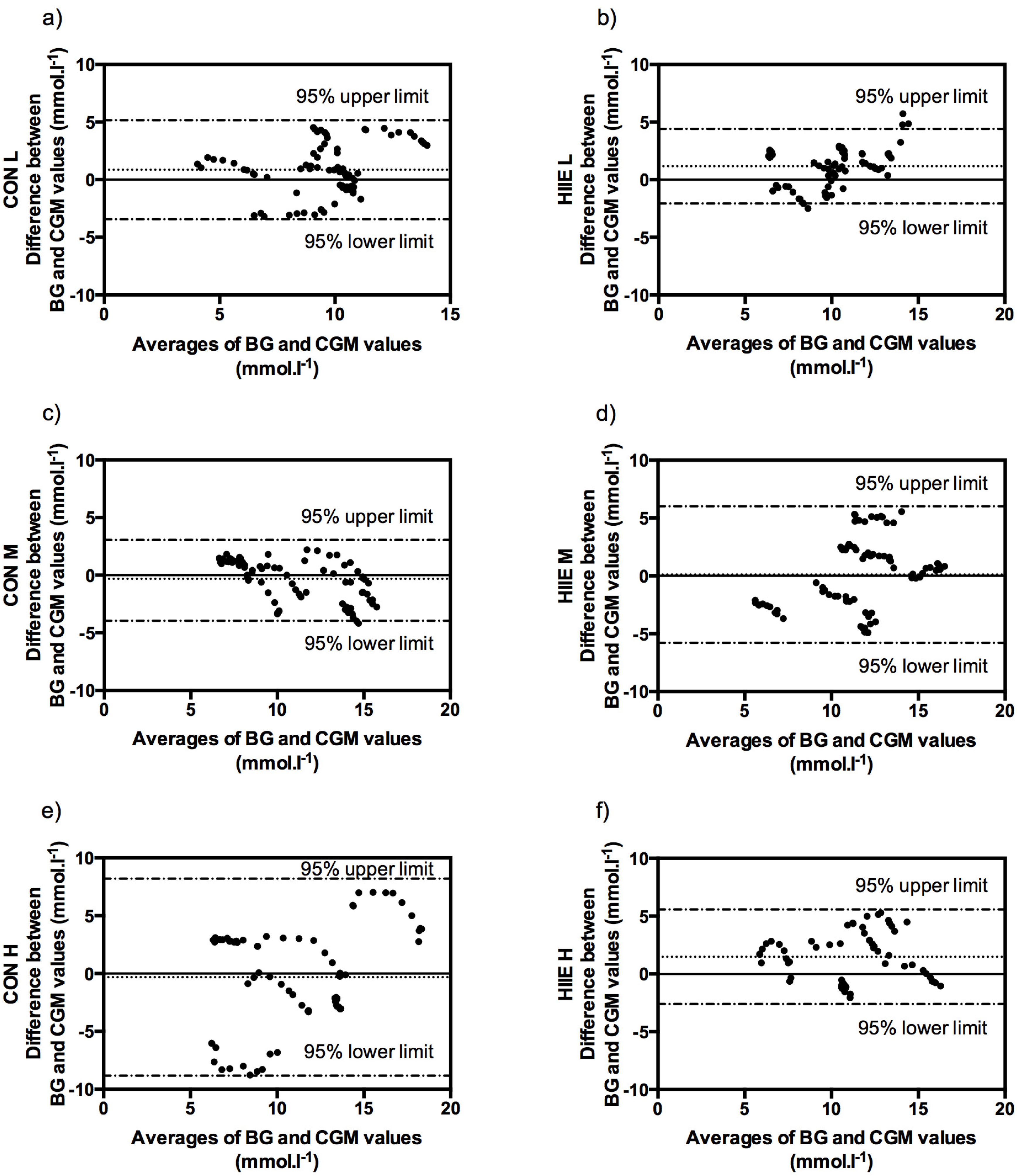
3.4. Clinical Acceptance of CGM in Comparison to BG
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| T1DM | Type 1 diabetes mellitus |
| BG | Blood glucose |
| CGM | Continuous glucose monitoring |
| Pmean | Mean workload power |
| CON | Continuous exercise |
| HIIE | High intensity interval exercise |
| HbA1c | Glycated hemoglobin (A1c) |
| C-peptide | Connecting peptide |
| GCP | Good Clinical Practice |
| Pmax | Maximal power output |
| LTP1 | First lactate turn point |
| LTP2 | Second lactate turn point |
| PLTP1 | Power output at the first lactate turn point |
| PLTP2 | Power output at the second lactate turn point |
| L | Mean exercise intensity 5% of Pmax from IET below PLTP1 |
| M | Mean exercise intensity 5% of Pmax from IET above PLTP1 |
| H | Mean exercise 5% of Pmax from IET below PLTP2 |
| Ppeak | Peak workload during HIIE |
| tpeak | Peak workload duration during HIIE |
| Prec | Recovery load during HIIE |
| trec | Recovery duration during HIIE |
| CRF | Case report form |
| SD | Standard deviation |
| MD | Mean difference |
| MARD | Mean absolute relative difference |
| BMI | Body mass index |
| VO2max | Maximal oxygen uptake |
| W | Watt |
| GLUT 4 | Glucose-transporter type 4 |
| ISO | International Organization for Standardization |
References
- Galassetti, P.; Riddell, M.C. Exercise and type 1 diabetes (T1DM). Compr. Physiol. 2013, 3, 1309–1336. [Google Scholar] [PubMed]
- Schwingshackl, L.; Missbach, B.; Dias, S.; König, J.; Hoffmann, G. Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: A systematic review and network meta-analysis. Diabetologia 2014, 57, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Tanaka, S.; Heianza, Y.; Fujihara, K.; Horikawa, C.; Shimano, H.; Saito, K.; Yamada, N.; Ohashi, Y.; Sone, H. Association between physical activity and risk of all-cause mortality and cardiovascular disease in patients with diabetes: A meta-analysis. Diabetes Care 2013, 36, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Moser, O.; Tschakert, G.; Mueller, A.; Groeschl, W.; Pieber, T.R.; Obermayer-Pietsch, B.; Koehler, G.; Hofmann, P. Effects of High-Intensity Interval Exercise versus Moderate Continuous Exercise on Glucose Homeostasis and Hormone Response in Patients with Type 1 Diabetes Mellitus Using Novel Ultra-Long-Acting Insulin. PLoS ONE 2015, 10, e0136489. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.D.; West, D.J.; Bain, S.C.; Kingsley, M.I.C.; Foley, P.; Kilduff, L.; Turner, D.; Gray, B.; Stephens, J.W.; Bracken, R.M. Simulated games activity vs. continuous running exercise: A novel comparison of the glycemic and metabolic responses in T1DM patients. Scand. J. Med. Sci. Sports 2015, 25, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Riddell, M.; Perkins, B.A. Exercise and glucose metabolism in persons with diabetes mellitus: Perspectives on the role for continuous glucose monitoring. J. Diabetes Sci. Technol. 