The Effects of Reduced Gluten Barley Diet on Humoral and Cell-Mediated Systemic Immune Responses of Gluten-Sensitive Rhesus Macaques
Abstract
:1. Introduction
2. Experimental Section
2.1. Ethics Approval
2.2. Pre-Screening and Selection of Rhesus Macaques for the Study
2.3. Diets Used
2.4. Dietary Time Periods and Samples Collected
2.5. Histopathological Evaluation, AGA, TG2 and Anti-Hordein Antibody (AHA) Serum Responses
2.6. Fluorescent-Activated Cell Sorting (FACS) of Cytokine-Producing PBMCs
2.7. Statistical Analysis
3. Results
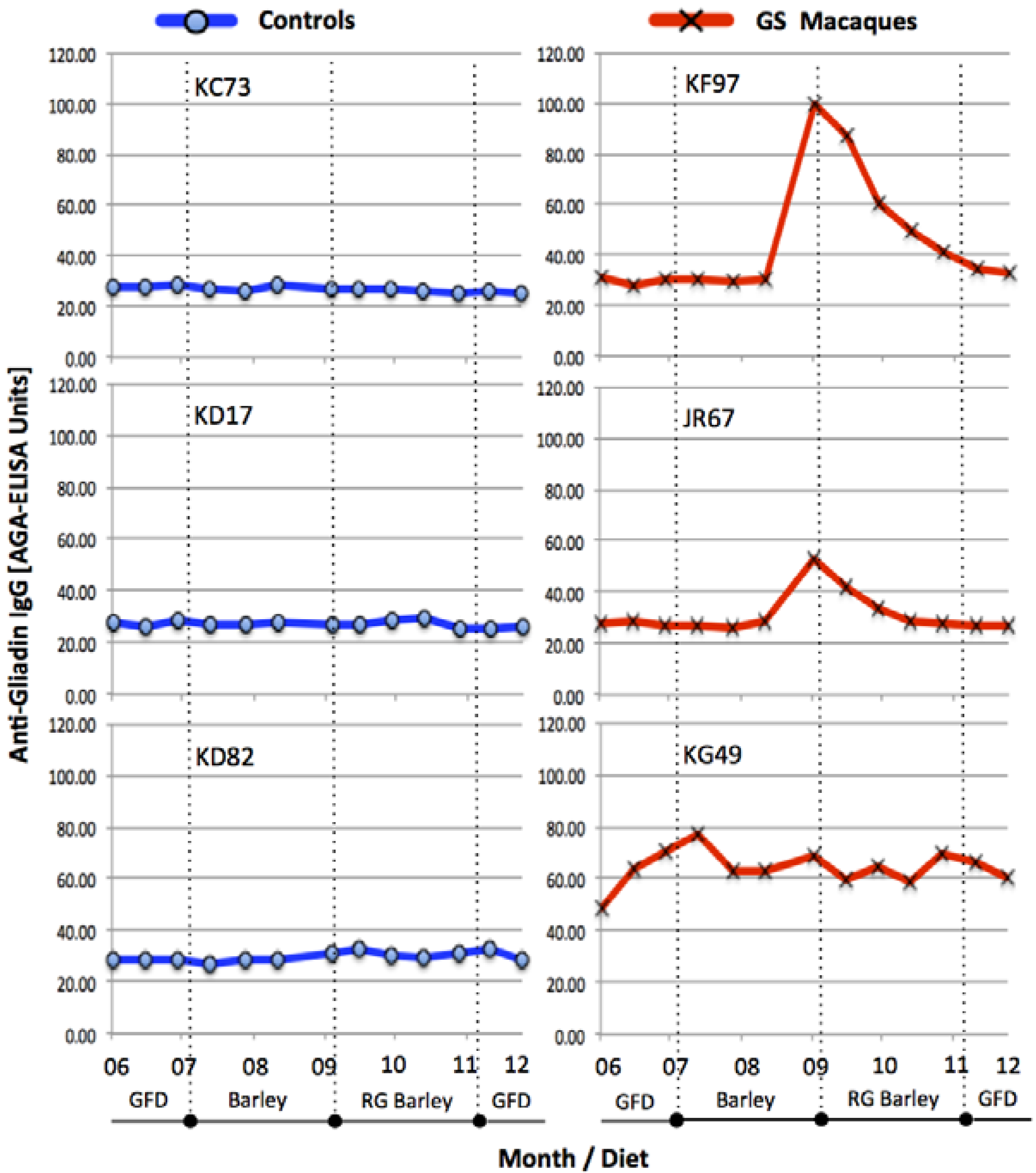
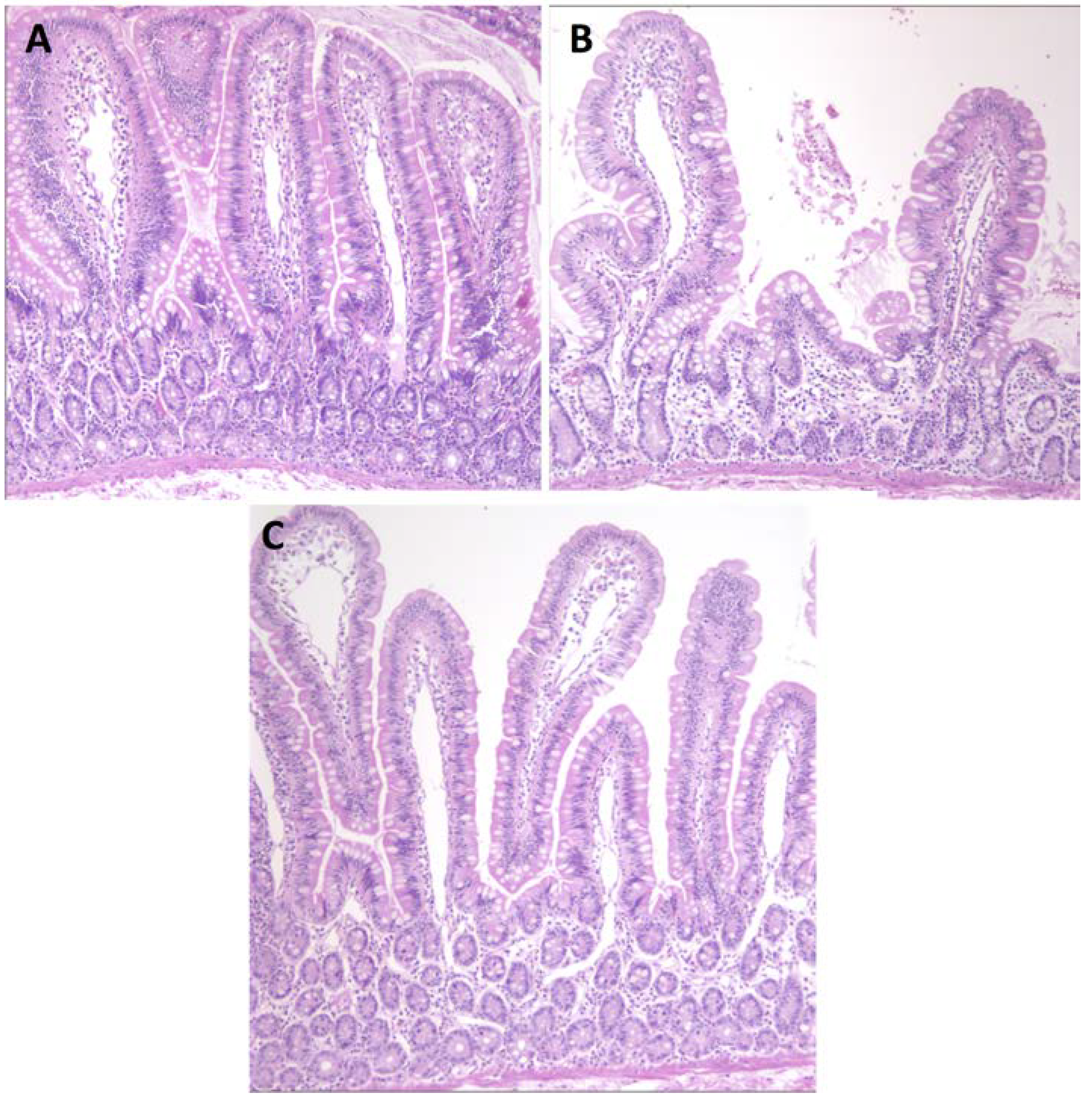
3.1. Serum Antibody Responses, Intestinal Histopathology and Diarrhea
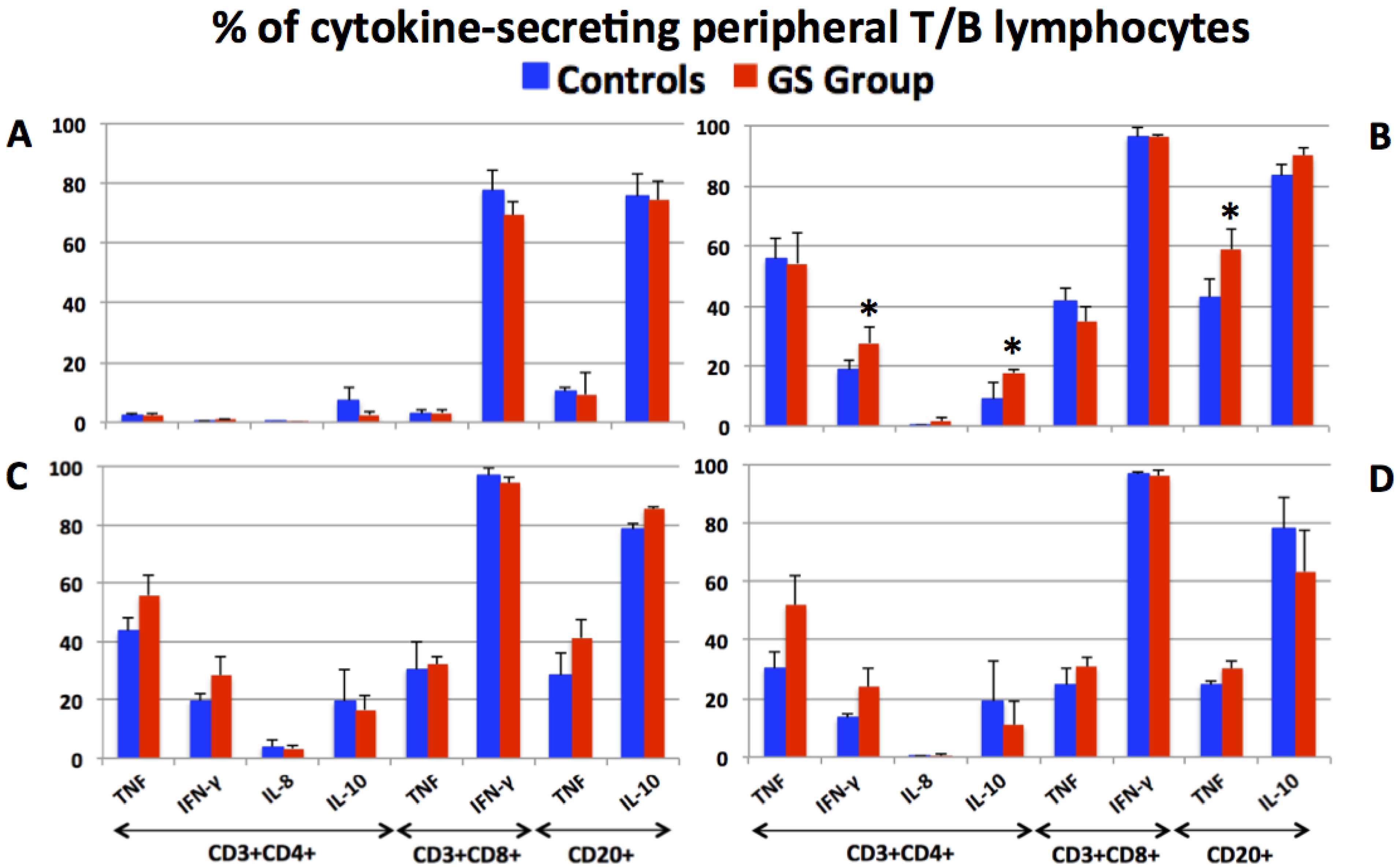
3.2. Production of Pro- and Anti-Inflammatory Cytokines by Peripheral T and B Lymphocytes
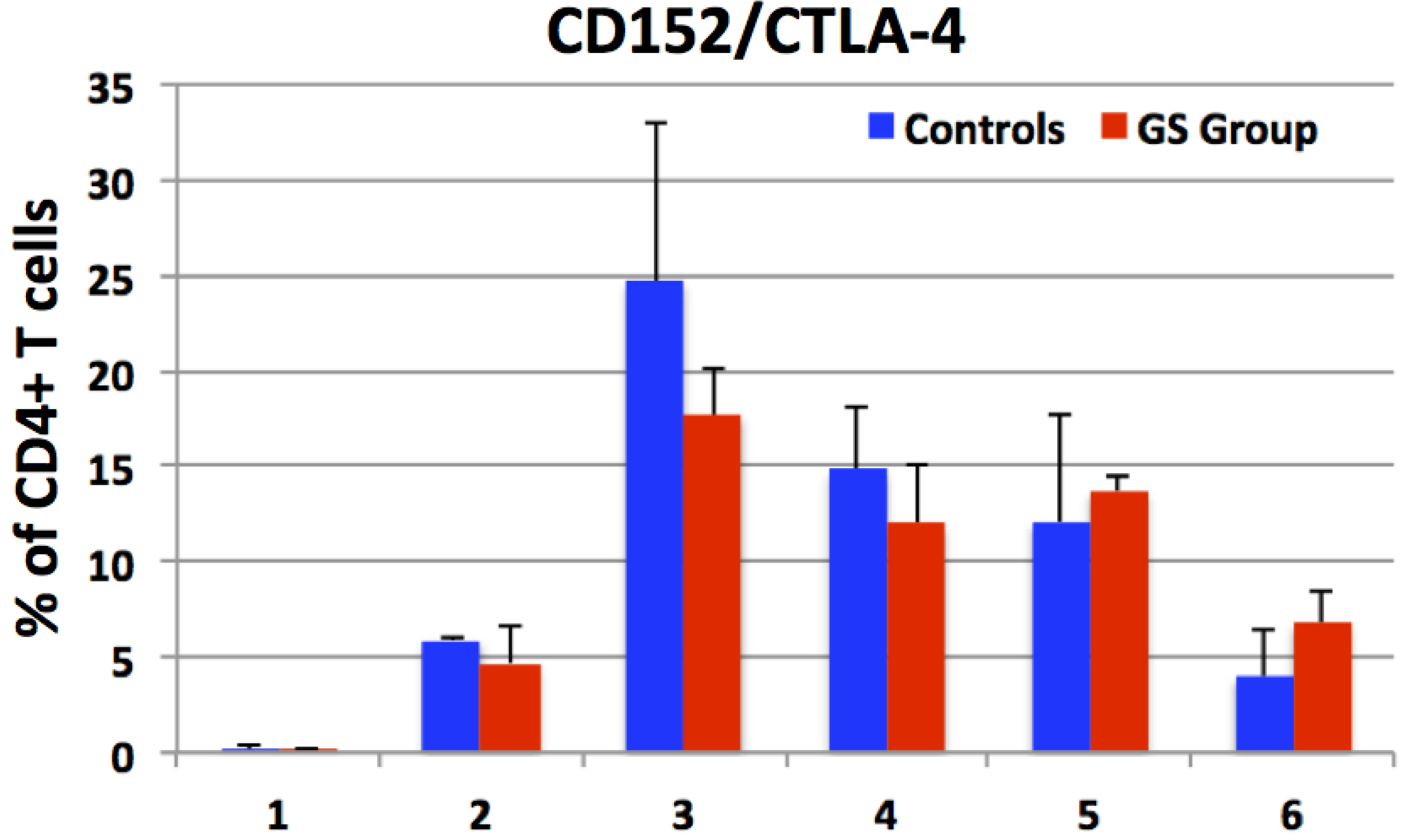
3.3. Expression of CD152 by Peripheral CD4+ Cells e.g., Enumeration of CD4+CTLA-4+ T Helper Cells
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Catassi, C.; Fasano. Celiac disease. Curr. Opin. Gastroenterol. 2008, 24, 687–691. [Google Scholar] [CrossRef]
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sestak, K.; Fortgang, I. Celiac and non-celiac forms of gluten sensitivity: Shifting paradigms of an old disease. Br. Microb. Res. J. 2013, 3, 585–589. [Google Scholar] [CrossRef]
- Sollid, L.M.; Khosla, C. Novel therapies for coeliac disease. J. Int. Med. 2011, 269, 604–613. [Google Scholar] [CrossRef]
- Sollid, L.M.; Khosla, C. Future therapeutic options for celiac disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Molberg, O.; Parrot, I.; Hausch, F.; Filiz, F.; Gray, G.M.; Sollid, L.M.; Khosla, C. Structural basis for gluten intolerance in celiac sprue. Science 2002, 297, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Qiao, S.W.; Arentz-Nansen, H.; Molberg, O.; Gray, G.M.; Sollid, L.M.; Khosla, C. Identification and analysis of multivalent proteolytically resistant peptides from gluten: Implications for celiac sprue. J. Proteom. Res. 2005, 4, 1732–1741. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Stewart, J.A.; Dromey, J.A.; Beissbarth, T.; van Heel, D.A.; Tatham, A.; Henderson, K.; Mannering, S.I.; Gianfrani, C.; Jewell, D.P. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci. Transl. Med. 2010, 2. [Google Scholar] [CrossRef]
- Molberg, O.; Uhlen, A.K.; Jensen, T.; Flaete, N.S.; Fleckenstein, B.; Arentz-Hansen, H.; Raki, M.; Lundin, K.E.A.; Sollid, L.M. Mapping of gluten T-cell epitopes in the bread wheat ancestors: Implications for celiac disease. Gastroenterology 2005, 128, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Spaenij-Dekking, L.; Kooy-Winkelaar, Y.; van Veelen, P.; Wouter-Drijhout, J.; Jonker, H.; van Soest, L.; Smulders, M.J.M.; Bosch, D.; Gilissen, L.J.W.J.; Koning, F. Natural variation in toxicity of wheat: Potential for selection of nontoxic varieties for celiac disease patients. Gastroenterology 2005, 129, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeck, H.; van Herpen, T.; Schuit, C.; Salentijn, E.; Dekking, L.; Bosch, D.; Hamer, R.; Smulders, M.; Gilissen, L.; van der Meer, I. Removing celiac disease-related gluten proteins from bread wheat while retaining technological properties: a study with Chinese Spring deletion lines. BMC Plant Biol. 2009, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Comino, I.; Real, A.; de Lorenzo, L.; Cornell, H.; Lopez-Casado, M.A.; Barro, F.; Lorite, P.; Torres, M.I.; Cebolla, A.; Sousa, C. Diversity in oat potential immunogenicity: