The Role of Gangliosides in Neurodevelopment
Abstract
:1. Introduction
2. Structure of Gangliosides
3. Gangliosides in Human Brain
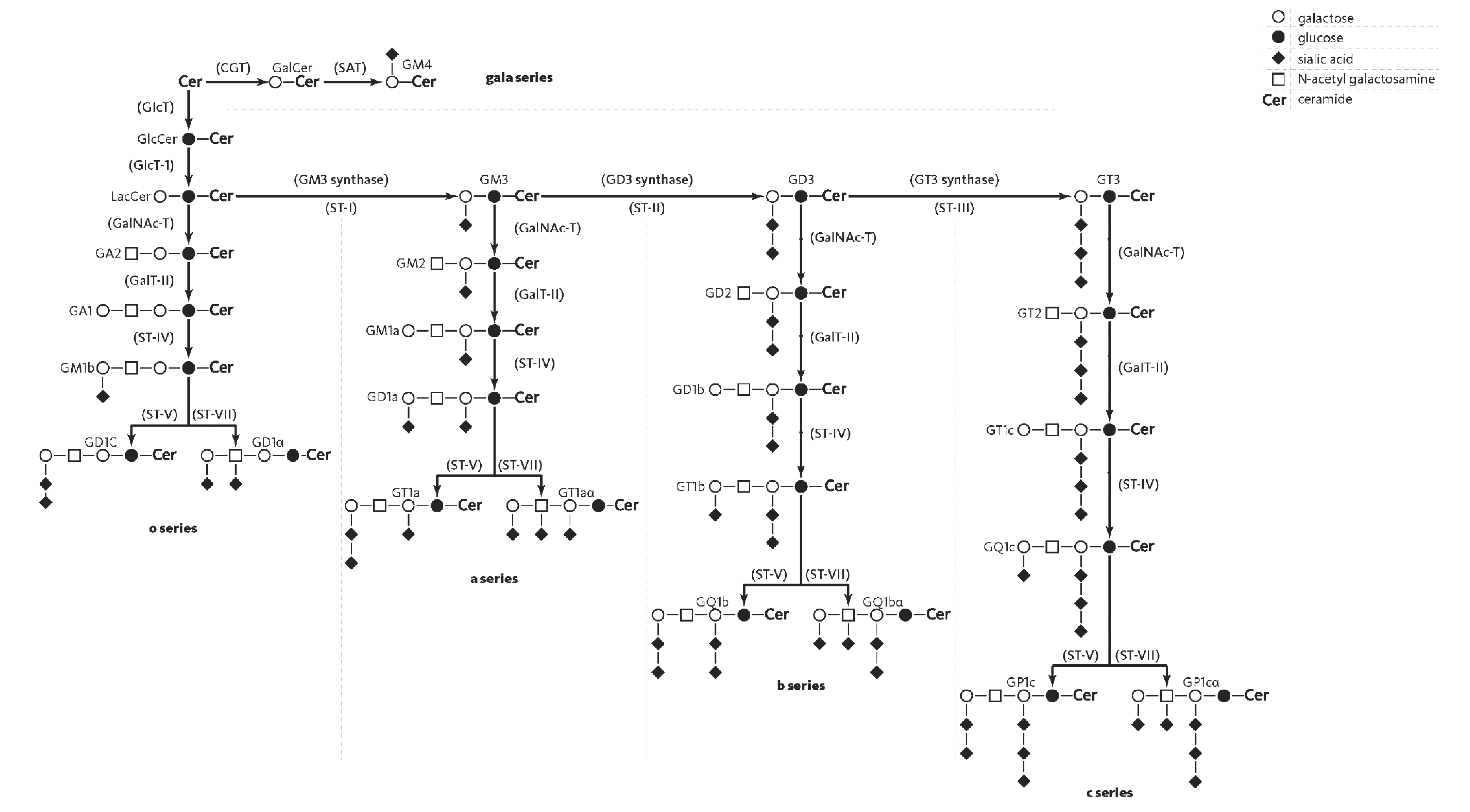
4. Role of Gangliosides in Neurodevelopment and Neuro-maintenance
5. Gangliosides in Neuroplasticity and Memory Formation
6. Influences of Diet on Brain Gangliosides
7. Dietary Gangliosides and Cognitive Functions
8. Mechanistic Studies of Dietary Ganglioside Effect on Cognitive Functions
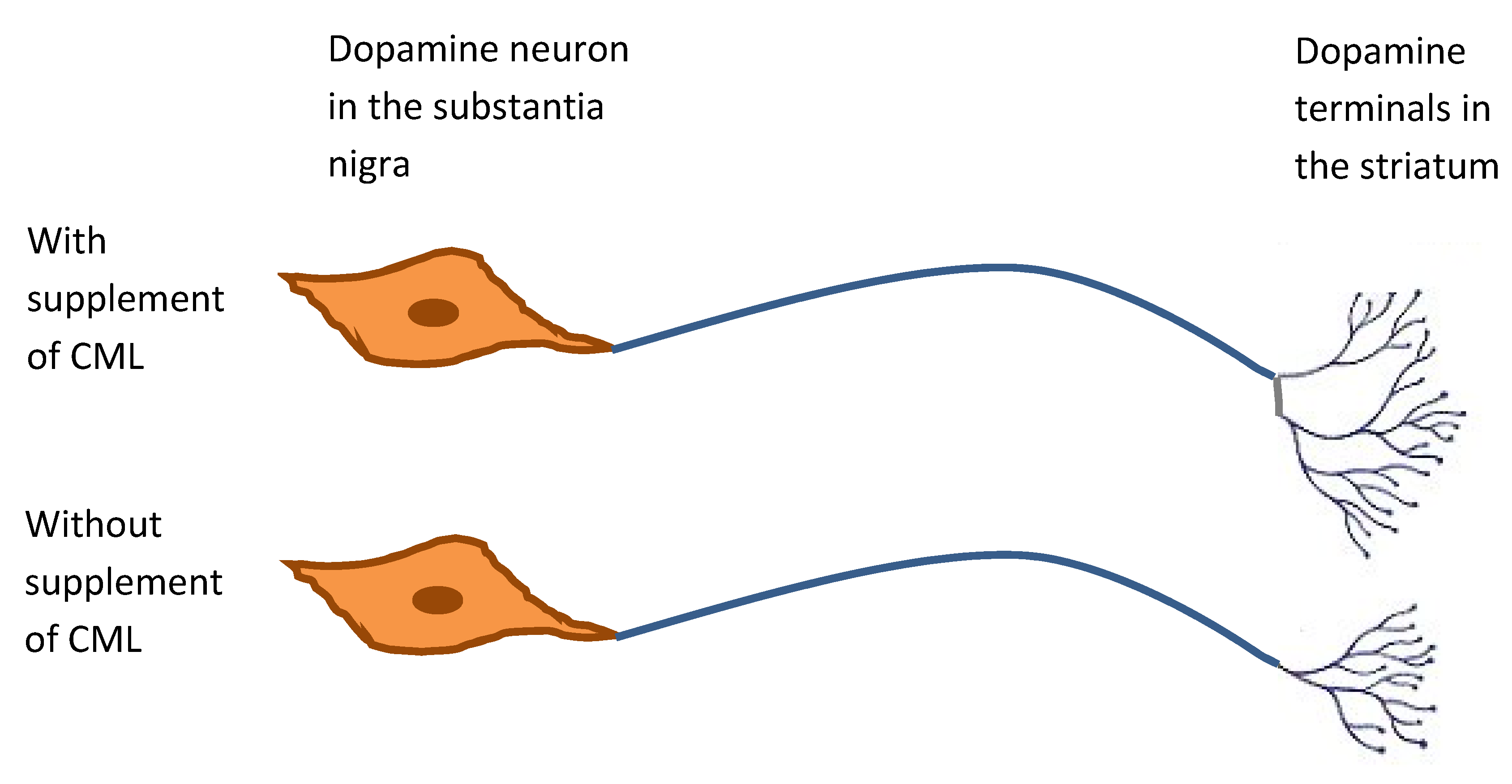
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lucki, N.C.; Sewer, M.B. Nuclear sphingolipid metabolism. Ann. Rev. Physiol. 2012, 74, 131–151. [Google Scholar] [CrossRef]
- Ledeen, R.W.; Yu, R.K. Gangliosides-structure, isolation, and analysis. Methods Enzymol. 1982, 83, 139–191. [Google Scholar] [PubMed]
- Kracun, I.; Rosner, H.; Drnovsek, V.; Vukelic, Z.; Cosovic, C.; Trbojeviccepe, M.; Kubat, M. Ganglioisides in the human brain-development and aging. Neurochem. Int. 1992, 20, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.K.; Nakatani, Y.; Yanagisawa, M. The role of glycosphingolipid metabolism in the developing brain. J. Lipid. Res. 2009, 50, S440–S445. [Google Scholar] [CrossRef] [PubMed]
- De Chaves, E.P.; Sipione, S. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett. 2010, 584, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Kolter, T. Ganglioside biochemistry. ISRN Biochem. 2012, 2012, 1–36. [Google Scholar] [CrossRef]
- Ledeen, R. Gangliosides of the neuron. Trends Neurosci. 1985, 8, 169–174. [Google Scholar] [CrossRef]
- Prinetti, A.; Loberto, N.; Chigorno, V.; Sonnino, S. Glycosphingolipid behaviour in complex membranes. Biochim. Biophy. Acta-Biomembranes 2009, 1788, 184–193. [Google Scholar]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic acid in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef]
- Lopez, P.H.H.; Schnaar, R.L. Gangliosides in cell recognition and membrane protein regulation. Curr. Opin. Struct. Biol. 2009, 19, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, J.-I. Membrane microdomains and insulin resistance. FEBS Lett. 2010, 584, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Nowycky, M.C.; Wu, G.; Ledeen, R.W. Glycobiology of ion transport in the nervous system. Adv. Neurobiol. 2014, 9, 321–342. [Google Scholar] [PubMed]
- Todeschini, A.R.; Hakomori, S.-I. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim. Biophys. Acta-Gen. Subj. 2008, 1780, 421–433. [Google Scholar] [CrossRef]
- Morgan, B.L.G. Nutritional requirements for normative development of the brain and behavior. Ann. NY. Acad. Sci. 1990, 602, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K. Nutrition and the developing brain: Nutrient priorities and measurement. Amer. J. Clin. Nutr. 2007, 85, 614S–620S. [Google Scholar] [PubMed]
- Uauy, R.; Mena, P.; Peirano, P. Mechanisms for nutrient effects on brain development and cognition. Nestle Nutr. Workshop Ser. Clin. Perform. Program. 2001, 5, 41–70. [Google Scholar]
- Chiavegatto, S.; Sun, J.; Nelson, R.J.; Schnaar, R.L. A functional role for complex gangliosides: Motor deficits in GM2/GD2 synthase knockout mice. Exp. Neurol. 2000, 166, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Kracun, I.; Rosner, H.; Drnovsek, V.; Heffer-Lauc, M.; Cosovic, C.; Lauc, G. Human brain gangliosides in development, aging and disease. Int. J. Devel. Biol. 1991, 35, 289–295. [Google Scholar]
