A Comparison of Nutritional Antioxidant Content in Breast Milk, Donor Milk, and Infant Formulas
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- ESPGHAN Committee on Nutrition; Arslanoglu, S.; Corpeleijn, W.; Moro, G.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellöf, M.; Fewtrell, M.; et al. Donor human milk for preterm infants: Current evidence and research directions. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E.; Giuliani, F.; Baricco, M.; Di Nicola, P.; Peila, C.; Vassia, C.; Chiale, F.; Pirra, A.; Cresi, F.; Martano, C.; et al. Benefits of donor milk in the feeding of preterm infants. Early Hum. Dev. 2013, 89 (Suppl. 2), S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.A.; Soares, F.V.; Pimenta, H.P.; Abranches, A.D.; Moreira, M.E. Analysis of the influence of pasteurization, freezing/thawing, and offer processes on human milk’s macronutrient concentrations. Early Hum. Dev. 2011, 87, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E.; Arslanoglu, S.; Martano, C.; Di Nicola, P.; Giuliani, F.; Peila, C.; Cester, E.; Pirra, A.; Coscia, A.; Moro, G. Biological, nutritional and clinical aspects of feeding preterm infants with human milk. J. Biol. Regul. Homeost. Agents 2012, 26 (Suppl. 3), 9–13. [Google Scholar] [PubMed]
- Sandal, G.; Uras, N.; Gokmen, T.; Oguz, S.S.; Erdeve, O.; Dilmen, U. Assessment of oxidant/antioxidant system in newborns and their breast milks. J. Matern. Fetal Neonatal Med. 2013, 26, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Stuetz, W.; Bernhard, W.; Franz, A.; Raith, M.; Grune, T.; Breusing, N. Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. Eur. J. Clin. Nutr. 2014, 68, 215–222. [Google Scholar] [CrossRef] [PubMed]
- El-Sohemy, A.; Baylin, A.; Kabagambe, E.; Ascherio, A.; Spiegelman, D.; Campos, H. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. Am. J. Clin. Nutr. 2002, 76, 172–179. [Google Scholar] [PubMed]
- O’Connor, D.L.; Ewaschuk, J.B.; Unger, S. Human milk pasteurization: Benefits and risks. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Peila, C.; Coscia, A.; Bertino, E.; Cavaletto, M.; Spertino, S.; Icardi, S.; Tortone, C.; Visser, G.H.A.; Gazzolo, D. Effects of holder pasteurization on the protein profile of human milk. Ital. J. Pediatr. 2016, 42. [Google Scholar] [CrossRef] [PubMed]
- Ewaschuk, J.B.; Unger, S.; Harvey, S.; O’Connor, D.L.; Field, C.J. Effect of pasteurization on immune components of milk: Implications for feeding preterm infants. Appl. Physiol. Nutr. Metab. 2011, 36, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Ewaschuk, J.B.; Unger, S.; O’Connor, D.L.; Stone, D.; Harvey, S.; Clandinin, M.T.; Field, C.J. Effect of pasteurization on selected immune components of donated human breast milk. J. Perinatol. 