Long-Term Fructose Intake Increases Adipogenic Potential: Evidence of Direct Effects of Fructose on Adipocyte Precursor Cells
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals and Treatment
2.2. Peripheral Metabolite Measurements
2.3. RPAT Stromal Vascular Fraction (SVF) Cell and Adipocyte Isolation
2.4. Adipocyte Size Analysis
2.5. RPAT SVF Cell Culture
2.6. SVF Cell Composition Analysis by Flow Cytometry (FACS)
2.7. Cell Differentiation
2.8. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
2.9. Leptin Measurement
2.10. Cellular Lipid Content
2.11. Percentages of Cell Differentiation and Maturation
2.12. Statistical Analysis
3. Results
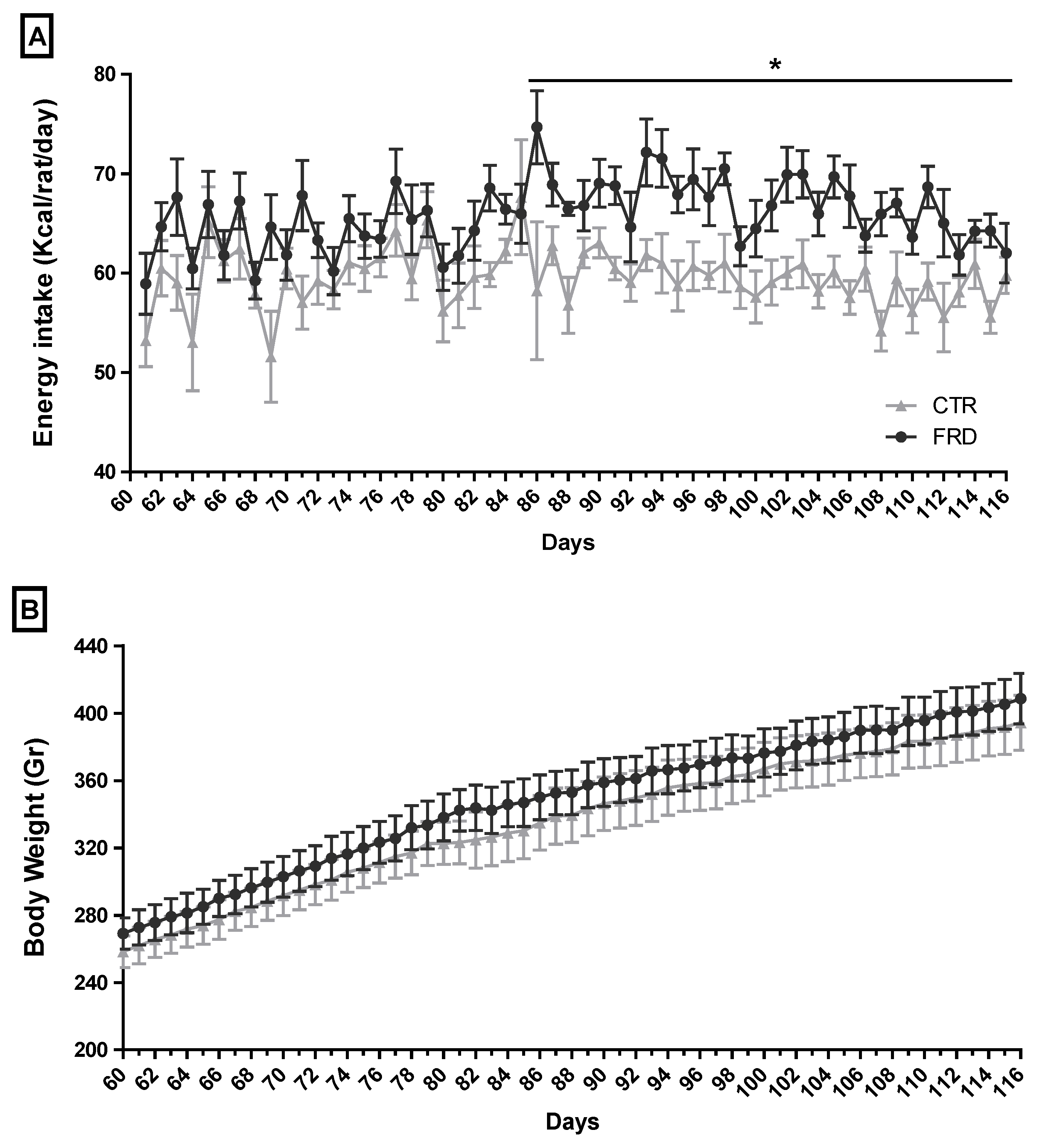
3.1. Effect of FRD Intake on Metabolic Parameters
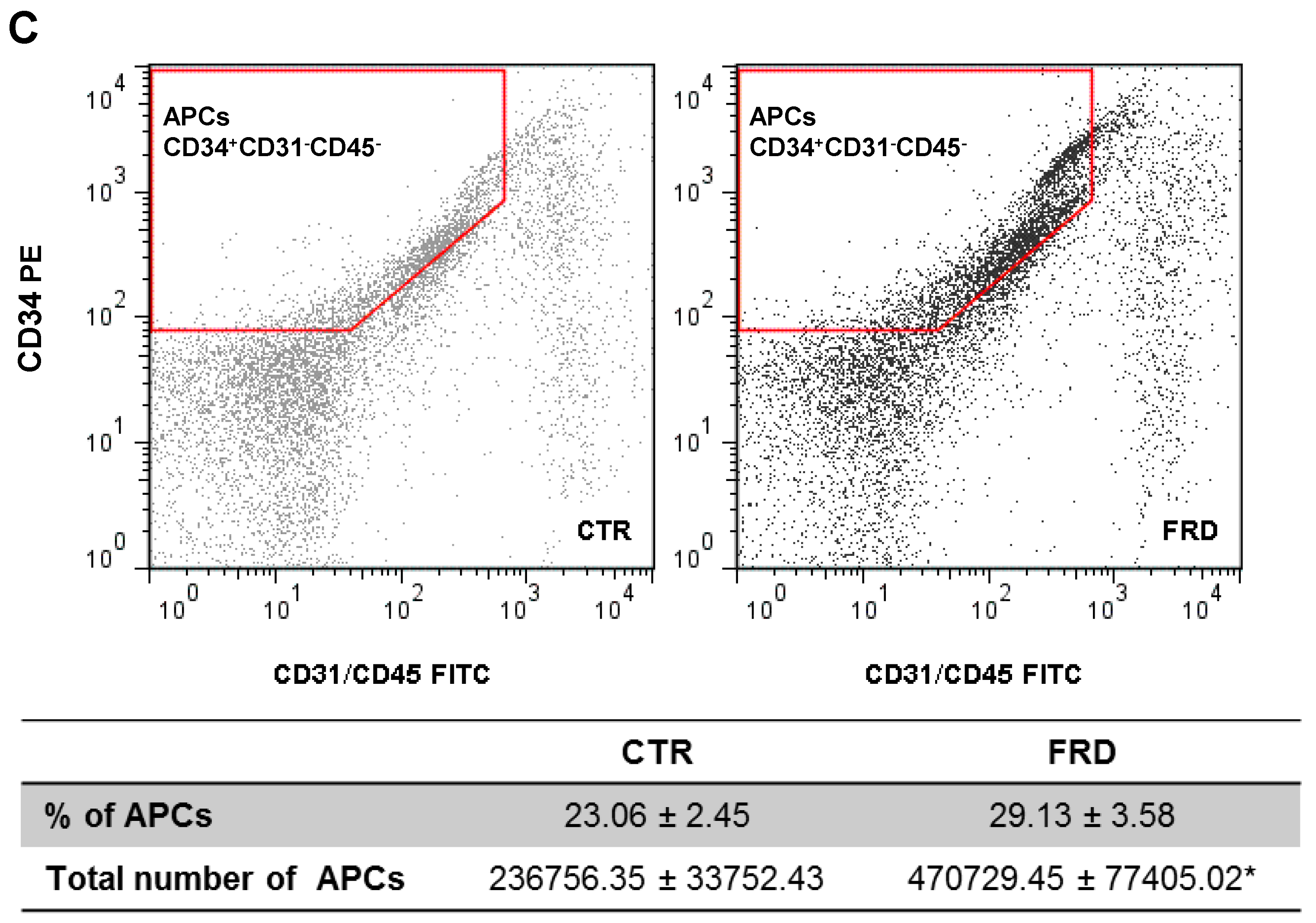
3.2. FRD Modifies APCs Number and Adipogenic Potential
3.3. In vitro Adipocyte Differentiation
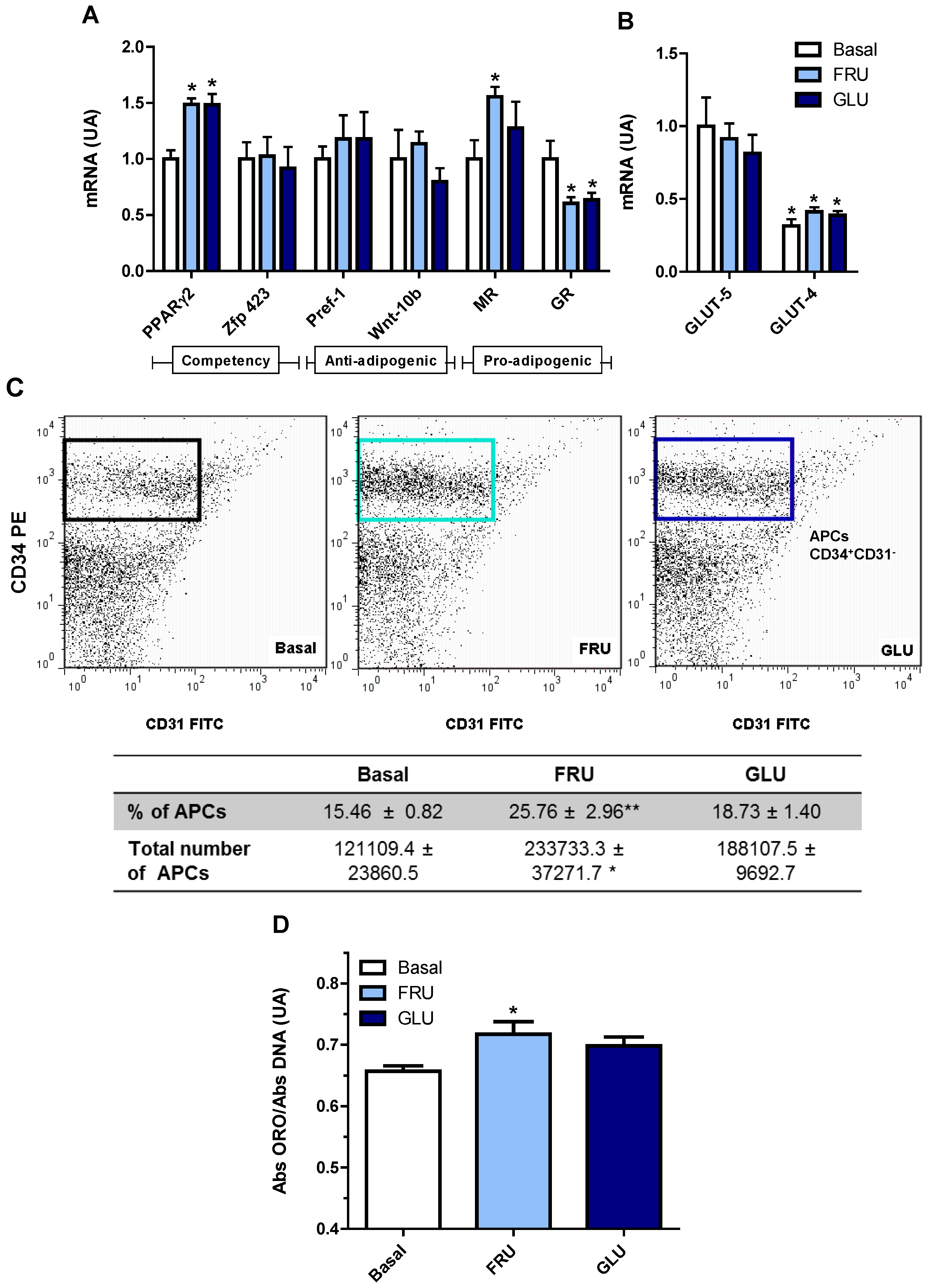
3.4. Direct Effect of Fructose Exposure on the Adipogenic Potential of Normal APCs
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.-P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Høstmark, A.T. The Oslo health study: Soft drink intake is associated with the metabolic syndrome. Appl. Physiol. Nutr. Metab. 2010, 35, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.-H.; Gersch, M.S.; Benner, S.; Sánchez-Lozada, L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar] [PubMed]
- Tappy, L.; Lê, K.A.; Tran, C.; Paquot, N. Fructose and metabolic diseases: New findings, new questions. Nutrition 2010, 26, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Parks, E.J.; Skokan, L.E.; Timlin, M.T.; Dingfelder, C.S. Dietary sugars stimulate fatty acid synthesis in adults. J. Nutr. 2008, 138, 1039–1046. [Google Scholar] [PubMed]
- Samuel, V.T. Fructose induced lipogenesis: From sugar to fat to insulin resistance. Trends Endocrinol. Metab. 2011, 22, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Douard, V.; Ferraris, R.P. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E227–E237. [Google Scholar] [CrossRef] [PubMed]
