Challenges in Analyzing the Biological Effects of Resveratrol
Abstract
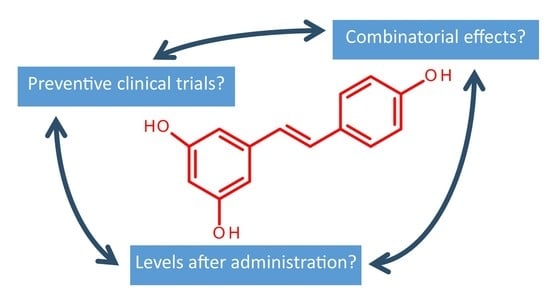
:1. Introduction
2. The Combinatory Effect—A Boosted Effect?
2.1. Drug–Drug Interaction with Resveratrol
2.2. Combinatory Effect—Do the Dietary Components Work in Cooperation?
2.3. Complex Mixtures—Effect of Resveratrol
2.4. How to Get Further?
3. In Vivo Exposure to Resveratrol: What Is the Relevant Dose and What Is the Actual Dose?
How to Increase and Target the Bioavailability?
4. Preventive vs. Therapeutic Effects of Resveratrol
4.1. Studies Including Healthy Subjects
4.2. Studies Including Subjects with Metabolic Challenges
4.3. Studies Including Subjects with Cardiovascular Challenges
4.4. Studies Including Subjects with Cancer
4.5. Studies Including Subjects with Alzheimers
5. Concluding Remarks
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Semba, R.D.; Ferrucci, L.; Bartali, B.; Urpi-Sarda, M.; Zamora-Ros, R.; Sun, K.; Cherubini, A.; Bandinelli, S.; Andres-Lacueva, C. Resveratrol levels and all-cause mortality in older community-dwelling adults. JAMA Intern. Med. 2014, 174, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Rufini, A.; Gescher, A. Do not throw out the resveratrol with the bath water. JAMA Intern. Med. 2015, 175, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, M. Resveratrol, a new phenolic compound from Veratrum grandiflorum. Nippon Kagaku Kaichi 1939, 60, 1090–1100. [Google Scholar] [CrossRef]
- Chow, H.H.; Garland, L.L.; Hsu, C.H.; Vining, D.R.; Chew, W.M.; Miller, J.A.; Perloff, M.; Crowell, J.A.; Alberts, D.S. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev. Res. 2010, 3, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhou, R.; Wang, B.; Mi, M.-T. Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2014, 99, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Brantley, S.; Argikar, A.; Lin, Y.S.; Nagar, S.; Paine, M.F. Herb-drug interactions: Challenges and opportunities for improved predictions. Drug Metab. Dispos. 2014, 24, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Bai, Y.; Lin, Y.W.; Zheng, X.Y.; Qin, J.; Yang, K.; Xie, L.P. Resveratrol confers resistance against taxol via induction of cell cycle arrest in human cancer cell lines. Mol. Nutr. Food Res. 2010, 54, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Nessa, M.U.; Beale, P.; Chan, C.; Yu, J.Q.; Huq, F. Combinations of resveratrol, cisplatin and oxaliplatin applied to human ovarian cancer cells. Anticancer Res. 2012, 32, 53–59. [Google Scholar] [PubMed]
- Diaz-Chavez, J.; Fonseca-Sanchez, M.A.; Arechaga-Ocampo, E.; Flores-Perez, A.; Palacios-Rodriguez, Y.; Dominguez-Gomez, G.; Marchat, L.A.; Fuentes-Mera, L.; Mendoza-Hernandez, G.; Gariglio, P.; et al. Proteomic profiling reveals that resveratrol inhibits HSP27 expression and sensitizes breast cancer cells to doxorubicin therapy. PLoS ONE 2013, 8, e64378. [Google Scholar]
- Heiduschka, G.; Lill, C.; Seemann, R.; Brunner, M.; Schmid, R.; Houben, R.; Bigenzahn, J.; Thurnher, D. The effect of resveratrol in combination with irradiation and chemotherapy: Study using Merkel cell carcinoma cell lines. Strahlenther. Onkol. 2014, 190, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Podhorecka, M.; Halicka, D.; Klimek, P.; Kowal, M.; Chocholska, S.; Dmoszynska, A. Resveratrol increases rate of apoptosis caused by purine analogues in malignant lymphocytes of chronic lymphocytic leukemia. Ann. Hematol. 2011, 90, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Lee, C.C.; Shih, Y.L.; Lin, T.Y.; Wang, S.H.; Lin, Y.F.; Shih, C.M. Resveratrol enhances the therapeutic effect of temozolomide against malignant glioma in vitro and in vivo by inhibiting autophagy. Free Radic. Biol. Med. 2012, 52, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.F.; Liu, B.Q.; Du, Z.X.; Gao, Y.Y.; Li, C.; Li, N.; Guan, Y.; Wang, H.Q. Resveratrol protects leukemic cells against cytotoxicity induced by proteasome inhibitors via induction of FOXO1 and p27Kip1. BMC Cancer 2011, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Huq, F.; Yu, J.Q.; Beale, P.; Chan, C.; Arzuman, L.; Nessa, M.U.; Mazumder, M.E.H. Combinations of platinums and selected phytochemicals as a means of overcoming resistance in ovarian cancer. Anticancer Res. 2014, 34, 541–545. [Google Scholar] [PubMed]
- Amiri, F.; Zarnani, A.H.; Zand, H.; Koohdani, F.; Jeddi-Tehrani, M.; Vafa, M. Synergistic anti-proliferative effect of resveratrol and etoposide on human hepatocellular and colon cancer cell lines. Eur. J. Pharmacol. 2013, 718, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, B.; Zahid, M.; Saeed, M.; Ali, M.F.; Cavalieri, E.L.; Rogan, E.G. Formation of diethylstilbestrol-DNA adducts in human breast epithelial cells and inhibition by resveratrol. J. Steroid Biochem. Mol. Biol. 2011, 127, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Pichardo, L.; Dharmawardhane, S.F. Grape polyphenols inhibit Akt/mammalian target of rapamycin signaling and potentiate the effects of gefitinib in breast cancer. Nutr. Cancer 2012, 64, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.J.; Goussetis, D.J.; Beauchamp, E.; Kosciuczuk, E.M.; Altman, J.K.; Eklund, E.A.; Platanias, L.C. Resveratrol enhances the suppressive effects of arsenic trioxide on primitive leukemic progenitors. Cancer Biol. Ther. 2014, 15, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Kai, L.; Levenson, A.S. Combination of resveratrol and antiandrogen flutamide has synergistic effect on androgen receptor inhibition in prostate cancer cells. Anticancer Res. 2011, 31, 3323–3330. [Google Scholar] [PubMed]