2011, 31, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, D.; Miranda, M.; Muriach, M.; Almansa, I.; Jareno, E.; Romero, F.J. Antioxidant capacity of human milk: Effect of thermal conditions for the pasteurization. Acta Paediatr. 2008, 97, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.P.; Shaw, P.N.; Whitfield, K.; Koorts, P.; McConachy, H.; Hewavitharana, A.K. Effect of pasteurisation on the concentrations of vitamin D compounds in donor breastmilk. Int. J. Food Sci. Nutr. 2016, 67, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Turhan, A.H.; Atici, A.; Muslu, N. Antioxidant capacity of breast milk of mothers who delivered prematurely is higher than that of mothers who delivered at term. Int. J. Vitam. Nutr. Res. 2011, 81, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Friel, J.K.; Martin, S.M.; Langdon, M.; Herzberg, G.R.; Buettner, G.R. Milk from mothers of both premature and full-term infants provides better antioxidant protection than does infant formula. Pediatr. Res. 2002, 51, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Oveisi, M.R.; Sadeghi, N.; Jannat, B.; Hajimahmoodi, M.; Behfar, A.O.; Jannat, F.; MokhtariNasab, F. Human breast milk provides better antioxidant capacity than infant formula. Iran. J. Pharm. Res. 2010, 9, 445–449. [Google Scholar] [PubMed]
- Sommerburg, O.; Meissner, K.; Nelle, M.; Lenhartz, H.; Leichsenring, M. Carotenoid supply in breast-fed and formula-fed neonates. Eur. J. Pediatr. 2000, 159, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.M.; Chan, M.M.; Gellermann, W.; Ermakov, I.; Ermakova, M.; Bhosale, P.; Bernstein, P.; Rau, C. Resonance Raman spectroscopy and the preterm infant carotenoid status. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.P.; Chan, G.M.; Barrett-Reis, B.M.; Fulton, A.B.; Hansen, R.M.; Ashmeade, T.L.; Oliver, J.S.; Mackey, A.D.; Dimmit, R.A.; Hartmann, E.E. Effect of carotenoid supplementation on plasma carotenoids, inflammation and visual development in preterm infants. J. Perinatol. 2012, 32, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Cser, M.A.; Majchrzak, D.; Rust, P.; Rust, P.; Sziklai-Laszlo, I.; Kovacs, I.; Bocskai, E.; Elmadfa, I. Serum carotenoid and retinol levels during childhood infections. Ann. Nutr. Metab. 2004, 48, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Vogelsang, A.; van Lingen, R.A.; Slootstra, J.; Dikkeschei, B.D.; Kollen, B.J.; Schaafsma, A.; van Zoeren-Grobben, D. Antioxidant role of plasma carotenoids in bronchopulmonary dysplasia in preterm infants. Int. J. Vitam. Nutr. Res. 2009, 79, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Kuchan, M.J.; Sen, S.; Johnson, E.J. Lutein and preterm infants with decreased concentrations of brain carotenoids. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Lipkie, T.E.; Morrow, A.L.; Jouni, Z.E.; McMahon, R.J.; Ferruzzi, M.G. Longitudinal survey of carotenoids in human milk from urban cohorts in China, Mexico, and the USA. PLoS ONE 2015, 10, e0127729. [Google Scholar] [CrossRef] [PubMed]