2009, 3, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Monsod, T.P.; Flanagan, D.E.; Rife, F.; Saenz, R.; Caprio, S.; Sherwin, R.S.; Tamborlane, W.V. Do sensor glucose levels accurately predict plasma glucose concentrations during hypoglycemia and hyperinsulinemia? Diabetes Care 2002, 25, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Herrington, S.J.; Gee, D.L.; Dow, S.D.; Monosky, K.A.; Davis, E.; Pritchett, K.L. Comparison of glucose monitoring methods during steady-state exercise in women. Nutrients 2012, 4, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Yardley, J.E.; Sigal, R.J.; Kenny, G.P.; Riddell, M.C.; Lovblom, L.E.; Perkins, B.A. Point accuracy of interstitial continuous glucose monitoring during exercise in type 1 diabetes. Diabetes Technol. Ther. 2013, 15, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, P.; Nilsson, S.; Lindblad, B. Continuous glucose monitoring system during physical exercise in adolescents with type 1 diabetes. Acta Paediatr. 2011, 100, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Bally, L.; Zueger, T.; Pasi, N.; Carlos, C.; Paganini, D.; Stettler, C. Accuracy of continuous glucose monitoring during differing exercise conditions. Diabetes Res. Clin. Pract. 2016, 112, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fayolle, C.; Brun, J.F.; Bringer, J.; Mercier, J.; Renard, E. Accuracy of continuous subcutaneous glucose monitoring with the GlucoDay in type 1 diabetic patients treated by subcutaneous insulin infusion during exercise of low versus high intensity. Diabetes Metab. 2006, 32, 313–320. [Google Scholar] [CrossRef]
- Iscoe, K.E.; Campbell, J.E.; Jamnik, V.; Perkins, B.A.; Riddell, M.C. Efficacy of continuous real-time blood glucose monitoring during and after prolonged high-intensity cycling exercise: Spinning with a continuous glucose monitoring system. Diabetes Technol. Ther. 2006, 8, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Helgerud, J.; Høydal, K.; Wang, E.; Karlsen, T.; Berg, P.; Bjerkaas, M.; Simonsen, T.; Helgesen, C.; Hjorth, N.; Bach, R.; Hoff, J. Aerobic high-intensity intervals improve VO2max more than moderate training. Med. Sci. Sports Exerc. 2007, 39, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Daussin, F.N.; Zoll, J.; Dufour, S.P.; Ponsot, E.; Lonsdorfer-Wolf, E.; Doutreleau, S.; Mettauer, B.; Piquard, F.; Geny, B.; Richard, R. Effect of interval versus continuous training on cardiorespiratory and mitochondrial functions: Relationship to aerobic performance improvements in sedentary subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R264–R272. [Google Scholar] [CrossRef] [PubMed]
- Kemi, O.J.; Wisloff, U. High-intensity aerobic exercise training improves the heart in health and disease. J. Cardiopulm. Rehabil. Prev. 2009, 30, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Iellamo, F.; Manzi, V.; Caminiti, G.; Vitale, C.; Castagna, C.; Massaro, M.; Franchini, A.; Rosano, G.; Volterrani, M. Matched dose interval and continuous exercise training induce similar cardiorespiratory and metabolic adaptations in patients with heart failure. Int. J. Cardiol. 2013, 167, 2561–2565. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 5, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; Gillen, J.B.; Percival, M.E. Physiological and health-related adaptations to low-volume interval training: Influences of nutrition and sex. Sports Med. 2014, 44, S127–S137. [Google Scholar] [CrossRef] [PubMed]