- Bruckbauer, A.; Zemel, M.B. Synergistic effects of metformin, resveratrol, and hydroxymethylbutyrate on insulin sensitivity. Diabetes Metab. Syndr. Obes. 2013, 6, 93–102. [Google Scholar] [PubMed]
- Thandapilly, S.J.; Louis, X.L.; Behbahani, J.; Movahed, A.; Yu, L.; Fandrich, R.; Zhang, S.; Kardami, E.; Anderson, H.D.; Netticadan, T. Reduced hemodynamic load aids low-dose resveratrol in reversing cardiovascular defects in hypertensive rats. Hypertens. Res. 2013, 36, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wang, W.; He, G.; Kuick, R.D.; Gossner, G.; Kueck, A.S.; Wahl, H.; Opipari, A.W.; Liu, J.R. Resveratrol inhibits ovarian tumor growth in an in vivo mouse model. Cancer 2016, 122, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Shin, Y.J.; Won, A.J.; Lee, B.M.; Choi, W.S.; Jung, J.H.; Chung, H.Y.; Kim, H.S. Resveratrol enhances chemosensitivity of doxorubicin in multidrug-resistant human breast cancer cells via increased cellular influx of doxorubicin. Biochim. Biophys. Acta 2014, 1840, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Farrand, L.; Byun, S.; Kim, J.Y.; Im-Aram, A.; Lee, J.; Lim, S.; Lee, K.W.; Suh, J.Y.; Lee, H.J.; Tsang, B.K. Piceatannol enhances cisplatin sensitivity in ovarian cancer via modulation of p53, X-linked inhibitor of apoptosis protein (XIAP), and mitochondrial fission. J. Biol. Chem. 2013, 288, 23740–23750. [Google Scholar] [CrossRef] [PubMed]
- Berbee, J.F.; Wong, M.C.; Wang, Y.; van der Hoorn, J.W.; Khedoe, P.P.; van Klinken, J.B.; Mol, I.M.; Hiemstra, P.S.; Tsikas, D.; Romijn, J.A.; et al. Resveratrol protects against atherosclerosis, but does not add to the antiatherogenic effect of atorvastatin, in APOE*3-Leiden.CETP mice. J. Nutr. Biochem. 2013, 24, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Carpene, C.; Gomez-Zorita, S.; Gupta, R.; Gres, S.; Rancoule, C.; Cadoudal, T.; Mercader, J.; Gomez, A.; Bertrand, C.; Iffiu-Soltesz, Z. Combination of low dose of the anti-adipogenic agents resveratrol and phenelzine in drinking water is not sufficient to prevent obesity in very-high-fat diet-fed mice. Eur. J. Nutr. 2014, 53, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Erdem, T.; Bayindir, T.; Filiz, A.; Iraz, M.; Selimoglu, E. The effect of resveratrol on the prevention of cisplatin ototoxicity. Eur. Arch. Otorhinolaryngol. 2012, 269, 2185–2188. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Song, Z.P.; Gui, D.M.; Hu, W.; Chen, Y.G.; Zhang, D.D. Resveratrol attenuates doxorubicin-Induced cardiomyocyte apoptosis in lymphoma nude mice by heme oxygenase-1 induction. Cardiovasc. Toxicol. 2012, 12, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Mikstacka, R.; Rimando, A.M.; Ignatowicz, E. Antioxidant effect of trans-resveratrol, pterostilbene, quercetin and their combinations in human erythrocytes in vitro. Plant Foods Hum. Nutr. 2010, 65, 57–63. [Google Scholar] [CrossRef] [PubMed]
- De Maria, S.; Scognamiglio, I.; Lombardi, A.; Amodio, N.; Caraglia, M.; Carteni, M.; Ravagnan, G.; Stiuso, P. Polydatin, a natural precursor of resveratrol, induces cell cycle arrest and differentiation of human colorectal Caco-2 cell. J. Transl. Med. 2013, 11, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Q.; Hu, B.; An, H.M.; Shen, K.P.; Xu, L.; Deng, S.; Wei, M.M. Synergistic anticancer effects of curcumin and resveratrol in Hepa1–6 hepatocellular carcinoma cells. Oncol. Rep. 2013, 29, 1851–1858. [Google Scholar] [PubMed]
- Dhandayuthapani, S.; Marimuthu, P.; Hormann, V.; Kumi-Diaka, J.; Rathinavelu, A. Induction of apoptosis in HeLa cells via caspase activation by resveratrol and genistein. J. Med. Food 2013, 16, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Pallares, V.; Calay, D.; Cedo, L.; Castell-Auvi, A.; Raes, M.; Pinent, M.; Ardevol, A.; Arola, L.; Blay, M. Enhanced anti-inflammatory effect of resveratrol and EPA in treated endotoxin-activated RAW 264.7 macrophages. Br. J. Nutr. 2012, 108, 1562–1573. [Google Scholar] [CrossRef] [PubMed]
- Gazova, Z.; Siposova, K.; Kurin, E.; Mucaji, P.; Nagy, M. Amyloid aggregation of lysozyme: The synergy study of red wine polyphenols. Proteins 2013, 81, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Sapere, N.; Visentin, M.; Zella, D.; Scapagnini, G. Enhancement of mitochondrial biogenesis with polyphenols: combined effects of resveratrol and equol in human endothelial cells. Immun. Ageing 2013, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y. Nitric oxide triggers apoptosis in A375 human melanoma cells treated with capsaicin and resveratrol. Mol. Med. Rep. 2012, 5, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Lasa, A.; Miranda, J.; Churruca, I.; Simon, E.; Arias, N.; Milagro, F.; Martinez, J.A.; Portillo, M.D. The combination of resveratrol and CLA does not increase the delipidating effect of each molecule in 3T3-L1 adipocytes. Nutr. Hosp. 2011, 26, 997–1003. [Google Scholar] [PubMed]
- Bruckbauer, A.; Zemel, M.B.; Thorpe, T.; Akula, M.R.; Stuckey, A.C.; Osborne, D.; Martin, E.B.; Kennel, S.; Wall, J.S. Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutr. Metab. 2012, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Bruckbauer, A.; Zemel, M.B. Synergistic Effects of polyphenols and methylxanthines with leucine on AMPK/sirtuin-mediated metabolism in muscle cells and adipocytes. PLoS ONE 2014, 9, e89166. [Google Scholar] [CrossRef] [PubMed]