- Bettler, J.; Zimmer, J.P.; Neuringer, M.; DeRusso, P.A. Serum lutein concentrations in healthy term infants fed human milk or infant formula with lutein. Eur. J. Nutr. 2010, 49, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Longini, M.; Marzocchi, B.; Picardi, A.; Bellieni, C.V.; Proietti, F.; Rodriguez, A.; Turrisi, G.; Buonocore, G. Effects of lutein on oxidative stress in the term newborn: A pilot study. Neonatology 2010, 97, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Guardione, R.; Bonetti, P.; Priolo, C.; Maestri, A.; Mansoldo, C.; Mostert, M.; Anselmetti, G.; Sardei, D.; Bellettato, M.; et al. Lutein and zeaxanthin supplementation in preterm very low-birth-weight neonates in neonatal intensive care units: A multicenter randomized controlled trial. Am. J. Perinatol. 2013, 30, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Giannantonio, C.; Romagnoli, C.; Barone, G.; Gervasoni, J.; Perri, A.; Zecca, E. Lutein and zeaxanthin concentrations in formula and human milk samples from Italian mothers. Eur. J. Clin. Nutr. 2015, 69, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academy Press: Washington, WA, USA, 2000. [Google Scholar]
- Tanaka, H.; Mino, M.; Takeuchi, T. A nutritional evaluation of vitamin E status in very low birth weight infants with respect to changes in plasma and red blood cell tocopherol levels. J. Nutr. Sci. Vitaminol. (Tokyo) 1988, 34, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Cook-Mills, J.M. Isoforms of vitamin E differentially regulate PKC and inflammation: A review. J. Clin. Cell Immunol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Han, S.N.; Meydani, M.; Meydani, S.N. Effect of concomitant consumption of fish oil and vitamin E on T cell mediated function in the elderly: A randomized double-blind trial. J. Am. Coll. Nutr. 2006, 25, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Meydani, M.; Cohn, J.S.; Macauley, J.B.; McNamara, J.R.; Blumberg, J.B.; Schaefer, E.J. Postprandial changes in the plasma concentration of α- and γ-tocopherol in human subjects fed a fat-rich meal supplemented with fat-soluble vitamins. J. Nutr. 1989, 119, 1252–1258. [Google Scholar] [PubMed]