- Wu, G.S.; Lu, Z.H.; Wang, J.F.; Wang, Y.; Xie, X.; Meyenhofer, M.F.; Ledeen, R.W. Enhanced susceptibility to kainate-induced seizures, neuronal apoptosis, and death in mice lacking gangliotetraose gangliosides: Protection with LIGA 20, a membrane-permeant analog of GM1. J. Neurosci. 2005, 25, 11014–11022. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.K.; Tsai, Y.-T.; Ariga, T. Functional roles of gangliosides in neurodevelopment: An overview of recent advances. Neurochem. Res. 2012, 37, 1230–1244. [Google Scholar] [CrossRef] [PubMed]
- Bruel-Jungerman, E.; Davis, S.; Laroche, S. Brain plasticity mechanisms and memory: A party of four. Neuroscientist 2007, 13, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.J.; Watts, R.J. Connecting vascular and nervous system development: Angiogenesis and the blood-brain barrier. Ann. Rev. Neurosci. 2010, 33, 379–408. [Google Scholar] [CrossRef] [PubMed]
- Kolb, B.; Gibb, R. Brain plasticity and behaviour in the developing brain. J. Can. Acad. Child. Adolesc. Psychiatry 2011, 20, 265–276. [Google Scholar] [PubMed]
- Thompson, R.A.; Nelson, C.A. Developmental science and the media: Early brain development. Am. Psychol. 2001, 56, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.; Dias, G.P.; Thuret, S. Effects of diet on brain plasticity in animal and human studies: Mind the gap. Neural Plast. 2014, 2014, 563160–563160. [Google Scholar] [PubMed]
- Schauer, R. Sialic acids: Fascinating sugars in higher animals and man. Zool. 2004, 107, 49–64. [Google Scholar] [CrossRef]
- Rosenber, A.; Stern, N. Changes in sphingosine and fatty acid components of gangliosides in developing rat and human brain. J. Lipid. Res. 1966, 7, 122–131. [Google Scholar]
- Svennerholm, L. Chromatographic separation of human brain gangliosides. J. Neurochem. 1963, 10, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.K.; Tsai, Y.T.; Ariga, T.; Yanagisawa, M. Structures, biosynthesis, and functions of gangliosides—An overview. J. Oleo. Sci. 2011, 60, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Otero, R.; Pimentel, P.M.; Ukrainstev, S.; McJarrow, P. Role of gangliosides in neurological developmen. In Nutrition in infancy; Waton, R.R., Grimble, G., Preedy, V.R., Zibadi, S., Eds.; Humana Press: New York, NY, USA, 2013; Volume 2, pp. 105–118. [Google Scholar]
- Ngamukote, S.; Yanagisawa, M.; Ariga, T.; Ando, S.; Yu, R.K. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J. Neurochem. 2007, 103, 2327–2341. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.K.; Macala, L.J.; Taki, T.; Weinfeld, H.M.; Yu, F.S. Developmental-changes in ganglioside composition and synthesis in embryonic rat-brain. J. Neurochem. 1988, 50, 1825–1829. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Chang, N.C.; Yu, R.K. High-performance thin-layer chromatography and densitometric determination of brain ganglioside compositions of several species. Anal. Biochem. 1978, 89, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Rahmann, H. Brain gangliosides and memory formation. Behav. Brain Res. 1995, 66, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, L.; Bostrom, K.; Fredman, P.; Mansson, J.E.; Rosengren, B.; Rynmark, B.M. Human brain gangliosides-developmental changes from early fetal stage to advanced age. Biochim. Biophys. Acta 1989, 1005, 109–117. [Google Scholar] [CrossRef]
- Kinney, H.C. Human myelination and perinatal white matter disorders. J. Neurolog. Sci. 2005, 228, 190–192. [Google Scholar] [CrossRef]