- Lekli, I.; Ray, D.; Mukherjee, S.; Gurusamy, N.; Ahsan, M.K.; Juhasz, B.; Bak, I.; Tosaki, A.; Gherghiceanu, M.; Popescu, L.M.; et al. Co-ordinated autophagy with resveratrol and gamma-tocotrienol confers synergetic cardioprotection. J. Cell. Mol. Med. 2010, 14, 2506–2518. [Google Scholar] [CrossRef] [PubMed]
- Bano, M.; Bhatt, D.K. Ameliorative effect of a combination of vitamin E, vitamin C, alpha-lipoic acid and stilbene resveratrol on lindane induced toxicity in mice olfactory lobe and cerebrum. Indian J. Exp. Biol. 2010, 48, 150–158. [Google Scholar] [PubMed]
- Saleh, M.C.; Connell, B.J.; Rajagopal, D.; Khan, B.V.; Abd-El-Aziz, A.S.; Kucukkaya, I.; Saleh, T.M. Co-administration of resveratrol and lipoic acid, or their synthetic combination, enhances neuroprotection in a rat model of ischemia/reperfusion. PLoS ONE 2014, 9, e87865. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, M.C.; Kowalczyk, P.; Tolstykh, O.; Hanausek, M.; Walaszek, Z.; Slaga, T.J. Synergistic effects of combined phytochemicals and skin cancer prevention in SENCAR mice. Cancer Prev. Res. 2010, 3, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Nair, P.; Dhawan, D.K. Study to evaluate molecular mechanics behind synergistic chemo-preventive effects of curcumin and resveratrol during lung carcinogenesis. PLoS ONE 2014, 9, e93820. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Rho, O.; Junco, J.; Carbajal, S.; Siegel, D.; Slaga, T.J.; DiGiovanni, J. Effect of combined treatment with Ursolic acid and resveratrol on skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Prev. Res. 2015, 8, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Leontieva, O.V.; Paszkiewicz, G.; Demidenko, Z.N.; Blagosklonny, M.V. Resveratrol potentiates rapamycin to prevent hyperinsulinemia and obesity in male mice on high fat diet. Cell Death Dis. 2013, 4, e472. [Google Scholar] [CrossRef] [PubMed]
- Militaru, C.; Donoiu, I.; Craciun, A.; Scorei, I.D.; Bulearca, A.M.; Scorei, R.I. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: Effects on lipid profiles, inflammation markers, and quality of life. Nutrition 2013, 29, 178–183. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, L.S.; Miller, L.E.; Hosick, P.A.; Utter, A.C.; Quindry, J.C.; McAnulty, S.R. Effect of resveratrol and quercetin supplementation on redox status and inflammation after exercise. Appl. Physiol. Nutr. Metab. 2013, 38, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xu, Y.M.; Khaoustov, V.; Yoffe, B. Identification of components of grape powder with anti-apoptotic effects. Toxicol. Ind. Health 2011, 27, 19–28. [Google Scholar]
- San Miguel, S.M.; Opperman, L.A.; Allen, E.P.; Zielinski, J.; Svoboda, K.K. Antioxidants counteract nicotine and promote migration via RacGTP in oral fibroblast cells. J. Periodontol. 2010, 81, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bi, W.N.; Cheng, A.; Freire, D.; Vempati, P.; Zhao, W.; Gong, B.; Janle, E.M.; Chen, T.Y.; Ferruzzi, M.G.; et al. Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer’s disease-experimental approach and therapeutic implications. Front. Aging Neurosci. 2014, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Khan, D.A.; Mahjabeen, W.; Papasian, C.J.; Qureshi, N. Suppression of nitric oxide production and cardiovascular risk factors in healthy seniors and hypercholesterolemic subjects by a combination of polyphenols and vitamins. J. Clin. Exp. Cardiol. 2012, 5, 8. [Google Scholar]
- Qureshi, A.A.; Khan, D.A.; Mahjabeen, W.; Papasian, C.J.; Qureshi, N. Nutritional supplement-5 with a combination of proteasome inhibitors (resveratrol, quercetin, g-tocotrienol) modulate age-associated biomarkers and cardiovascular lipid parameters in human subjects. J. Clin. Exp. Cardiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Kurin, E.; Mucaji, P.; Nagy, M. In vitro antioxidant activities of three red wine polyphenols and their mixtures: An interaction study. Molecules 2012, 17, 14336–14348. [Google Scholar] [CrossRef] [PubMed]
- Mahida, J.P.; Antczak, C.; Decarlo, D.; Champ, K.G.; Francis, J.H.; Marr, B.; Polans, A.S.; Albert, D.M.; Abramson, D.H.; Djaballah, H. A synergetic screening approach with companion effector for combination therapy: Application to retinoblastoma. PLoS ONE 2013, 8, e59156. [Google Scholar] [CrossRef] [PubMed]
- Elmadhun, N.Y.; Sabe, A.A.; Robich, M.P.; Chu, L.M.; Lassaletta, A.D.; Sellke, F.W. The pig as a valuable model for testing the effect of resveratrol to prevent cardiovascular disease. Ann. N. Y. Acad. Sci. 2013, 1290, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.M.; Yan, J.; Soleas, G.J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef]
- Azorin-Ortuno, M.; Yanez-Gascon, M.J.; Pallares, F.J.; Vallejo, F.; Larrosa, M.; Garcia-Conesa, M.T.; Tomas-Barberan, F.; Espin, J.C. Pharmacokinetic study of trans-resveratrol in adult pigs. J. Agric. Food Chem. 2010, 58, 11165–11171. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.; Piccirilli, G.; et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef] [PubMed]
- Muzzio, M.; Huang, Z.; Hu, S.C.; Johnson, W.D.; McCormick, D.L.; Kapetanovic, I.M. Determination of resveratrol and its sulfate and glucuronide metabolites in plasma by LC-MS/MS and their pharmacokinetics in dogs. J. Pharm. Biomed. Anal. 2012, 59, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef] [PubMed]
- Rotches-Ribalta, M.; Andres-Lacueva, C.; Estruch, R.; Escribano, E.; Urpi-Sarda, M. Pharmacokinetics of resveratrol metabolic profile in healthy humans after moderate consumption of red wine and grape extract tablets. Pharmacol. Res. 2012, 66, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Stervbo, U.; Vang, O.; Bonnesen, C. A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chem. 2007, 101, 449–457. [Google Scholar] [CrossRef]