- Litherland, G.J.; Hajduch, E.; Gould, G.W.; Hundal, H.S. Fructose transport and metabolism in adipose tissue of Zucker rats: Diminished GLUT5 activity during obesity and insulin resistance. Mol. Cell. Biochem. 2004, 261, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Björkman, O.; Felig, P. Role of the kidney in the metabolism of fructose in 60-hour fasted humans. Diabetes 1982, 31, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Franck, N.; Stenkula, K.G.; Ost, A.; Lindström, T.; Strålfors, P.; Nystrom, F.H. Insulin-induced GLUT4 translocation to the plasma membrane is blunted in large compared with small primary fat cells isolated from the same individual. Diabetologia 2007, 50, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Wåhlen, K.; Sjölin, E.; Löfgren, P. Role of fat cell size for plasma leptin in a large population based sample. Exp. Clin. Endocrinol. Diabetes 2011, 119, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Alzamendi, A.; Giovambattista, A.; Raschia, A.; Madrid, V.; Gaillard, R.C.; Rebolledo, O.; Gagliardino, J.J.; Spinedi, E. Fructose-rich diet-induced abdominal adipose tissue endocrine dysfunction in normal male rats. Endocrine 2009, 35, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Zubiría, M.G.; Fariña, J.P.; Moreno, G.; Gagliardino, J.J.; Spinedi, E.; Giovambattista, A. Excess fructose intake-induced hypertrophic visceral adipose tissue results from unbalanced precursor cell adipogenic signals. FEBS J. 2013, 280, 5864–5874. [Google Scholar] [CrossRef] [PubMed]
- Fariña, J.P.; García, M.E.; Alzamendi, A.; Giovambattista, A.; Marra, C.A.; Spinedi, E.; Gagliardino, J.J. Antioxidant treatment prevents the development of fructose-induced abdominal adipose tissue dysfunction. Clin. Sci. 2013, 125, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Heaney, A.P. Regulation of adipose differentiation by fructose and GluT5. Mol. Endocrinol. 2012, 26, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Legeza, B.; Balázs, Z.; Odermatt, A. Fructose promotes the differentiation of 3T3-L1 adipocytes and accelerates lipid metabolism. FEBS Lett. 2014, 588, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Perelló, M.; Gaillard, R.C.; Chisari, A.; Spinedi, E. Adrenal enucleation in MSG-damaged hyperleptinemic male rats transiently restores adrenal sensitivity to leptin. Neuroendocrinology 2003, 78, 176–184. [Google Scholar] [PubMed]