Basis for the selection of oat varieties with no toxicity in coeliac disease. Gut 2011, 60, 915–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comino, I.; Real, A.; Gil-Humanes, J.; Piston, F.; de Lorenzo, L.; Moreno, M.L.; Lopez-Casado, M.A.; Lorite, P.; Cebolla, A.; Torres, M.I.; et al. Significant differences in coeliac immunotoxicity of barley varieties. Mol. Nutr. Food Res. 2012, 56, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Gil-Humanes, J.; Piston, F.; Tollefsen, S.; Sollid, L.M.; Barro, F. Effective shutdown in the expression of celiac disease-related wheat gliadin T-cell epitopes by RNA interference. Proc. Natl. Acad. Sci. USA 2010, 107, 17023–17028. [Google Scholar] [CrossRef] [PubMed]
- Piston, F.; Gil-Humanes, J.; Rodriguez-Quijano, M.; Barro, F. Down-regulating γ-gliadins in bread wheat leads to non-specific increases in other gluten proteins and has no major effects on dough gluten strength. PLoS One 2011, 6, e24754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Humanes, J.; Piston, F.; Altamirano-Fortoul, R.; Real, A.; Comino, I.; Sousa, C.; Rosell, C.M.; Barro, F. Reduced-gliadin wheat bread: an alternative to the gluten-free diet for consumers suffering gluten-related pathologies. PLoS One 2014, 9, e90898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doll, H. Inheritance of the high-lysine character of a barley mutant. Hereditas 1973, 74, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Doll, H.; Koie, B.; Eggum, B. Induced high lysine mutants in barley. Radiat. Bot. 1974, 14, 73–80. [Google Scholar] [CrossRef]
- Jorgensen, H.; Gabert, V.M.; Fernandez, J.A. Influence of nitrogen fertilization on the nutritional value of high-lysine barley determined in growing pigs. Anim. Feed Sci. Technol. 1999, 79, 79–91. [Google Scholar] [CrossRef]