- Pignatelli, P.; Ghiselli, A.; Buchetti, B.; Carnevale, R.; Natella, F.; Germano, G.; Fimognari, F.; di Santo, S.; Lenti, L.; Violi, F. Polyphenols synergistically inhibit oxidative stress in subjects given red and white wine. Atherosclerosis 2006, 188, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Hikami, S.; Iiduna, S.; Yoshimatsu, M.; Asama, T.; Ota, H.; Kimura, Y.; Tatefuji, T.; Hashimo, K.; Higaki, K. Pharmacokinetics and safety of resveratrol derivatives in humans after oral administration of melinjo (Gnetum gnemon L.) seed extract powder. J. Agric. Food Chem. 2014, 62, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Sforza, S.; Galaverna, G.; Ghidini, C.; Caporaso, N.; Vescovi, P.P.; Fogliano, V.; Marchelli, R. Bioavailability of trans-resveratrol from red wine in humans. Mol. Nutr. Food Res. 2005, 49, 495–504. [Google Scholar] [CrossRef] [PubMed]
- La Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-state pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Rotches-Ribalta, M.; Urpi-Sarda, M.; Llorach, R.; Boto-Ordonez, M.; Jauregui, O.; Chiva-Blanch, G.; Perez-Garcia, L.; Jaeger, W.; Guillen, M.; Corella, D.; et al. Gut and microbial resveratrol metabolite profiling after moderate long-term consumption of red wine versus dealcoholized red wine in humans by an optimized ultra-high-pressure liquid chromatography tandem mass spectrometry method. J. Chromatogr. A 2012, 1265, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Rotches-Ribalta, M.; Urpi-Sarda, M.; Marti, M.M.; Reglero, G.; Andres-Lacueva, C. Resveratrol metabolic fingerprinting after acute and chronic intakes of a functional beverage in humans. Electrophoresis 2014, 35, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef] [PubMed]
- El-Mohsen, M.A.; Bayele, H.; Kuhnle, G.; Gibson, G.; Debnam, E.; Kaila, S.S.; Rice-Evans, C.; Spencer, J.P. Distribution of [3H]trans-resveratrol in rat tissues following oral administration. Br. J. Nutr. 2006, 96, 62–70. [Google Scholar] [CrossRef]
- Lou, B.S.; Wu, P.S.; Hou, C.W.; Cheng, F.Y.; Chen, J.K. Simultaneous quantification of trans-resveratrol and its sulfate and glucuronide metabolites in rat tissues by stable isotope-dilution UPLC-MS/MS analysis. J. Pharm. Biomed. Anal. 2014, 94, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Azorin-Ortuno, M.; Yanez-Gascon, M.J.; Vallejo, F.; Pallares, F.J.; Larrosa, M.; Lucas, R.; Morales, J.C.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Metabolites and tissue distribution of resveratrol in the pig. Mol. Nutr. Food Res. 2011, 55, 1154–1168. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Scott, E.; Kholghi, A.; Andreadi, C.; Rufini, A.; Karmokar, A.; Britton, R.G.; Horner-Glister, E.; Greaves, P.; Jawad, D.; et al. Cancer chemoprevention: Evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Sci. Transl. Med. 2015, 7, 298ra117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Catana, F.; Yang, Y.N.; Roderick, R.; van Breemen, R.B. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J. Agric. Food Chem. 2002, 50, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Vian, M.A.; Tomao, V.; Gallet, S.; Coulomb, P.O.; Lacombe, J.M. Simple and rapid method for cis- and trans-resveratrol and piceid isomers determination in wine by high-performance liquid chromatography using chromolith columns. J. Chromatogr. A 2005, 1085, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nicolas, J.M.; Nunez-Delicado, E.; Perez-Lopez, A.J.; Barrachina, A.C.; Cuadra-Crespo, P. Determination of stoichiometric coefficients and apparent formation constants for beta-cyclodextrin complexes of trans-resveratrol using reversed-phase liquid chromatography. J. Chromatogr. A 2006, 1135, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Belguendouz, L.; Fremont, L.; Gozzelino, M.T. Interaction of transresveratrol with plasma lipoproteins. Biochem. Pharmacol. 1998, 55, 811–816. [Google Scholar] [CrossRef]
- Jannin, B.; Menzel, M.; Berlot, J.P.; Delmas, D.; Lancon, A.; Latruffe, N. Transport of resveratrol, a cancer chemopreventive agent, to cellular targets: Plasmatic protein binding and cell uptake. Biochem. Pharmacol. 2004, 68, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.; Gescher, A.J. Phase I randomised double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases-safety, pharmacokinetics andpharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Frozza, R.L.; Bernardi, A.; Paese, K.; Hoppe, J.B.; da Silva, T.; Battastini, A.M.; Pohlmann, A.R.; Guterres, S.S.; Salbego, C. Characterization of trans-resveratrol-loaded lipid-core nanocapsules and tissue distribution studies in rats. J. Biomed. Nanotechnol. 2010, 6, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Anju, S.S.; Cinu, T.A.; Aleykutty, N.A.; Thomas, S.; Souto, E.B. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. Int. J. Pharm. 2014, 474, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Penalva, R.; Esparza, I.; Larraneta, E.; Gonzalez-Navarro, C.J.; Gamazo, C.; Irache, J.M. Zein-based nanoparticles improve the oral bioavailability of resveratrol and its anti-inflammatory effects in a mouse model of endotoxic shock. J. Agric. Food Chem. 2015, 63, 5603–5611. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pai, R.S. In-vitro/in-vivo characterization of trans-resveratrol-loaded nanoparticulate drug delivery system for oral administration. J. Pharm. Pharmacol. 2014, 66, 1062–1076. [Google Scholar] [PubMed]