- Giovambattista, A.; Gaillard, R.C.; Spinedi, E. Ghrelin gene-related peptides modulate rat white adiposity. Vitam. Horm. 2008, 77, 171–205. [Google Scholar] [PubMed]
- Tchoukalova, Y.D.; Harteneck, D.A.; Karwoski, R.A.; Tarara, J.; Jensen, M.D. A quick, reliable, and automated method for fat cell sizing. J. Lipid Res. 2003, 44, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.; Sengenès, C.; Decaunes, P.; Zakaroff-Girard, A.; Bourlier, V.; Lafontan, M.; Galitzky, J.; Bouloumié, A. Evidence of in situ proliferation of adult adipose tissue-derived progenitor cells: Influence of fat mass microenvironment and growth. J. Clin. Endocrinol. Metab. 2008, 93, 4098–4106. [Google Scholar] [CrossRef] [PubMed]
- Giovambattista, A.; Piermaría, J.; Suescun, M.O.; Calandra, R.S.; Gaillard, R.C.; Spinedi, E. Direct effect of ghrelin on leptin production by cultured rat white adipocytes. Obesity 2006, 14, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dodson, M.V.; Jiang, Z. Cellular and molecular comparison of redifferentiation of intramuscular- and visceral-adipocyte derived progeny cells. Int. J. Biol. Sci. 2010, 6, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, F.; Todoroff, G.; Hauser, N.; Remacle, C. The stroma-vascular fraction of rat inguinal and epididymal adipose tissue and the adipoconversion of fat cell precursors in primary culture. Biol. Cell Auspices Eur. Cell Biol. Organ. 1990, 69, 215–222. [Google Scholar]
- Alzamendi, A.; Giovambattista, A.; García, M.E.; Rebolledo, O.R.; Gagliardino, J.J.; Spinedi, E. Effect of pioglitazone on the fructose-induced abdominal adipose tissue dysfunction. PPAR Res. 2012, 2012, 259093. [Google Scholar] [CrossRef] [PubMed]