- Newman, C.; Overland, M.; Newman, R.; Bang-Olsen, K.; Pedersen, B. Protein quality of a new high-lysine barley derived from Riso 1508. Canad. J. Anim. Sci. 1990, 70, 279–285. [Google Scholar] [CrossRef]
- Munck, L.; Pram Nielsen, J.; Moller, B.; Jacobsen, S.; Sondergaard, I.; Engelsen, S.; Norgaard, L.; Bro, R. Exploring the phenotypic expression of a regulatory proteome-altering gene by spectroscopy and chemometrics. Analyt. Chim. Acta 2001, 446, 169–184. [Google Scholar] [CrossRef]
- Mazumdar, K.; Alvarez, X.; Borda, J.T.; Dufour, J.; Martin, E.; Bethune, M.T.; Khosla, C.; Sestak, K. Visualization of transepithelial passage of the immunogenic 33-residue peptide from α-2 gliadin in gluten-sensitive macaques. PLoS One 2010, 5, e10228. [Google Scholar] [CrossRef] [PubMed]
- Sestak, K.; Merritt, C.K.; Borda, J.; Saylor, E.; Schwamberger, S.R.; Cogswell, F.; Didier, E.S.; Didier, P.J.; Plauche, G.; Bohm, R.P.; et al. Infectious agent and immune response characteristics of chronic enterocolitis in captive rhesus macaques. Infect. Immun. 2003, 71, 4079–4086. [Google Scholar] [CrossRef] [PubMed]
- Bethune, M.T.; Borda, J.T.; Ribka, E.; Liu, M.X.; Phillipi-Falkenstein, K.; Jandacek, R.J.; Doxiadis, G.G.M.; Gray, G.M.; Khosla, C.; Sestak, K. A non-human primate model for gluten sensitivity. PLoS One 2008, 3, e1614. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Feely, S.L.; Wang, X.; Liu, D.X.; Borda, J.T.; Dufour, J.; Li, W.; Aye, P.P.; Doxiadis, G.G.; Khosla, C.; et al. Gluten-sensitive enteropathy coincides with decreased capability of intestinal T cells to secrete IL-17 and IL-22 in a macaque model for celiac disease. Clin. Immunol. 