- Amiot, M.J.; Romier, B.; Dao, T.M.A.; Fanciullino, R.; Ciccolini, J.; Burcelin, R.; Pechere, L.; Emond, C.; Savouret, J.F.; Seree, E. Optimization of trans-Resveratrol bioavailability for human therapy. Biochimie 2013, 95, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, O.L.; Friesenhahn, G.; Javors, M.A.; Smoliga, J.M. Development of a lozenge for oral transmucosal delivery of trans-resveratrol in humans: Proof of concept. PLoS ONE 2014, 9, e90131. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, P.; Ko, Y.T. Improved oral delivery of resveratrol from N-trimethyl chitosan-g-palmitic acid surface-modified solid lipid nanoparticles. Colloids Surf. B. Biointerfaces 2016, 139, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Zhang, Y.; Wang, W.; Zhao, X.; Han, X.; Wang, K.; Ge, Y. Preparation and in vitro/in vivo evaluation of resveratrol-loaded carboxymethyl chitosan nanoparticles. Drug Deliv. 2016, 23, 981–991. [Google Scholar] [PubMed]
- Scalia, S.; Trotta, V.; Iannuccelli, V.; Bianchi, A. Enhancement of in vivo human skin penetration of resveratrol by chitosan-coated lipid microparticles. Colloids Surf. B Biointerfaces 2015, 135, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Parnes, H.L.; Brawley, O.W.; Minasian, L.M.; Ford, L.G. Phase III prostate cancer chemoprevention trials. Recent Results Cancer Res. 2014, 202, 73–77. [Google Scholar] [PubMed]
- Smoliga, J.M.; Colombo, E.S.; Campen, M.J. A healthier approach to clinical trials evaluating resveratrol for primary prevention of age-related diseases in healthy populations. Aging 2013, 5, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Robich, M.P.; Chu, L.M.; Chaudray, M.; Nezafat, R.; Han, Y.; Clements, R.T.; Laham, R.J.; Manning, W.J.; Coady, M.A.; Sellke, F.W. Anti-angiogenic effect of high-dose resveratrol in a swine model of metabolic syndrome. Surgery 2010, 148, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Robich, M.P.; Osipov, R.M.; Chu, L.M.; Han, Y.; Feng, J.; Nezafat, R.; Clements, R.T.; Manning, W.J.; Sellke, F.W. Resveratrol modifies risk factors for coronary artery disease in swine with metabolic syndrome and myocardial ischemia. Eur. J. Pharmacol. 2011, 664, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Robich, M.P.; Chu, L.M.; Burgess, T.A.; Feng, J.; Han, Y.; Nezafat, R.; Leber, M.P.; Laham, R.J.; Manning, W.J.; Sellke, F.W. Resveratrol preserves myocardial function and perfusion in remote nonischemic myocardium in a swine model of metabolic syndrome. J. Am. Coll. Surg. 2012, 215, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Robich, M.P.; Osipov, R.M.; Nezafat, R.; Feng, J.; Clements, R.T.; Bianchi, C.; Boodhwani, M.; Coady, M.A.; Laham, R.J.; Sellke, F.W. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation 2010, 122, S142–S149. [Google Scholar] [CrossRef] [PubMed]
- Dal-Pan, A.; Pifferi, F.; Marchal, J.; Picq, J.L.; Aujard, F. Cognitive performances are selectively enhanced during chronic caloric restriction or resveratrol supplementation in a primate. PLoS ONE 2011, 6, e16581. [Google Scholar] [CrossRef] [PubMed]
- Marchal, J.; Blanc, S.; Epelbaum, J.; Aujard, F.; Pifferi, F. Effects of chronic calorie restriction or dietary resveratrol supplementation on insulin sensitivity markers in a primate, Microcebus murinus. PLoS ONE 2012, 7, e34289. [Google Scholar] [CrossRef] [PubMed]
- Fiori, J.L.; Shin, Y.K.; Kim, W.; Krzysik-Walker, S.M.; Gonzalez-Mariscal, I.; Carlson, O.D.; Sanghvi, M.; Moaddel, R.; Farhang, K.; Gadkaree, S.K.; et al. Resveratrol prevents beta-cell dedifferentiation in non-human primates given a high fat/high sugar diet. Diabetes 2013, 62, 3500–3513. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Wang, M.; Bernier, M.; Zhang, J.; Park, S.S.; Maudsley, S.; An, S.S.; Santhanam, L.; Martin, B.; Faulkner, S.; et al. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014, 20, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Vang, O.; Ahmad, N.; Baile, C.A.; Baur, J.A.; Brown, K.; Csiszar, A.; Das, D.K.; Delmas, D.; Gottfried, C.; Lin, H.Y.; et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS ONE 2011, 6, e19881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshino, J.; Conte, C.; Fontana, L.; Mittendorfer, B.; Imai, S.I.; Schechtman, K.B.; Gu, C.; Kunz, I.; Fanelli, F.R.; Patterson, B.W.; et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012, 15, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Kerti, L.; Margulies, D.S.; Floel, A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, B.; Campen, M.J.; Channell, M.M.; Wherry, S.J.; Varamini, B.; Davis, J.G.; Baur, J.A.; Smoliga, J.M. Resveratrol for primary prevention of atherosclerosis: Clinical trial evidence for improved gene expression in vascular endothelium. Int. J. Cardiol. 2013, 188, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Brasnyo, P.; Molnar, G.A.; Mohas, M.; Marko, L.; Laczy, B.; Cseh, J.; Mikolas, E.; Szijarto, I.A.; Merei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Del Villar, M.; Gonzalez-Ortiz, M.; Martinez-Abundis, E.; Perez-Rubio, K.G.; Lizarraga-Valdez, R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2014, 12, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Gonzalvez, M.; Larrosa, M.; Garcia-Almagro, F.J.; Aviles-Plaza, F.; Parra, S.; Yanez-Gascon, M.J.; Ruiz-Ros, J.A.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A.; et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Gonzalvez, M.; Larrosa, M.; Yanez-Gascon, M.J.; Garcia-Almagro, F.J.; Ruiz-Ros, J.A.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A.; Espin, J.C. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am. J. Cardiol. 2012, 110, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Larrosa, M.; Yanez-Gascon, M.J.; Davalos, A.; Gil-Zamorano, J.; Gonzalvez, M.; Garcia-Almagro, F.J.; Ros, J.A.; Tomas-Barberan, F.A.; Espin, J.C.; et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol. Res. 2013, 72, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [PubMed]