- Segler-Stahl, K.; Webster, J.C.; Brunngraber, E.G. Changes in the concentration and composition of human brain gangliosides with aging. Gerontology 1983, 29, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Sandhoff, K.; Kolter, T. Biosynthesis and degradation of mammalian glycosphingolipids. Philos Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, H.J.F. Glycosylation of glycolipids in the golgi complex. J. Neurochem. 2007, 103, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Proia, R.L. Gangliosides help stabilize the brain. Nat. Genet. 2004, 36, 1147–1148. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv. Nutr. 2012, 3, 465S–472S. [Google Scholar] [CrossRef] [PubMed]
- Rueda, R.; Gil, A. Role of gangliosides in infant nutrition. In Lipids in infant nutrition; Huang, Y.S., Sinclair, A.J., Eds.; AOAC Press: Champaign, IL, USA, 1998; pp. 213–234. [Google Scholar]
- Sandhoff, K.; Harzer, K. Gangliosides and gangliosidoses: Principles of molecular and metabolic pathogenesis. J. Neurosci. 2013, 33, 10195–10208. [Google Scholar] [CrossRef] [PubMed]
- Derry, D.M.; Wolfe, L.S. Gangliosides in isolated neurons and glial cells. Science 1967, 158, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Allende, M.L.; Proia, R.L. Simplifying complexity: Genetically resculpting glycosphingolipid synthesis pathways in mice to reveal function. Glycoconj. J. 2014, 31, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, A.; Wakade, C.; Yu, R.K. Ganglioside GD3 is required for neurogenesis and long-term maintenance of neural stem cells in the postnatal mouse brain. J. Neurosci. 2014, 34, 13790–13800. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Resende, V.T.; Gomes, T.A.; de Lima, S.; Nascimento-Lima, M.; Bargas-Rega, M.; Santiago, M.F.; de Melo Reis, R.A.; de Mello, F.G. Mice lacking GD3 synthase display morphological abnormalities in the sciatic nerve and neuronal disturbances during peripheral nerve regeneration. PLoS One 2014, 9, e108919. [Google Scholar] [CrossRef] [PubMed]
- Tajima, O.; Egashira, N.; Ohmi, Y.; Fukue, Y.; Mishima, K.; Iwasaki, K.; Fujiwara, M.; Inokuchi, J.; Sugiura, Y.; Furukawa, K.; et al. Reduced motor and sensory functions and emotional response in GM3-only mice: Emergence from early stage of life and exacerbation with aging. Behav. Brain Res. 2009, 198, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Tajima, O.; Egashira, N.; Ohmi, Y.; Fukue, Y.; Mishima, K.; Iwasaki, K.; Fujiwara, M.; Sugiura, Y.; Furukawa, K.; Furukawa, K. Dysfunction of muscarinic acetylcholine receptors as a substantial basis for progressive neurological deterioration in GM3-only mice. Behav. Brain Res. 2010, 206, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.A.; Cross, H.; Proukakis, C.; Priestman, D.A.; Neville, D.C.A.; Reinkensmeier, G.; Wang, H.; Wiznitzer, M.; Gurtz, K.; Verganelaki, A.; et al. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat. Genet. 2004, 36, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Utz, J.R.J.; Crutcher, T.; Schneider, J.; Sorgen, P.; Whitley, C.B. Biomarkers of central nervous system inflammation in infantile and juvenile gangliosidoses. Mol. Gen. Metab. 2015, 114, 274–280. [Google Scholar] [CrossRef]