- Lowette, K.; Roosen, L.; Tack, J.; Vanden Berghe, P. Effects of high-fructose diets on central appetite signaling and cognitive function. Front. Nutr. 2015, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Baelemans, A.; Erlanson-Albertsson, C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul. Pept. 2008, 150, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Crescenzo, R.; Bianco, F.; Coppola, P.; Mazzoli, A.; Valiante, S.; Liverini, G.; Iossa, S. Adipose tissue remodeling in rats exhibiting fructose-induced obesity. Eur. J. Nutr. 2014, 53, 413–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengenès, C.; Lolmède, K.; Zakaroff-Girard, A.; Busse, R.; Bouloumié, A. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J. Cell. Physiol. 2005, 205, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Arany, Z.; Seale, P.; Mepani, R.J.; Ye, L.; Conroe, H.M.; Roby, Y.A.; Kulaga, H.; Reed, R.R.; Spiegelman, B.M. Transcriptional control of preadipocyte determination by Zfp423. Nature 2010, 464, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Spiegelman, B.M. Fat and beyond: the diverse biology of PPARgamma. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Caprio, M.; Fève, B.; Claës, A.; Viengchareun, S.; Lombès, M.; Zennaro, M.-C. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J. 2007, 21, 2185–2194. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Fried, S.K. The glucocorticoid receptor, not the mineralocorticoid receptor, plays the dominant role in adipogenesis and adipokine production in human adipocytes. Int. J. Obes. 2014, 38, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. How bad is fructose? Am. J. Clin. Nutr. 2007, 86, 895–896. [Google Scholar] [PubMed]
- Hajduch, E.; Darakhshan, F.; Hundal, H.S. Fructose uptake in rat adipocytes: GLUT5 expression and the effects of streptozotocin-induced diabetes. Diabetologia 1998, 41, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; del, M.; Sabater, D.; Fernández-López, J.A.; Remesar, X.; Alemany, M. Glycerol Production from Glucose and Fructose by 3T3-L1 Cells: A Mechanism of Adipocyte Defense from Excess Substrate. PLoS ONE 2015, 10, e0139502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darakhshan, F.; Hajduch, E.; Kristiansen, S.; Richter, E.A.; Hundal, H.S. Biochemical