- Van der Made, S.M.; Plat, J.; Mensink, R.P. Resveratrol does not influence metabolic risk markers related to cardiovascular health in overweight and slightly obese subjects: A randomized, placebo-controlled crossover trial. PLoS ONE 2015, 10, e0118393. [Google Scholar]
- Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C.; Holcombe, R.F. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag. Res. 2009, 1, 25–37. [Google Scholar] [PubMed]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013, 160, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Grzasko, N.; Morawska, M.; Hus, M. Optimizing the treatment of patients with multiple myeloma and renal impairment. Clin. Lymphoma Myeloma Leuk. 2015, 15, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stodkilde-Jorgensen, H.; Moller, N.; Jessen, N.; Pedersen, S.B.; Jorgensen, J.O. High-dose resveratrol supplementation in obese men: An investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 2013, 62, 1186–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dash, S.; Xiao, C.; Morgantini, C.; Szeto, L.; Lewis, G.F. High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2895–2901. [Google Scholar] [CrossRef] [PubMed]
- Movahed, A.; Nabipour, I.; Lieben, L.X.; Thandapilly, S.J.; Yu, L.; Kalantarhormozi, M.; Rekabpour, S.J.; Netticadan, T. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid. Based Complement. Altern. Med. 2013, 2013, 851267. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Crandall, J.P.; Oram, V.; Trandafirescu, G.; Reid, M.; Kishore, P.; Hawkins, M.; Cohen, H.W.; Barzilai, N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, X.; Ran, L.; Wan, J.; Wang, X.; Qin, Y.; Shu, F.; Gao, Y.; Yuan, L.; Zhang, Q.; et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Dig. Liver Dis. 2015, 47, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Faghihzadeh, F.; Adibi, P.; Rafiei, R.; Hekmatdoost, A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr. Res. 2014, 34, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Berry, N.M.; Coates, A.M.; Buckley, J.D.; Bryan, J.; Kunz, I.; Howe, P.R. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J. Hypertens. 2013, 31, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Gliemann, L.; Schmidt, J.F.; Olesen, J.; Bienso, R.S.; Peronard, S.L.; Grandjean, S.U.; Mortensen, S.P.; Nyberg, M.; Bangsbo, J.; Pilegaard, H.; et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. FASEB J. 2013, 27, 5047–5059. [Google Scholar] [CrossRef] [PubMed]
- Faghihzadeh, F.; Adibi, P.; Hekmatdoost, A. The effects of resveratrol supplementation on cardiovascular risk factors in patients with non-alcoholic fatty liver disease: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2015, 114, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.X.; Howe, P.R.C.; Buckley, J.D.; Coates, A.M.; Kunz, I.; Berry, N.M. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 851–856. [Google Scholar] [CrossRef] [PubMed]
| Participant Condition (Design of the Study) | Parameter | Parameter Change | Dose | Duration | Reference | |
|---|---|---|---|---|---|---|
| Heathy young adult (Double-blinded/Placebo-controlled/crossover) N = 22 | Total hemoglobin | ↑ | 250 and 500 mg | 2 separate days (7 ± 2 days between) | Kennedy et al., 2010 [119] | |
| - | |||||
| ↑ | |||||
| Task related differences | - | |||||
| Cognitive task performance and mental fatigue | - | |||||
| Healthy non-obese with healthy glucose tolerance (Randomized/Double-blinded/ Placebo-controlled) N = 29 | BMI, Free-fat index, Free fat, Subcutaneous abdominal fat volume, intra-abdominal fat volume, intra-hepatic triglyceride content | - | 75 mg/day | 12 weeks | Yoshino et al., 2012 [104] | |
| Glucose, Insulin, HOMA-IR | - | |||||
| Free fatty acids, Total cholesterol, LDL-c, HDL-c, Triglyceride | - | |||||
| Leptin | - | |||||
| Adiponectin, IL-6, CRP | - | |||||
| Resting metabolic rate | - | |||||
| Hepatic insulin sensitivity rate | - | |||||
| Systolic and Diastolic blood pressure | - | |||||
| White blood, Red blood cell count, Platelet | - | |||||
| Hemoglobin, Hematrocit, MCV, MCH, MCHC | - | |||||
| Blood urea nitrogen, Total protein, Albumin | - | |||||
| AST, ALT, Alkaline phosphatase, Bilirubin | - | |||||
| Muscle or adipose tissue gene expression for | ||||||
| - | |||||
| - | |||||
| - | |||||
| - | |||||
| Healthy older adults (Double-blinded/Placebo-controlled) N = 46 | Memory retention | ↑ | 200 mg/day | 26 weeks | Witte et al., 2014 [105] | |
| HbA1c | ↓ | |||||
| Insulin | ↑ | |||||
| Total cholesterol | ↑ | |||||
| Leptin | ↑ | |||||
| BDNF | - | |||||
| LDL:HDL ratio, Triacylglycerides | - | |||||
| IGF-1 | - | |||||
| TNF-α | ↓ | |||||
| IL-6, hsCRP | ↓ | |||||
| Body fat, BMI | - | |||||
| Systolic blood pressure | - | |||||
| Diastolic blood pressure | ↓ | |||||
| Subjects with normal heart rate and Blood pressure (Randomized/Triple-blinded/Placebo-controlled) N = 44 | Resveratrol | Placebo | 400 mg Resv + 400 mg grape skin extract + 100 mg quercetin | 30 days | Agarwal et al., 2013 [106] | |
| Insulin | ↓ | - | ||||