- Bisel, B.; Pavone, F.S.; Calamai, M. GM1 and GM2 gangliosides: Recent developments. Biomol. Concepts 2014, 5, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Suh, M.; Thomson, B.; Thomson, A.B.R.; Ramanujam, K.S.; Clandinin, M.T. Dietary ganglioside decreases cholesterol content, caveolin expression and inflammatory mediators in rat intestinal microdomains. Glycobiology 2005, 15, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Rueda, R.; Sabatel, J.L.; Maldonaldo, J.; Molina-Font, J.A.; Gil, A. Addition of gangliosides to an adapted milk formula modifies levels of fecal escherichia coli in preterm newborn infants. J. Pediatr. 1998, 133, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Gollomp, S.M.; Sendek, S.; Colcher, A.; Cambi, F.; Du, W. A randomized, controlled, delayed start trial of GM1 ganglioside in treated parkinson's disease patients. J. Neurol. Sci. 2013, 324, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Argentino, C.; Sacchetti, M.L.; Toni, D. GM1 ganglioside therapy in acute ischemic stroke-response. Stroke 1990, 21, 825–825. [Google Scholar] [CrossRef] [PubMed]
- Geisler, F.H.; Coleman, W.P.; Grieco, G.; Poonian, D.; Sygen Study, G. The sygen® multicenter acute spinal cord injury study. Spine 2001, 26, S87–S98. [Google Scholar] [CrossRef] [PubMed]
- Roisen, F.J.; Bartfeld, H.; Nagele, R.; Yorke, G. Ganglioside stimulation of axonal sprouting in vitro. Science 1981, 214, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.C.; Ledeen, R.W.; Roisen, F.J.; Yorke, G.; Sclafani, J.R. Ganglioside-induced neuritogenesis: Verification that gangliosides are the active agents, and comparison of molecular species. J. Neurochem. 1983, 41, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Arita, M.; Nagai, Y. GQ1b, a bioactive ganglioside that exhibits novel nerve growth factor (NGF)-like activities in the two neuroblastoma cell lines. J. Biochem. 1983, 94, 303–306. [Google Scholar] [PubMed]
- Gorio, A. Ganglioside enhancement of neuronal differentation, plasticity, and repair. CRC Crit. Rev. Clin. Neurobiol. 1986, 2, 241–296. [Google Scholar] [PubMed]
- Ferreira, A.; Busciglio, J.; Landa, C.; Caceres, A. Ganglioside-enhanced neurite growth: Evidence for a selective induction of high-molecular-weight MAP-2. J. Neurosci. 1990, 10, 293–302. [Google Scholar] [PubMed]
- Doherty, P.; Ashton, S.V.; Skaper, S.D.; Leon, A.; Walsh, F.S. Ganglioside modulation of neural cell-adhesion molecule and N-cadherin-dependent neurite outgrowth. J. Cell Biol. 1992, 117, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.; Lu, Z.H.; Ledeen, R.W. Induction of axon-like and dendrite-like processes in neuroblastoma cells. J. Neurocytol. 1998, 27, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, N.; Iwabuchi, K.; Kurihara, H.; Ishii, K.; Kobayashi, T.; Sasaki, T.; Hattori, N.; Mizuno, Y.; Hozumi, K.; Yamada, Y.; et al. Binding of laminin-1 to monosialoganglioside GM1 in lipid rafts is crucial for neurite outgrowth. J. Cell Sci. 2009, 122, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, R.K. Interaction of ganglioside GD3 with an EGF receptor sustains the self-renewal ability of mouse neural stem cells in vitro. Proc. Nat. Acad. Sci. 2013, 110, 19137–19142. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Otero, R.; Santiago, M.F. Functional role of a specific ganglioside in neuronal migration and neurite outgrowth. Brazil. J. Med. Biol. Res. 2003, 36, 1003–1013. [Google Scholar]
- Santiago, M.F.; Liour, S.S.; Mendez-Otero, R.; Yu, R.K. Glial-guided neuronal migration in P19 embryonal carcinoma stem cell aggregates. J. Neurosci. Res. 2005, 81, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, M.; Blagojevic, T.; Vukosavic, S.; Zvezdina, N.; Pekovic, S.; Nikezic, G.; Rakic, L. Ganglioside GM1 and GM3 in early brain development: An immunocytochemical study. Int. J. Dev. Neurosci. 1996, 14, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Mochetti. Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins. Cell Mol. Life Sci. 2005, 62, 2283–2294.