- Guelfi, K.J.; Jones, T.W.; Fournier, P.A. New insights into managing the risk of hypoglycaemia associated with intermittent high-intensity exercise in individuals with type 1 diabetes mellitus: Implications for existing guidelines. Sports Med. 2007, 37, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Iscoe, K.E.; Riddell, M.C. Continuous moderate-intensity exercise with or without intermittent high-intensity work: Effects on acute and late glycaemia in athletes with Type1 diabetes mellitus. Diabet. Med. 2011, 28, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Tschakert, G.; Kroepfl, J.; Hofmann, P.; Mueller, A.; Moser, O.; Groeschl, W. How to Regulate the Acute Physiological Response to “Aerobic” High-Intensity Interval Exercise. J. Sports Sci. Med. 2015, 14, 29–36. [Google Scholar] [PubMed]
- ClinicalTrials.gov. Available online: http://clinicaltrials.gov/ct2/show/NCT02075567 (accessed on 5 August 2016).
- Koehler, G.; Heller, S.; Korsatko, S.; Roepstorff, C.; Rasmussen, S.; Haahr, H.; Pieber, T.R. Insulin degludec is not associated with a delayed or diminished response to hypoglycaemia compared with insulin glargine in type 1 diabetes: A double-blind randomised crossover study. Diabetologia 2014, 57, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Tschakert, G.; Hofmann, P. High-intensity intermittent exercise: Methodological and physiological aspects. Int. J. Sports Physiol. Perform. 2013, 8, 600–610. [Google Scholar] [PubMed]
- Hofmann, P.; Tschakert, G. Special needs to prescribe exercise intensity for scientific studies. Cardiol. Res. Pract. 2011, 2011, 209302. [Google Scholar] [CrossRef] [PubMed]
- Tschakert, G.; Kroepfl, J.M.; Mueller, A.; Harpf, H.; Harpf, L.; Traninger, H.; Wallner-Liebmann, S.; Stojakovic, T.; Scharnagl, H.; Meinitzer, A.; et al. Acute Physiological Responses to Short- and Long-Stage High-Intensity Interval Exercise in Cardiac Rehabilitation: A Pilot Study. J. Sports Sci. Med. 2016, 15, 80–91. [Google Scholar] [PubMed]
- Langendam, M.; Luijf, Y.M.; Hooft, L.; Devries, J.H.; Mudde, A.H.; Scholten, R.J. Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst. Rev. 2012, 1, CD008101. [Google Scholar] [PubMed]
- Rabasa-Lhoret, R.; Bourque, J.; Ducros, F.; Chiasson, J.L. Guidelines for premeal insulin dose reduction for postprandial exercise of different intensities and durations in type 1 diabetic subjects treated intensively with a basal-bolus insulin regimen (ultralente-lispro). Diabetes Care 2001, 24, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Clarke, W.L.; Cox, D.; Gonder-Frederick, L.A.; Carter, W.; Pohl, S.L. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care 1987, 10, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Roberts, R.; Bailey, T. Guidelines for optimal bolus calculator settings in adults. J. Diabetes Sci. Technol. 2011, 5, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Keenan, D.B.; Mastrototaro, J.J.; Voskanyan, G.; Steil, G.M. Delays in minimally invasive continuous glucose monitoring devices: A review of current technology. J. Diabetes Sci. Technol. 2009, 3, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Scharhag-Rosenberger, F.; Meyer, T.; Gäßler, N.; Faude, O.; Kindermann, W. Exercise at given percentages of VO2max: Heterogeneous metabolic responses between individuals. J. Sci. Med. Sport 2010, 13, 74–79. [Google Scholar] [CrossRef] [PubMed]