- Redlich, C.A.; Grauer, J.N.; van Bennekum, A.M.; Clever, S.L.; Ponn, R.B.; Blaner, W.S. Characterization of carotenoid, vitamin A, and α-tocopheral levels in human lung tissue and pulmonary macrophages. Am. J. Respir. Crit. Care Med. 1996, 154, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Uauy, R.; Hoffman, D.R.; Birch, E.E.; Birch, D.G.; Jameson, D.M.; Tyson, J. Safety and efficacy of omega-3 fatty acids in the nutrition of very low birth weight infants: Soy oil and marine oil supplementation of formula. J. Pediatr. 1994, 124, 612–620. [Google Scholar] [CrossRef]
- Martysiak-Zurowska, D.; Szlagatys-Sidorkiewicz, A.; Zagierski, M. Concentrations of α- and γ-tocopherols in human breast milk during the first months of lactation and in infant formulas. Matern. Child. Nutr. 2013, 9, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Elisia, I.; Kitts, D.D. Differences in vitamin E and C profile between infant formula and human milk and relative susceptibility to lipid oxidation. Int. J. Vitam. Nutr. Res. 2013, 83, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.F.; Hansen, N.I.; Brion, L.P.; Ehrenkranz, R.A.; Kennedy, K.A.; Walsh, M.C.; Shankaran, S.; Acarregui, M.J.; Johnson, K.J.; Hale, E.C.; et al. Serum tocopherol levels in very preterm infants after a single dose of vitamin E at birth. Pediatrics 2013, 132, e1626–e1633. [Google Scholar] [CrossRef] [PubMed]
- Cook-Mills, J.M.; Abdala-Valencia, H.; Hartert, T. Two faces of vitamin E in the lung. Am. J. Respir. Crit. Care Med. 2013, 188, 279–284. [Google Scholar] [CrossRef] [PubMed]
- McCary, C.A.; Yoon, Y.; Panagabko, C.; Cho, W.; Atkinson, J.; Cook-Mills, J.M. Vitamin E isoforms directly bind PKCα and differentially regulate activation of PKCα. Biochem. J. 2012, 441, 189–198. [Google Scholar] [CrossRef] [PubMed]
- McCary, C.A.; Abdala-Valencia, H.; Berdnikovs, S.; Cook-Mills, J.M. Supplemental and highly elevated tocopherol doses differentially regulate allergic inflammation: Reversibility of α-tocopherol and γ-tocopherol’s effects. J. Immunol. 2011, 186, 3674–3685. [Google Scholar] [CrossRef] [PubMed]
- Berdnikovs, S.; Abdala-Valencia, H.; McCary, C.; McCary, C.; Somand, M.; Cole, R.; Garcia, A.; Bryce, P.; Cook-Mills, J.M. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J. Immunol. 2009, 182, 4395–4405. [Google Scholar] [CrossRef] [PubMed]
- Abdala-Valencia, H.; Berdnikovs, S.; Cook-Mills, J.M. Vitamin E isoforms as modulators of lung inflammation. Nutrients. 2013, 5, 4347–4363. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, H.; Kanazawa, T.; Mori, R.; Hirano, S.; Ogihara, T.; Fujimura, M. Long-term α-tocopherol supplements may improve mental development in extremely low birthweight infants. Acta Paediatr. 2015, 104, e82–e89. [Google Scholar] [CrossRef] [PubMed]