- Bieberich, E.; MacKinnon, S.; Silva, J.; Yu, R.K. Regulation of apoptosis during neuronal differentiation by ceramide and b-series complex gangliosides. J. Biol. Chem. 2001, 276, 44396–44404. [Google Scholar] [CrossRef]
- Nakatsuji, Y.; Miller, R.H. Selective cell-cycle arrest and induction of apoptosis in proliferating neural cells by ganglioside GM3. Exp. Neurol. 2001, 168, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Ohmi, Y.; Tajima, O.; Ohkawa, Y.; Furukawa, K.; Furukawa, K. Gangliosides play pivotal roles in the regulation of complement systems and in the maintenance of integrity in nerve tissues. J. Neurochem. 2010, 113, 21. [Google Scholar]
- Ohmi, Y.; Ohkawa, Y.; Yamauchi, Y.; Tajima, O.; Furukawa, K.; Furukawa, K. Essential roles of gangliosides in the formation and maintenance of membrane microdomains in brain tissues. Neurochem. Res. 2012, 37, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Ohmi, Y.; Tajima, O.; Ohkawa, Y.; Yamauchi, Y.; Sugiura, Y.; Furukawa, K.; Furukawa, K. Gangliosides are essential in the protection of inflammation and neurodegeneration via maintenance of lipid rafts: Elucidation by a series of ganglioside-deficient mutant mice. J. Neurochem. 2011, 116, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.S.; Zeller, C.B.; Shaper, N.L.; Kiso, M.; Hasegawa, A.; Shapiro, R.E.; Schnaar, R.L. Gangliosides are neuronal ligands for myelin-associated glycoprotein. Proc. Nat. Acad. Sci. 1996, 93, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, K.A.; Sun, J.; Liu, Y.J.; Kawai, H.; Crawford, T.O.; Proia, R.L.; Griffin, J.W.; Schnaar, R.L. Mice lacking complex gangliosides develop wallerian degeneration and myelination defects. Proc. Nat. Acad. Sci. 1999, 96, 7532–7537. [Google Scholar] [CrossRef] [PubMed]
- Vajn, K.; Viljetic, B.; Degmecic, I.V.; Schnaar, R.L.; Heffer, M. Differential distribution of major brain gangliosides in the adult mouse central nervous system. PLoS One 2013, 8, e75720. [Google Scholar] [CrossRef] [PubMed]
- Ledeen, R.W.; Wu, G.S. Ganglioside function in calcium homeostasis and signaling. Neurochem. Res. 2002, 27, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Ledeen, R.W.; Wu, G. Nuclear sphingolipids: Metabolism and signaling. J. Lipid Res. 2008, 49, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Waki, H.; Kon, K.; Ando, S. Gangliosides enhance KCl-induced Ca2+ influx and acetylcholine release in brain synaptosomes. Neuroreport 1997, 8, 2203–2207. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, A.; Kuroda, Y.; Muramoto, K.; Kobayashi, K.; Yamagishi, K.; Inokuchi, J. Effects of glucosylceramide synthase inhibitor and ganglioside GQ1b on synchronous oscillations of intracellular Ca2+ in cultured cortical neurons. Biochem. Biophys. Res. Comm. 1996, 222, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.F.; Bliss, T.V.P. Plasticity in the human central nervous system. Brain 2006, 129, 1659–1673. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, M.; Fujii, H.; Kim, R.; Kawashima, T.; Okuno, H.; Bito, H. Untangling the two-way signalling route from synapses to the nucleus, and from the nucleus back to the synapses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369. [Google Scholar] [CrossRef] [PubMed]
- Kesner, R.P. Behavioral functions of the CA3 subregion of the hippocampus. Learn. Mem. 2007, 14, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Egorushkina, N.V.; Ratushnyak, A.S.; Egorushkin, I.V. The influence of exogenous gangliosides on the dynamics of the development of prolonged posttetanic potentiation. Neurosci. Behav. Phys. 1993, 23, 435–438. [Google Scholar] [CrossRef]
- Bliss, T.V.P.; Collingridge, G.L. A synaptic model of memory-long term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Igarashi, K.; Sasaki, H.; Furuse, H.; Ito, K.; Kaneko, K.; Kato, H.; Inokuchi, J.; Waki, H.; Ando, S. Effects of the mono- and tetrasialogangliosides GM1 and GQ1b on ATP-induced long-term potentiation in hippocampal CA1 neurons. Glycobiology 2002, 12, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, K.; Fujiwara, H.; Yamazaki, Y.; Goto, J.-I.; Kaneko, K.; Kato, H.; Fujii, S.; Sasaki, H.; Fukumoto, S.; Furukawa, K.; et al. Impaired hippocampal long-term potentiation and failure of learning in beta 1,4-N-acetylgalactosaminyltransferase gene transgenic mice. Glycobiology 2011, 21, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Ikarashi, K.; Yamazaki, Y.; Goto, J.-I.; Kaneko, K.; Sugita, M.; Kato, H.; Sasaki, H.; Inokuchi, J.-I.; Furukawa, K.; et al. Impairment of hippocampal long-term potentiation and failure of learning in mice treated with d-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol. Biomed. Res. 2012, 33, 265–271. [Google Scholar] [CrossRef] [PubMed]
- She, J.Q.; Wang, M.; Zhu, D.M.; Sun, L.G.; Ruan, D.Y. Effect of ganglioside on synaptic plasticity of hippocampus in lead-exposed rats in vivo. Brain Res. 2005, 1060, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.J.; Hogan, E.L. Brain gangliosides: Increased incorporation of (1–3 h) glucosamine during training. Pharmacol. Biochem. Behav. 1975, 3, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Irwin, L.N.; Samson, F.E., Jr. Content and turnover of gangliosides in rat brain following behavioural stimulation. J. Neurochem. 1971, 18, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Savaki, H.E.; Levis, G.M. Changes in rat brain gangliosides following active avoidance conditioning. Pharmacol. Biochem. Behav. 1977, 7, 7–12. [Google Scholar] [CrossRef] [PubMed]