- González-Alonso, J. Human thermoregulation and the cardiovascular system. Exp. Physiol. 2012, 97, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Gallen, I.W.; Hume, C.; Lumb, A. Fuelling the athlete with type 1 diabetes. Diabetes Obes. Metab. 2011, 13, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, I.; Weber, K.S.; Roden, M.; Herrmann, M.-E.; Müssig, K. Type 1 Diabetes Mellitus and Exercise in Competitive Athletes. Exp. Clin. Endocrinol. Diabetes 2015, 123, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Devadoss, M.; Kennedy, L.; Herbold, N. Endurance athletes and type 1 diabetes. Diabetes Educ. 2011, 37, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Brazeau, A.S.; Rabasa-Lhoret, R.; Strychar, I.M.H. Barriers to Physical Activity Among Patients With Type 1 Diabetes. Diabetes Care 2008, 31, 2108–2109. [Google Scholar] [CrossRef] [PubMed]
- Liebl, A.; Henrichs, H.R.; Heinemann, L.; Freckmann, G.; Biermann, E.; Thomas, A. Continuous glucose monitoring: Evidence and consensus statement for clinical use. J. Diabetes Sci. Technol. 2013, 7, 500–519. [Google Scholar] [CrossRef] [PubMed]
- Marliss, E.B.; Vranic, M. Intense exercise has unique effects on both insulin release and its roles in glucoregulation: Implications for diabetes. Diabetes 2002, 51, 271–283. [Google Scholar] [CrossRef]
- Anzalone, P. Equivalence of earlobe site blood glucose testing with finger stick. Clin. Nurs. Res. 2008, 17, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Maahs, D.M.; de Salvo, D.; Pyle, L.; Ly, T.; Messer, L.; Clinton, P.; Westfall, E.; Wadwa, R.P.; Buckingham, B.; Zhang, Y.; et al. Effect of acetaminophen on CGM glucose in an outpatient setting. Diabetes Care 2015, 38, e158–e159. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moser, O.; Mader, J.K.; Tschakert, G.; Mueller, A.; Groeschl, W.; Pieber, T.R.; Koehler, G.; Messerschmidt, J.; Hofmann, P. Accuracy of Continuous Glucose Monitoring (CGM) during Continuous and High-Intensity Interval Exercise in Patients with Type 1 Diabetes Mellitus. Nutrients 2016, 8, 489. https://doi.org/10.3390/nu8080489
Moser O, Mader JK, Tschakert G, Mueller A, Groeschl W, Pieber TR, Koehler G, Messerschmidt J, Hofmann P. Accuracy of Continuous Glucose Monitoring (CGM) during Continuous and High-Intensity Interval Exercise in Patients with Type 1 Diabetes Mellitus. Nutrients. 2016; 8(8):489. https://doi.org/10.3390/nu8080489
Chicago/Turabian StyleMoser, Othmar, Julia K. Mader, Gerhard Tschakert, Alexander Mueller, Werner Groeschl, Thomas R. Pieber, Gerd Koehler, Janin Messerschmidt, and Peter Hofmann. 2016. "Accuracy of Continuous Glucose Monitoring (CGM) during Continuous and High-Intensity Interval Exercise in Patients with Type 1 Diabetes Mellitus" Nutrients 8, no. 8: 489. https://doi.org/10.3390/nu8080489