2013, 147, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Bethune, M.T.; Ribka, E.; Khosla, C.; Sestak, K. Transepithelial transport and enzymatic detoxification of gluten in gluten-sensitive rhesus macaques. PLoS One 2008, 3, e1857. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Liu, D.X.; Rasmussen, T.; Lackner, A.A.; Veazey, R.S. IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques. Mucos. Immunol. 2012, 5, 658–669. [Google Scholar] [CrossRef]
- Anderson, R.P.; van Heel, D.A.; Tye-Din, J.A.; Barnardo, M.; Salio, M.; Jewell, D.P.; Hill, A.V.S. T cells in peripheral blood after gluten challenge in coeliac disease. Gut 2005, 54, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Abadie, V.; Sollid, L.M.; Barreiro, L.B.; Jabri, B. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu. Rev. Immunol. 2011, 29, 493–525. [Google Scholar] [CrossRef] [PubMed]
- DiRaimondo, T.R.; Klock, C.; Khosla, C. Interferon-γ activates transglutaminase 2 via a phosphatidylinositol-3-kinase-dependent pathway: Implications for celiac sprue therapy. J. Pharmacol. Exp. Therapeut. 2012, 341, 104–114. [Google Scholar] [CrossRef]
- Schuppan, D.; Junker, Y.; Barisani, D. Celiac disease: From pathogenesis to novel therapies. Gastroenterology 2009, 137, 1912–1933. [Google Scholar] [CrossRef] [PubMed]
- Spatola, B.N.; Kaukinen, K.; Collin, P.; Maki, M.; Kagnoff, M.F.; Daugherty, P.S. Persistence of elevated deamidated gliadin peptide antibodies on a gluten-free diet indicates nonresponsive coeliac disease. Aliment. Pharmacol. Ther. 2014, 39, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Troncone, R.; Jabri, B. Coeliac disease and gluten sensitivity. J. Intern. Med. 2011, 269, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Klock, C.; DiRaimondo, T.R.; Khosla, C. Role of transglutaminase 2 in celiac disease pathogenesis. Semin. Immunopathol. 