| Glucose | - | - | ||||
| INF-γ | ↓ | - | ||||
| IL-1β | - | ↓ | ||||
| IL-6, TNF-α | - | - | ||||
| Leptin | - | - | ||||
| ICAM, VCAM | ↓ | - | ||||
| IL-8 | ↓ | - | ||||
| Condition of the Subject (Design of the Study) | Parameter | Parameter Change | Dose | Duration | Reference |
|---|---|---|---|---|---|
| Healthy obese subjects (Randomized/double-blinded/ /crossover design with 4-weeks washout) N = 11 | Glucose | ↓ | 150 mg/day | 30 days * | Timmers et al., 2011 [107] |
| Insulin | ↓ | ||||
| HOMA index | ↓ | ||||
| Triglycerides | ↓ | ||||
| Leptin | ↓ | ||||
| TNF-α | ↓ | ||||
| Leukocytes | ↓ | ||||
| ALT | ↓ | ||||
| Non-esterified fatty acids | - | ||||
| Adiponectin, CRP, IL-1β, IL-6, IL-8 | - | ||||
| Hemoglobin, Erythrocytes, Thrombocytes | - | ||||
| Leukocytes | - | ||||
| Urea, Creatinine AST, Bilirubin, Total protein, Albumin | - | ||||
| Healthy obese subjects (Randomized/double-blinded/placebo-controlled/parallel-group trial) N = 24 | Glucose, Insulin, HOMA index, HbA1c | - | 3 × 500 mg/day | 4 weeks * | Poulsen et al., 2013 [120] |
| Cholesterol, HDL, LDL, Triglycerides | - | ||||
| Leptin, | - | ||||
| hsCRP, IL-6, TNF-α, MCP-1 | - | ||||
| Leukocytes, ALT | - | ||||
| Obese with mild/ moderate hyperglycemia (Randomized/double-blinded/crossover trial) N = 8 | Glucose, Insulin, HOMA-IR | - | 1 g (week 1)
2 g (week 2) | 2 weeks * | Dash et al., 2013 [121] |
| Triglycerides | - | ||||
| ApoB-48 | ↓ | ||||
| - | ||||
| ApoB-100 | ↓ | ||||
| ↓ | ||||
| Type 2 Diabetic patients (Randomized/Placebo-controlled/double-blinded) N = 66 | Body weight, BMI | - | 2 × 500 mg/day | 45 days ** | Movahed et al., 2013 [122] |
| Systolic blood pressure | ↓ | ||||
| Diastolic blood pressure | - | ||||
| Fasting glucose, Insulin, HOMA-IR | ↓ | ||||
| HbA1c | ↓ | ||||
| HOMA-β | ↓ | ||||
| Triglyceride, Total cholesterol | - | ||||
| HDL-c | ↑ | ||||
| LDL-c | - | ||||
| Creatinine | - | ||||
| Type 2 Diabetic patients (Prospective/open-label/randomized/controlled study) N = 62 | BMI | - | 250 mg/day | 3 months * | Bhatt et al., 2012 [123] |
| Fasting glucose | ↓ | ||||
| HbA1c | ↓ | ||||
| Systolic and diastolic blood pressure | ↓ | ||||
| Total cholesterol, LDL-c, Triglycerides | ↓ | ||||
| HDL-c | - | ||||
| Urea nitrogen | ↓ | ||||
| Creatinine | - | ||||
| Total protein | ↓ | ||||
| Patients with impaired glucose tolerance (Randomized/open-label/pilot study) N = 10 | Body weight, Body fat | - | 1–2 g/day | 4 weeks ** | Crandall et al., 2012 [124] |
| Cholesterol, HDL-c, LDL-c, Triglycerides | - | ||||
| hsCRP, Adiponectin | - | ||||
| Fasting glucose, Fasting insulin | - | ||||
| HOMA-IR | - | ||||
| Insulin sensitivity (Matsuda index) | - | ||||
| Serum creatinine, AST, ALT | - | ||||
| Systolic and diastolic blood pressure | - | ||||
| Glucose (AUC- 3 h after meal) | ↓ | ||||
| Insulin (AUC- 3 h after meal) | ↓ | ||||
| Patients with Metabolic syndrome (Randomized/double-blinded/placebo-controlled) N = 24 | Body weight, BMI, Fat mass | ↓ | 3 × 500 mg/day | 90 days ** | Mendez-Del Villar et al., 2014 [109] |
| Waist circumference | ↓ | ||||
| Systolic and diastolic blood pressure | - | ||||
| Glucose | - | ||||
| Triglycerides, HDL-c, LDL-c | - | ||||
| Glucose (AUC) | - | ||||
| Insulin (AUC) | ↓ | ||||
| Insulinogenic index | ↓ | ||||
| Stumvoll index | - | ||||
| Insulin sensitivity (Matsuda index) | - | ||||
| Patients with Non-alcoholic fatty liver disease (Randomized/double-blinded/placebo-controlled) N = 60 | Body weight, BMI, Waist circumference, Hip circumference | - | 2 × 150 mg | 3 months * | Chen et al., 2015 [125] |
| Systolic and diastolic blood pressure | - | ||||
| Red blood cell, White blood cell and Platelet | - | ||||
| Blood urea nitrogen, Creatinine | - | ||||
| ALT, AST | ↓ | ||||
| GGT | - | ||||
| Glucose | ↓ | ||||
| Insulin | - | ||||
| HOMA-IR | ↓ | ||||
| C-peptide | - | ||||
| Total cholesterol, HDL-c, LDL-c | ↓ | ||||
| Triacylglycerol | ↓ | ||||
| ApoB, ApoA-I | - | ||||
| TNF-α | ↓ | ||||
| Adiponectin | ↑ | ||||
| Cytokeratin 18 | ↓ | ||||
| Fibroblast growth factor 21 | ↓ | ||||
| Patients with Non-alcoholic fatty liver disease (Randomized/double-blinded/placebo-controlled) N = 50 | Body weight, BMI, Waist circumference, Energy intake | - | 500 mg/day | 12 weeks *** | Faghihzadeh et al., 2014 [126] |
| Hip circumference | - | ||||
| Waist:hip ratio, Metabolic equivalent task | - | ||||
| ALT | ↓ | ||||
| AST, GGT | - | ||||
| Bilirubin direct, Bilirubin total | - | ||||
| hsCRP, TNF-α, IL-6, Cytokeratin-18, NF-κB | ↓ | ||||
| Steatosis | ↓ |
| Condition of the Subject (Design of the Study) | Parameter | Parameter Change | Dose | Duration | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Patients treated with primary prevention of cardiovascular disease (3 groups: A: control; B: Grape extract; C: Grape extract + Resv) (Randomized/Triple-blinded/Placebo-controlled/parallel) N = 75 | A vs. B | A vs. C | B vs. C | 8 mg (6 month)–16 mg (next 6 months) | 12 months * | Tome-Carneiro et al., 2012 [111] | |||
| TNF-α, Adiponectin, IL-6, | - | - | - | ||||||
| IL-10 | - | - | ↓ | ||||||
| IL-6/IL-10, IL-18, hsCRP | - | - | - | ||||||
| sICAM-1 | - | ↓ | - | ||||||
| PAI-1 | - | ↓ | ↓ | ||||||
| Patients treated with primary prevention of cardiovascular disease (3 groups: A: control; B: Grape extract; C: Grape extract + Resv) (Randomized/Triple-blinded/Placebo-controlled/parallel) N = 75 | A vs. B | A vs. C | B vs. C | 8 mg | 6 months * | Tome-Carneiro et al., 2012 [110] | |||