- McJarrow, P.; Schnell, N.; Jumpsen, J.; Clandinin, T. Influence of dietary gangliosides on neonatal brain development. Nutr. Rev. 2009, 67, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, P.E.; Lomanowska, A.M.; McCutcheon, D.; Park, E.J.; Clandinin, M.T.; Ramanujam, K.S. Postnatal dietary supplementation with either gangliosides or choline: Effects on spatial short-term memory in artificially-reared rats. Nutr. Neurosci. 2007, 10, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.R.; Kim, H.G.; Kim, K.L. Ganglioside GQ1b improves spatial learning and memory of rats as measured by the y-maze and the morris water maze tests. Neurosci. Lett. 2008, 439, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.T.; Zheng, J.Z. Effects of exogenous gangliosides on learning and memory in rats. Jpn. J. Physiol. 1993, 43, S295–S299. [Google Scholar] [PubMed]
- Yang, R.; Wang, Q.; Min, L.; Sui, R.; Li, J.; Liu, X. Monosialoanglioside improves memory deficits and relieves oxidative stress in the hippocampus of rat model of alzheimer’s disease. Neurolog. Sci. 2013, 34, 1447–1451. [Google Scholar] [CrossRef]
- Karpiak, S.; Graf, L.; Rapport, M. Passive avoidance learning is inhibited by antiserum to brain ganglioside. Neurosci. Abstr. 1976, 2, 443. [Google Scholar]
- Karpiak, S.E.; Rapport, M.M. Inhibition of consolidation and retrieval stages of passive-avoidance learning by antibodies to gangliosides. Behav. Neural. Biol. 1979, 27, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Elias, M.F.; Dore, G.A.; Robbins, M.A. Relation between dairy food intake and cognitive function: The maine-syracuse longitudinal study. Int. Dairy J. 2012, 22, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Murphy, K.J.; Howe, P.R.C.; Buckley, J.D.; Bryan, J. Dairy consumption and working memory performance in overweight and obese adults. Appetite 2012, 59, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Landon, J.; Davison, M.; Krageloh, C.U.; Thompson, N.M.; Miles, J.L.; Vickers, N.H.; Fraser, M.; Breier, B.H. Global undernutrition during gestation influences learning during adult fife. Learn. Behav. 2007, 35, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.L.; Winick, M. Effects of administration of N-acetylneuraminic acid (NANA) on brain nana content and behavior. J. Nutr. 1980, 110, 416–424. [Google Scholar] [PubMed]
- Morgan, B.L.; Winick, M. The subcellular localization of administered N-acetylneuraminic acid in the brains of well-fed and protein restricted rats. Brit. J. Nutr. 1981, 46, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.L.; Winick, M. Effects of environmental stimulation on brain N-acetylneuraminic acid content and behavior. J. Nutr. 1980, 110, 425–432. [Google Scholar]
- Morgan, B.L.; Naismith, D.J. The effect of early postnatal undernutrition on the growth and development of the rat brain. Brit. J. Nutr. 1982, 48, 15–23. [Google Scholar] [CrossRef]
- Morgan, B.L.; Oppenheimer, J.; Winick, M. Effects of essential fatty acid deficiency during late gestation on brain N-acetylneuraminic acid metabolism and behaviour in the progeny. Brit. J. Nutr. 1981, 46, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Vaswani, K.K.; Sharma, M. Effect of neonatal undernutrition on rat brain gangliosides. Int. J. Vit. Nutr. Res. 1985, 55, 323–329. [Google Scholar]
- Wang, B.; McVeagh, P.; Petocz, P.; Brand-Miller, J. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. Am. J. Clin. Nutr. 2003, 78, 1024–1029. [Google Scholar] [PubMed]
- Lacomba, R.; Salcedo, J.; Alegria, A.; Barbera, R.; Hueso, P.; Matencio, E.; Jesus Lagarda, M. Sialic acid (N-acetyl and N-glycolylneuraminic acid) and ganglioside in whey protein concentrates and infant formulae. Int. Dairy J. 2011, 21, 887–895. [Google Scholar] [CrossRef]
- Rueda, R. Gangliosides, immunity, infection and inflammation. In Diet, Immunity and Inflammation; Calder, P.C., Yaqoob, P., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2013; pp. 341–354. [Google Scholar]
- Zhang, J.; Ren, Y.; Huang, B.; Tao, B.; Pedersen, M.R.; Li, D. Determination of disialoganglioside GD3 and monosialoganglioside GM3 in infant formulas and whey protein concentrates by ultra-performance liquid chromatography/electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2012, 35, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sosa, S.; Martin, M.J.; Castro, M.D.; Cabezas, J.A.; Hueso, P. Lactational changes in the fatty acid composition of human milk gangliosides. Lipids 2004, 39, 111–116. [Google Scholar] [PubMed]
- Pan, X.L.; Izumi, T. Variation of the ganglioside compositions of human milk, cow’s milk and infant formulas. Early Human Dev. 2000, 57, 25–31. [Google Scholar] [CrossRef]