2012, 34, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Tanner, G.J.; Blundell, M.J.; Colgrave, M.L.; Howitt, C.A. Quantification of hordeins by ELISA: The correct standard makes a magnitude of difference. PLoS One 2013, 8, e56456. [Google Scholar] [CrossRef] [PubMed]
- Tanner, G.J.; Colgrave, M.L.; Blundell, M.J.; Goswami, H.P.; Howitt, C.A. Measuring hordein (gluten) in beer-A comparison of ELISA and mass spectrometry. PLoS One 2013, 8, e56452. [Google Scholar] [CrossRef] [PubMed]
- Meresse, B.; Curran, S.A.; Ciszewski, C.; Orbelyan, G.; Setty, M.; Bhagat, G.; Lee, L.; Tretiakova, M.; Semrad, C.; Kistner, E.; et al. Reprogramming of CTLs into natural killer-like cells in celiac disease. J. Exp. Med. 2006, 203, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Sollid, L.M.; Jabri, B. Triggers and drivers of autoimmunity: Lessons from coeliac disease. Nat. Rev. Imunol. 2013, 13, 294–302. [Google Scholar] [CrossRef]
- Forsberg, G.; Hernell, O.; Melgar, S.; Israelsson, A.; Hammarstrom, S.; Hammarstrom, M.L. Paradoxical coexpression of proinflammatory and down-regulatory cytokines in intestinal T cells in childhood celiac disease. Gastroenterology 2002, 123, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, G.; Hernell, O.; Hammarstrom, S.; Hammarstrom, M.L. Concomitant increase of IL-10 and pro-inflammatory cytokines in intraepithelial lymphocyte subsets in celiac disease. Internat. Immunol. 2007, 19, 993–1001. [Google Scholar] [CrossRef]
- Diosdado, B.; Wijmenga, C. Molecular mechanisms of the adaptive, innate and regulatory immune responses in the intestinal mucosa of celiac disease patients. Expert Rev. Mol. Diagn. 2005, 5, 681–700. [Google Scholar] [CrossRef] [PubMed]