and functional characterization of the GLUT5 fructose transporter in rat skeletal muscle. Biochem. J. 1998, 336, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Robubi, A.; Huber, K.R.; Krugluger, W. Extra fructose in the growth medium fuels lipogenesis of adipocytes. J. Obes. 2014, 2014, 647034. [Google Scholar] [CrossRef] [PubMed]
- Wood, I.S.; Hunter, L.; Trayhurn, P. Expression of Class III facilitative glucose transporter genes (GLUT-10 and GLUT-12) in mouse and human adipose tissues. Biochem. Biophys. Res. Commun. 2003, 308, 43–49. [Google Scholar] [CrossRef]
- Kovačević, S.; Nestorov, J.; Matić, G.; Elaković, I. Dietary fructose-related adiposity and glucocorticoid receptor function in visceral adipose tissue of female rats. Eur. J. Nutr. 2014, 53, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Joe, A.W.B.; Yi, L.; Even, Y.; Vogl, A.W.; Rossi, F.M. V Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells 2009, 27, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Macotela, Y.; Emanuelli, B.; Mori, M.A.; Gesta, S.; Schulz, T.J.; Tseng, Y.-H.; Kahn, C.R. Intrinsic Differences in Adipocyte Precursor Cells From Different White Fat Depots. Diabetes 2012, 61, 1691–1699. [Google Scholar] [CrossRef] [PubMed]



| Primers (5′-3′) | GBAN | bp | |
|---|---|---|---|
| ACTβ | se, AGCCATGTACGTAGCCATCC | NM_031144 | 115 |
| as, ACCCTCATAGATGGGCACAG | |||
| ADIPOQ | se, AATCCTGCCCAGTCATGAAG | NM_144744 | 159 |
| as, TCTCCAGGAGTGCCATCTCT | |||
| C/EBPα | se, CTGCGAGCACGAGACGTCTATAG | NM_012524 | 159 |
| as, TCCCGGGTAGTCAAAGTCACC | |||
| GLUT-4 | se, GCTTCTGTTGCCCTTCTGTC | NM_012751.1 | 166 |
| as, TGGACGCTCTCTTTCCAACT | |||
| GLUT-5 | se, CTTGCAGAGCAACGATGGAG | NM_031741.1 | 145 |
| as, AACTCTGAGGGCGAGTTGAC | |||
| GR | se, TGCCCAGCATGCCGCTATCG | NW_047512 | 170 |
| as, GGGGTGAGCTGTGGTAATGCTGC | |||
| IRS-1 | se, TGTGCCAAGCAACAAGAAAG | NM_012969.1 | 176 |
| as, ACGGTTTCAGAGCAGAGGAA | |||
| IRS-2 | se, CTACCCACTGAGCCCAAGAG | NM_001168633.1 | 151 |
| as, CCAGGGATGAAGCAGGACTA | |||
| MR | se, TCGCTCCGACCAAGGAGCCA | NM_013131 | 193 |