- Laegreid, A.; Otnaess, A.B.K.; Fuglesang, J. Human and bovine-milk: Comparison of ganglioside composition and enterotoxin-inhibitory activity. Pediatr. Res. 1986, 20, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Takamizawa, K.; Iwamori, M.; Mutai, M.; Nagai, Y. Selective changes in gangliosides of human milk during lactation: A molecular indicator for the period of lactation. Biochim. Biophys. Acta 1986, 879, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.-i.; Sekiguchi, K.; Akaishi, M.; Anan, A.; Maeda, T.; Izumi, T. Characterization and chronological changes of preterm human milk gangliosides. Nutrition 2011, 27, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, F.; Elmelegy, I.M.; Thakkar, S.K.; Marmet, C.; Destaillats, F. Longitudinal evolution of the conentration of gangliosides GM3 and GD3 in human milk. Lipids 2014, 49, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Rueda, R.; Puente, R.; Hueso, P.; Maldonado, J.; Gil, A. New data on content and distribution of gangliosides in human milk. Bioli. Chemi. 1995, 376, 723–727. [Google Scholar]
- Park, E.J.; Suh, M.; Clandinin, M.T. Dietary ganglioside and long-chain polyunsaturated fatty acids increase ganglioside GD3 content and alter the phospholipid profile in neonatal rat retina. Invest. Ophthalmol. Vis. Sci. 2005, 46, 2571–2575. [Google Scholar] [CrossRef] [PubMed]
- Rueda, R.; Garcia Salmeron, J.L.; Maldonado, J.; Gil, A. Changes during lactation in ganglioside distribution in human milk from mothers delivering preterm and term infants. Bioli. Chemi. 1996, 377, 599–601. [Google Scholar]
- Puente, R.; Garciapardo, L.A.; Hueso, P. Gangliosides in bovine-milk-changes in content and distribution of individual ganglioside levels during lactation. Bioli. Chemi. 1992, 373, 283–288. [Google Scholar]
- Anderson, J.W.; Johnstone, B.M.; Remley, D.T. Breast-feeding and cognitive development: A meta-analysis. Am. J. Clin. Nutr. 1999, 70, 525–535. [Google Scholar] [PubMed]
- Horwood, L.J.; Darlow, B.A.; Mogridge, N. Breast milk feeding and cognitive ability at 7–8 years. Arch. Dis. Child. Fetal Neonatal Ed. 2001, 84, F23–F27. [Google Scholar] [CrossRef] [PubMed]
- Syahrir, L.; Fadlyana, E.; Effendi, S.H. Comparison of language and visual-motor developments between exclusively and non-exclusively breastfed infants through cognitive adaptive test/clinical linguistic and auditory milestone scale. Paediatr. Indones. 2009, 49, 337–341. [Google Scholar]
- Lucas, A.; Morley, R.; Cole, T.J. Randomised trial of early diet in preterm babies and later intelligence quotient. Brit. Med. J. 1998, 317, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, E.B.; Fischl, B.R.; Quinn, B.T.; Chong, W.K.; Gadian, D.G.; Lucas, A. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr. Res. 2010, 67, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Willatts, P. Long chain polyunsaturated fatty acids improve cognitive development. J. Fam. Health Care 2002, 12, 5. [Google Scholar] [PubMed]
- Helland, I.B.; Smith, L.; Saarem, K.; Saugstad, O.D.; Drevon, C.A. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics 2003, 111, e39–e44. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Jorgensen, M.H.; Olsen, S.F.; Straarup, E.M.; Michaelsen, K.F. Maternal fish oil supplementation in lactation: Effect on developmental outcome in breast-fed infants. Reprod. Nutr. Devel. 2005, 45, 535–547. [Google Scholar] [CrossRef]
- Park, E.J.; Suh, M.; Ramanujam, K.; Steiner, K.; Begg, D.; Clandinin, T.T. Diet-induced changes in membrane gangliosides in rat intestinal mucosa, plasma and brain. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Hungund, B.L.; Morishima, H.O.; Gokhale, V.S.; Cooper, T.B. Placental-transfer of (H-3)-GM1 and its distribution to maternal and fetal tissues of the rat. Life Sci. 1993, 53, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.B.; Clandinin, T. Dietary ganglioside and neurochemistry in the developing rat. Glycobiology 2006, 16, 1148–1149. [Google Scholar]
- Mitchell, M.D.; Henare, K.; Balakrishnan, B.; Lowe, E.; Fong, B.Y.; McJarrow, P. Transfer of gangliosides across the human placenta. Placenta 2012, 33, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H.; Guan, J.; Gustavsson, M.; Krageloh, C.U.; Breier, B.H.; Davison, M.; Fong, B.; Norris, C.; McJarrow, P.; Hodgkinson, S.C. Supplementation with a mixture of complex lipids derived from milk to growing rats results in improvements in parameters related to growth and cognition. Nutr. Res. 2009, 29, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, M.; Hodgkinson, S.C.; Fong, B.; Norris, C.; Guan, J.A.; Krageloh, C.U.; Breier, B.H.; Davison, M.; McJarrow, P.; Vickers, M.H. Maternal supplementation with a complex milk lipid mixture during pregnancy and lactation alters neonatal brain lipid composition but lacks effect on cognitive function in rats. Nutr. Res. 2010, 30, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Salvati, S.; Attorri, L.; Avellino, C.; Di Biase, A.; Sanchez, M. Diet, lipids and brain development. Devel. Neurosci. 2000, 22, 481–487. [Google Scholar] [CrossRef]
- Dobbing, J.; Sands, J. Comparative aspects of the brain growth spurt. Early Human Devel. 1979, 3, 79–83. [Google Scholar] [CrossRef]