- Tack, G.J.; van Wanrooij, R.L.; Von Bloomberg, B.M.; Amini, H.; Coupe, V.M.; Bonnet, P.; Mulder, C.J.; Schreurs, M.W. Serum parameters in the spectrum of coeliac disease: Beyond standard antibody testing—A cohort study. BMC Gastroenterol. 2012, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Brottveit, M.; Beitnes, A.C.; Tollefsen, S.; Bratlie, J.E.; Jahnsen, F.L.; Johansen, F.E.; Sollid, L.M.; Lundin, K.E. Mucosal cytokine responses after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am. J. Gastroenterol. 2013, 108, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Bjorck, S.; Lindehammer, S.R.; Fex, M.; Agardh, D. Serum cytokine pattern in young children with screening detected celiac disease. Clin. Exp. Immunol. 2015, 179, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Pesce, G.; Auricchio, R.; Bagnasco, M.; Saverino, D. Oversecretion of soluble CTLA-4 in various autoimmune diseases overlapping celiac disease. Genet. Test. Mol. Biomark. 2014, 18, 8–11. [Google Scholar] [CrossRef]
- Wen, S.; Wen, N.; Pang, J.; Langen, G.; Brew-Appiah, R.A.; Mejias, J.H.; Osorio, C.; Yang, M.; Gemini, R.; Moehs, C.P.; et al. Structural genes of wheat and barley 5-methylcytosine DNA glycosylases and their potential applications for human health. Proc. Natl. Acad. Sci. USA 2012, 109, 20543–20548. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, M.B. Methylation of B-hordein genes in barley endosperm is inversely correlated with gene activity and affected by the regulatory gene Lys3. Proc. Natl. Acad. Sci. USA 1992, 89, 4119–4123. [Google Scholar] [CrossRef] [PubMed]