| as, TTCGCTGCCAGGCGGTTGAG | |||
| Ob | se, GAGACCTCCTCCATCTGCTG | NM_013076 | 192 |
| as, CTCAGCATTCAGGGCTAAGG | |||
| PPAR-γ2 | se, AGGGGCCTGGACCTCTGCTG | NW_047696 | 185 |
| as, TCCGAAGTTGGTGGGCCAGA | |||
| Pref-1 | se, TGCTCCTGCTGGCTTTCGGC | NM_053744 | 113 |
| as, CCAGCCAGGCTCACACCTGC | |||
| Wnt-10b | se, AGGGGCTGCACATCGCCGTTC | NW_047784 | 175 |
| as, ACTGCGTGCATGACACCAGCAG | |||
| Zfp423 | se, CCGCGATCGGTGAAAGTTG | NM_053583.2 | 121 |
| as, CACGGCTGGATTTCCGATCA |
| CTR | FRD | |
|---|---|---|
| Body Weight (g) | 403.87 ± 10.54 | 408.69 ± 10.95 |
| LEP (ng/mL) | 5.34 ± 0.68 | 9.39 ± 1.66 * |
| CORT (nM) | 345.21 ± 32.32 | 282.67 ± 23.36 |
| INS (nM) | 0.29 ± 0.04 | 0.46 ± 0.03 * |
| Glu (mM) | 6.94 ± 0.11 | 7.05 ± 0.22 |
| Tg (mM) | 1.29 ± 0.09 | 2.51 ± 0.16 * |
| RPAT mass (g) | 3.92 ± 0.29 | 5.30 ± 0.51 * |
| RPAT Adipocyte diameter (µm) | 69.24 ± 0.67 | 77.36 ± 0.77 * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubiría, M.G.; Alzamendi, A.; Moreno, G.; Rey, M.A.; Spinedi, E.; Giovambattista, A. Long-Term Fructose Intake Increases Adipogenic Potential: Evidence of Direct Effects of Fructose on Adipocyte Precursor Cells. Nutrients 2016, 8, 198. https://doi.org/10.3390/nu8040198
Zubiría MG, Alzamendi A, Moreno G, Rey MA, Spinedi E, Giovambattista A. Long-Term Fructose Intake Increases Adipogenic Potential: Evidence of Direct Effects of Fructose on Adipocyte Precursor Cells. Nutrients. 2016; 8(4):198. https://doi.org/10.3390/nu8040198
Chicago/Turabian StyleZubiría, María Guillermina, Ana Alzamendi, Griselda Moreno, María Amanda Rey, Eduardo Spinedi, and Andrés Giovambattista. 2016. "Long-Term Fructose Intake Increases Adipogenic Potential: Evidence of Direct Effects of Fructose on Adipocyte Precursor Cells" Nutrients 8, no. 4: 198. https://doi.org/10.3390/nu8040198
APA StyleZubiría, M. G., Alzamendi, A., Moreno, G., Rey, M. A., Spinedi, E., & Giovambattista, A. (2016). Long-Term Fructose Intake Increases Adipogenic Potential: Evidence of Direct Effects of Fructose on Adipocyte Precursor Cells. Nutrients, 8(4), 198. https://doi.org/10.3390/nu8040198