- Liu, H.; Radlowski, E.C.; Conrad, M.S.; Li, Y.; Dilger, R.N.; Johnson, R.W. Early supplementation of phospholipids and gangliosides affects brain and cognitive development in neonatal piglets. J. Nutr. 2014, 144, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Z.; Zhu, T.-C. Effect of ganglioside in repairing the neurological function of chinese children with cerebral palsy: Analysis of the curative efficacy in 2230 cases. Chin. J. Clin. Rehab. 2005, 9, 122–123. [Google Scholar]
- Gurnida, D.A.; Rowan, A.M.; Idjradinata, P.; Muchtadi, D.; Sekarwana, N. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Human Devel. 2012, 88, 595–601. [Google Scholar] [CrossRef]
- Bouhours, D.; Bouhours, J.F. Developmental changes of hematoside of rat small intestine. Postnatal hydroxylation of fatty acids and sialic acid. J. Biol. Chem. 1983, 258, 299–304. [Google Scholar] [PubMed]
- Holgersson, J.; Stromberg, N.; Breimer, M.E. Glycolipids of human large-intestine: Difference in glycolipid expression related to anatomical localization, epithelial non-epithelial tissue and the ABO, LE and SE phenotypes of the donors. Biochimie 1988, 70, 1565–1574. [Google Scholar] [CrossRef]
- Wu, C.; Bai, L.; Wang, W. Neurobehavior effect of GM-1 on LBW infants. Hei Long Jiang Med. J. 2010, 34, 410–412. [Google Scholar]
- Hungund, B.L.; Ross, D.C.; Gokhale, V.S. Ganglioside GM1 reduces fetal alcohol effects in rat pups exposed to ethanol in utero. Alcohol. Clin. Exp. Res. 1994, 18, 1248–1251. [Google Scholar] [CrossRef]
- Laev, H.; Karpiak, S.E.; Gokhale, V.S.; Hungund, B.L. In-utero ethanol exposure retards growth and alters morphology of cortical cultures: GM1 reverses effects. Alcohol. Clin. Exp. Res. 1995, 19, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Mao, R.F.; Wang, R.; Vadasz, C.; Saito, M. Effects of gangliosides on ethanol-induced neurodegeneration in the developing mouse brain. Alcohol. Clin. Exp. Res. 2007, 31, 665–674. [Google Scholar] [PubMed]
- Svennerholm, L.; Brane, G.; Karlsson, I.; Lekman, A.; Ramstrom, I.; Wikkelso, C. Alzheimer disease: Effect of continuous intracerebroventricular treatment with GM1 ganglioside and a systematic activation programme. Dement. Geriatr. Cogn. Disord. 2002, 14, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Guillermo, R.B.; Yang, P.; Vickers, M.H.; McJarrow, P.; Guan, J. Supplementation with complex milk lipids during brain development promotes neuroplasticity without altering myelination or vascular density. Food Nutr. Res. 2015, 59, 25765. [Google Scholar] [CrossRef] [PubMed]
- Agnati, L.F.; Fuxe, K.; Calza, L.; Benfenati, F.; Cavicchioli, L.; Toffano, G.; Goldstein, M. Gangliosides increase the survival of lesioned nigral dopamine neurons and favour the recovery of dopaminergic synaptic function in striatum of rats by collateral sprouting. Acta Physiol. Scand. 1983, 119, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.G.; Neff, N.H.; Hadjiconstantinou, M. GM1 ganglioside improves spatial learning and memory of aged rats. Behav. Brain Res. 1997, 85, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.H.; Felicio, L.F.; Nasello, A.G.; Vital, M.; Frussa, R. Effect of ganglioside (GM1) on memory in senescent rats. Neurobiol. Aging 1996, 17, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; MacGibbon, A.; Zhang, R.; Elliffe, D.M.; Moon, S.; Liu, D.-X. Supplementation of complex milk lipid concentrate (CMLc) improved the memory of aged rats. Nutr. Neurosci. 2015, 18, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Glutamate and neurotrophic factors in neuronal plasticity and disease. In Neural signaling: Opportunities for novel diagnostic approaches and therapies; Goetzl, E.J., Ed.; Wiley-Blackwell: Boston, MA, USA, 2008; Volume 1144, pp. 97–112. [Google Scholar]
- Backman, L.; Lindenberger, U.; Li, S.-C.; Nyberg, L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: Recent data and future avenues. Neurosci. Biobehav. Rev. 2010, 34, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Kuo, M.-F.; Grosch, J.; Bergner, C.; Monte-Silva, K.; Paulus, W. d-1-receptor impact on neuroplasticity in humans. J. Neurosci. 2009, 29, 2648–2653. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.M.; Rice, G.E.; Mitchell, M.D. The role of gangliosides in brain development and the potential benefits of perinatal supplementation. Nutr. Res. 2013, 33, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Poppitt, S.D.; McGregor, R.A.; Wiessing, K.R.; Goyal, V.K.; Chitkara, A.J.; Gupta, S.; Palmano, K.; Kuhn-Sherlock, B.; McConnell, M.A. Bovine complex milk lipid containing gangliosides for prevention of rotavirus infection and diarrhoea in northern indian infants. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 167–171. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmano, K.; Rowan, A.; Guillermo, R.; Guan, J.; McJarrow, P. The Role of Gangliosides in Neurodevelopment. Nutrients 2015, 7, 3891-3913. https://doi.org/10.3390/nu7053891
Palmano K, Rowan A, Guillermo R, Guan J, McJarrow P. The Role of Gangliosides in Neurodevelopment. Nutrients. 2015; 7(5):3891-3913. https://doi.org/10.3390/nu7053891
Chicago/Turabian StylePalmano, Kate, Angela Rowan, Rozey Guillermo, Jian Guan, and Paul McJarrow. 2015. "The Role of Gangliosides in Neurodevelopment" Nutrients 7, no. 5: 3891-3913. https://doi.org/10.3390/nu7053891