| Total cholesterol, TGs, HDL-c, Non-HDL-c | - | - | - | ||||||
| LDL-c, LDL-ox, LDL-c/ApoB, LDL-c/HDL-c | - | - | - | ||||||
| LDL-c/LDL-ox | ↑ | ↑ | - | ||||||
| ApoB, | - | - | - | ||||||
| LDL-ox/ApoB | - | ↓ | - | ||||||
| Non-HDL-c/ApoB | - | - | - | ||||||
| Hypertensive patients with coronary artery disease (3 groups: A: control; B: Grape extract; C: Grape extract + Resv) (Randomized/Triple-blinded/Placebo-controlled/parallel) N = 35 | A | B | C | 8 mg (6 month)–16 mg (next 6 months) | 12 months * | Tome-Carneiro et al., 2012 [112] | |||
| Systolic and diastolic blood pressure | - | - | - | ||||||
| Total cholesterol, LDL-c, HDL,c, Non-HDL-c, TGs, LDL-c/HDL-c, | - | - | - | ||||||
| Glucose | - | - | - | ||||||
| HbA1c | - | - | - | ||||||
| GGT, ALT, AST | - | - | - | ||||||
| ALP | - | ↑ | ↓ | ||||||
| Creatinine, Albumin, Urate | - | - | - | ||||||
| PAI-I | - | - | - | ||||||
| Adiponectin | ↓ | - | - | ||||||
| IL-6 | - | - | ↓ | ||||||
| hsCRP, TNF-α | - | - | - | ||||||
| IL-10, IL6/IL-10 | ↑ | - | - | ||||||
| Patients with stable coronary artery disease (Randomized/Triple-blinded/Placebo-controlled/parallel) N = 40 | Resv vs. baseline | Resv vs. placebo | 10 mg/day | 3 months | Magyar et al., 2012 [113] | ||||
| Systolic and diastolic left ventricular function | - | - | |||||||
| Diastolic left ventricular function | ↑ | ↑ | |||||||
| 12-h fasting white blood cell count | - | - | |||||||
| CRP, TNF-α | - | - | |||||||
| Glucose | - | - | |||||||
| HbA1c | - | - | |||||||
| Total cholesterol, HDL-c, TGs | - | - | |||||||
| LDL-c | ↑ | - | |||||||
| Brachial artery FMD | ↑ | ↑ | |||||||
| Healthy obese adults (Randomized/Double-blinded/Placebo-controlled/cross over) N = 24 | Systolic and diastolic blood pressure | - | 75 mg/day Resv (ResVida) | 12 weeks | Wong et al., 2013 [127] | ||||
| Large artery elasticity index | - | ||||||||
| Small artery elasticity index | - | ||||||||
| Chronic FMD responses | |||||||||
| Resting branchial diameter | - | ||||||||
| Peak branchial diameter | - | ||||||||
| Chronic FMD | ↑ | ||||||||
| Acute FMD responses | |||||||||
| Resting branchial diameter | - | ||||||||
| Peak branchial diameter | - | ||||||||
| Acute FMD | ↑ | ||||||||
| Healthy aged physically inactive man (Randomized/Double-blinded/Placebo-controlled) N = 27 | Placebo | Resv | Resv vs. Placebo | 250 mg/day | 8 weeks | Gliemann et al., 2013 [128] | |||
| Mean arterial pressure | ↓ | - | - | ||||||
| Heart rate (rest) | ↓ | - | - | ||||||
| Heart rate (max) | - | - | - | ||||||
| ↑ | ↑ | ↓ | |||||||
| n.a. | n.a. | ↓ | |||||||
| ↓ | ↑ | ↓ | |||||||
| Glucose | - | - | - | ||||||
| Total cholesterol, HDL | - | - | - | ||||||
| LDL | ↓ | - | - | ||||||
| Total cholesterol/HDL | ↓ | - | - | ||||||
| TGs | ↓ | - | - | ||||||
| VCAM-1 | ↓ | ↓ | - | ||||||
| Overweight and Slightly Obese Subjects (Randomized/Placebo-Controlled/Crossover) N = 45 | Total cholesterol, LDL-c, HDL-c, Total:HDL-c ratio, | - | 150 mg/day | 4 weeks ** | Van der Made et al., 2015 [114] | ||||
| Triacylglycerol | - | ||||||||
| ApoA-I, ApoB-100 | - | ||||||||
| BMI | - | ||||||||
| Insulin, Glucose, HOMA-IR | - | ||||||||
| Systolic and diastolic blood pressure | - | ||||||||
| Heart rate, Mean arterial pressure | - | ||||||||
| hsCRP, IL-6, TNF-α | - | ||||||||
| E-selectin, Thrombomodulin, P-selectin | - | ||||||||
| ICAM-3, sICAM-1, sVCAM-1 | - | ||||||||
| Subjects with non-alcoholic fatty liver disease (Randomized/Placebo-Controlled/Double-blinded) N = 50 | ALT | ↓ | 500 mg/day | 12 weeks ** | Faghihzadeh et al., 2015 [129] | ||||
| AST, GGT | - | ||||||||
| Bilirubin direct, Bilirubin total | - | ||||||||
| Steatosis | ↓ | ||||||||
| TAG, Total cholesterol, LDL-c, HDL | - | ||||||||
| LDL/HDL, Non-HDL-c | ↑ | ||||||||
| Apo-A1 | - | ||||||||
| Glucose, Insulin, HOMA-IR, HOMA-β | - | ||||||||
| Systolic and diastolic blood pressure | - | ||||||||
| Overweight/obese individuals with elevated blood pressure (Randomized/Placebo-Controlled/Double-blinded/Crossover) N = 19 | FMD | ↑ | 30, 90 and 270 mg or placebo at each weekly visit | 4 weeks | Wong et al., 2011 [130] | ||||
| Design of the Study | Parameter | Parameter Change | Dose | Duration | Reference |
|---|---|---|---|---|---|
| Phase I (Open labelled/Pilot) N = 8 | Wnt pathway target genes | 0.073 mg (80 mg grape extract) | 14 days | Nguyen et al., 2009 [115] | |
| ↓ | ||||
| - | ||||
| Phase I (Randomized/Double-blinded) N = 6 | VEGF in serum | - | 5 g/day (SRT501) | 14 days (mean) (10-21 days) | Howells et al., 2011 [82] |
| Prostaglandin E2 in plasma | - | ||||
| IGF-1 in liver | - | ||||
| Cleaved caspase 3 | ↑ | ||||
| Phase I (Controlled) N = 20 | AMPK signaling (Tissue from patient) | - | 5 mg/day or 1 g/day | 6 days | Cai et al., 2015 [76] |
| NQO1 (Colorectal mucosa) | ↑ (5 mg dose) | ||||
| Protein carbonyl (Colorectal mucosa) | ↑ (5 mg dose) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdogan, C.S.; Vang, O. Challenges in Analyzing the Biological Effects of Resveratrol. Nutrients 2016, 8, 353. https://doi.org/10.3390/nu8060353
Erdogan CS, Vang O. Challenges in Analyzing the Biological Effects of Resveratrol. Nutrients. 2016; 8(6):353. https://doi.org/10.3390/nu8060353
Chicago/Turabian StyleErdogan, Cihan Suleyman, and Ole Vang. 2016. "Challenges in Analyzing the Biological Effects of Resveratrol" Nutrients 8, no. 6: 353. https://doi.org/10.3390/nu8060353
APA StyleErdogan, C. S., & Vang, O. (2016). Challenges in Analyzing the Biological Effects of Resveratrol. Nutrients, 8(6), 353. https://doi.org/10.3390/nu8060353