- Quigley, M.; McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Shankaran, S.; Laptook, A.R.; Walsh, M.C.; Hale, E.C.; Newman, N.S.; Schibler, K.; Carlo, W.A.; et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics 2010, 126, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; Wright, L.L.; Wrage, L.A.; Poole, W.K. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006, 117, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Corpeleijn, W.E.; de Waard, M.; Christmann, V.; van Goudoever, J.B.; der Jansen-van Weide, M.C.; Kooi, E.M.; Koper, J.F.; Kouwenhoven, S.M.; Lafeber, H.N.; Mank, E.; et al. Effect of donor milk on severe infections and mortality in very low-birth-weight infants: The Early Nutrition Study randomized clinical trial. JAMA Pediatr. 2016, 170, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Verd, S.; Porta, R.; Botet, F.; Gutierrez, A.; Ginovart, G.; Barbero, A.H.; Ciurana, A.; Plata, I.I. Hospital outcomes of extremely low birth weight infants after introduction of donor milk to supplement mother’s milk. Breastfeed Med. 2015, 10, 150–155. [Google Scholar] [CrossRef] [PubMed]
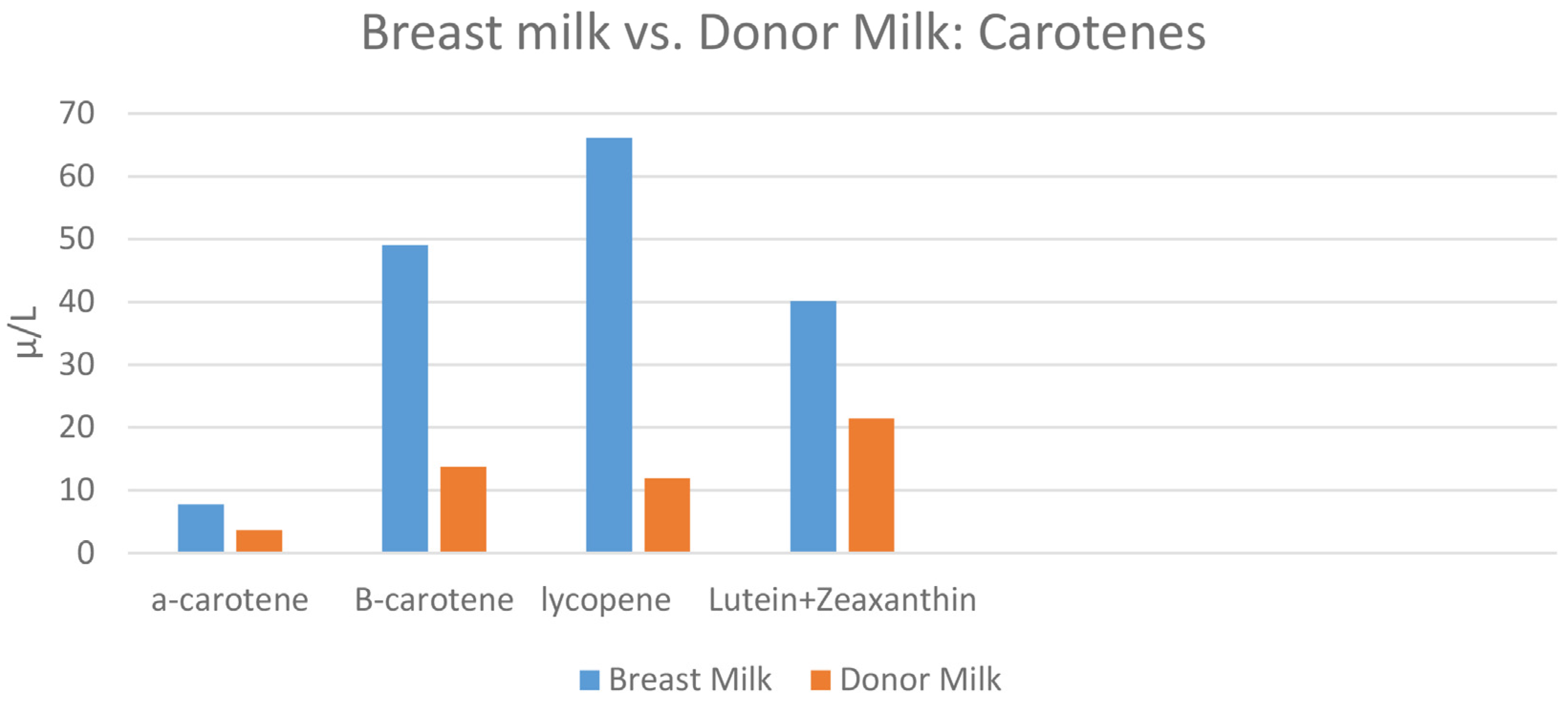
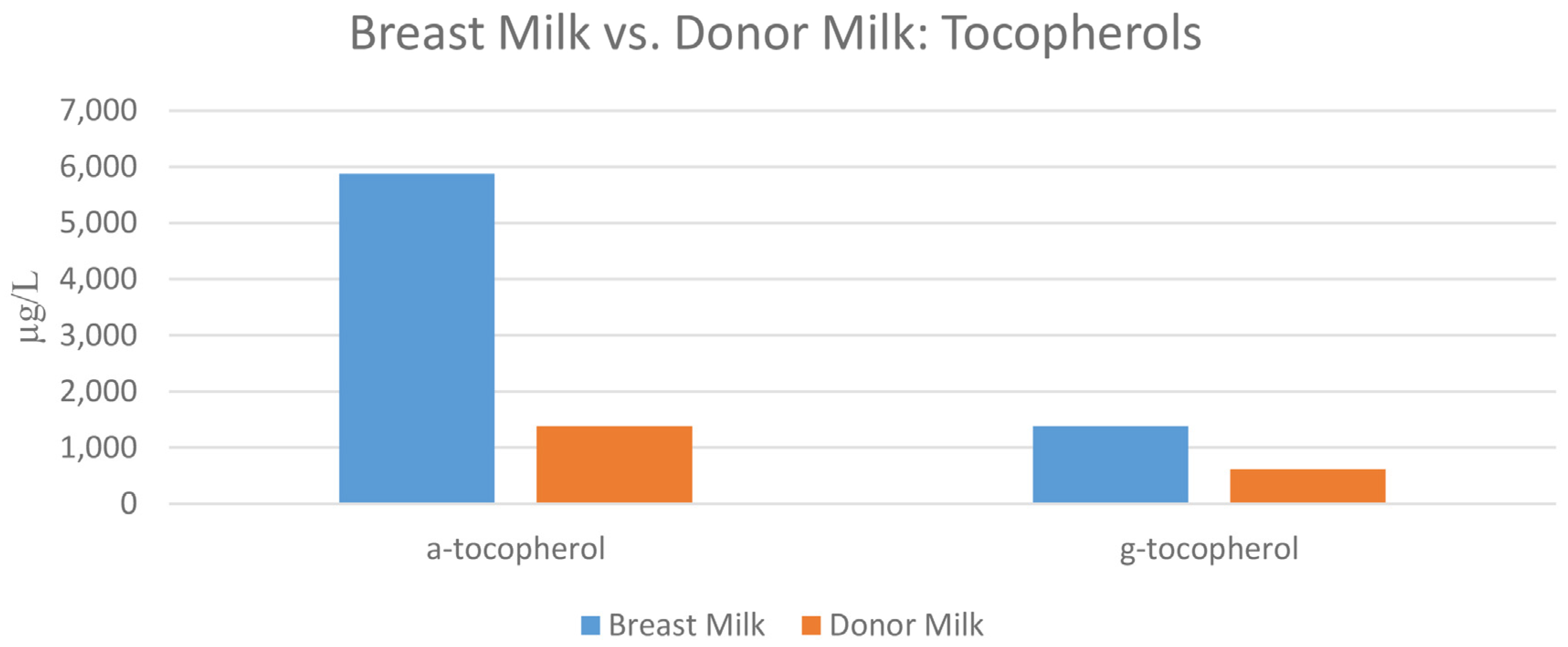
| Nutritional Antioxidant (µg/L) | Premature Formula | Transitional Formula | Term Standard Formula | Breast Milk Mean (SD) N = 12 | Donor Milk |
|---|---|---|---|---|---|
| α-carotene | 0.51 | 1.40 | 0.5 | 7.7 (14.5) | 3.6 |
| β-carotene | 71.1 | 63.9 | 25.0 | 49.1 (75.5) | 13.7 |
| β-cryptoxanthin | 0.9 | 0.9 | 0.48 | 21.7 (40.0) | 3.8 |
| Lycopene | 1.5 | 5.8 | 79.8 | 66.1 (55.9) | 11.9 |
| Lutein + zeaxanthin | 65.5 | 56.9 | 58.4 | 40.1 (42.5) | 21.4 |
| Retinol | 3086.2 | 911.8 | 571.2 | 401.6 (516.3) | 185.8 |
| α-tocopherol | 20,109.1 | 13,360.2 | 8520.0 | 5880.8 (4971.7) | 1381.9 |
| γ-tocopherol | 6787.1 | 6561.6 | 4204.0 | 1207.1 (668.4) | 622.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanson, C.; Lyden, E.; Furtado, J.; Van Ormer, M.; Anderson-Berry, A. A Comparison of Nutritional Antioxidant Content in Breast Milk, Donor Milk, and Infant Formulas. Nutrients 2016, 8, 681. https://doi.org/10.3390/nu8110681
Hanson C, Lyden E, Furtado J, Van Ormer M, Anderson-Berry A. A Comparison of Nutritional Antioxidant Content in Breast Milk, Donor Milk, and Infant Formulas. Nutrients. 2016; 8(11):681. https://doi.org/10.3390/nu8110681
Chicago/Turabian StyleHanson, Corrine, Elizabeth Lyden, Jeremy Furtado, Matthew Van Ormer, and Ann Anderson-Berry. 2016. "A Comparison of Nutritional Antioxidant Content in Breast Milk, Donor Milk, and Infant Formulas" Nutrients 8, no. 11: 681. https://doi.org/10.3390/nu8110681
APA StyleHanson, C., Lyden, E., Furtado, J., Van Ormer, M., & Anderson-Berry, A. (2016). A Comparison of Nutritional Antioxidant Content in Breast Milk, Donor Milk, and Infant Formulas. Nutrients, 8(11), 681. https://doi.org/10.3390/nu8110681