- Kreis, M.; Shewry, P.; Forde, B.; Rahman, S.; Miflin, B. Molecular analysis of a mutation conferring the high-lysine phenotype on the grain of barley (Hordeum. vulgare). Cell 1983, 34, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Tanner, G.; Howitt, C.; Forrester, R.; Campbell, P.; Tye-Din, J.; Anderson, R. Dissecting the T-cell response to hordeins in coeliac disease can develop barley with reduced immunotoxicity. Aliment. Pharmacol. Ther. 2010, 32, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Stepniak, D.; Spaenij-Dekking, L.; Mitea, C.; Moester, M.; de Ru, A.; Baak-Pablo, R.; van Veelen, P.; Edens, L.; Koning, F. Highly efficient gluten degradation with a newly identified prolyl endoprotease: Implications for celiac disease. Am. J. Physiol. Gastrointest Liver Physiol. 2006, 291, G621–G629. [Google Scholar] [CrossRef] [PubMed]
- Tack, G.J.; van de Water, J.M.; Bruins, M.J.; Kooy-Winkelaar, E.M.; van Bergen, J.; Bonnet, P.; Vreugdenhil, A.C.; Korponay-Szabo, I.; Edens, L.; von Blomberg, B.M.E. Consumption of gluten with gluten-degrading enzyme by celiac patients: A pilot study. World J. Gastroenterol. 2013, 19, 5837–5847. [Google Scholar] [CrossRef] [PubMed]
- Toft-Hansen, H.; Rasmussen, K.S.; Staal, A.; Roggen, E.L.; Sollid, L.M.; Lillevang, S.T.; Barington, T.; Husby, S. Treatment of both native and deamidated gluten peptides with an endo-peptidase from Aspergillus niger prevents stimulation of gut-derived gluten-reactive T cells from either children or adults with celiac disease. Clin. Immunol. 2014, 153, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Lahdeaho, M.-L.; Kaukinen, K.; Laurila, K.; Vuotikka, P.; Koivurova, O.-P.; Karja-Lahdensuu, T.; Marcantonio, A.; Adekman, D.C.; Maki, M. Glutenase ALV003 attenuates gluten-induced mucosal injury in patients with celiac disease. Gastroenterology 2014, 146, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sestak, K.; Thwin, H.; Dufour, J.; Aye, P.P.; Liu, D.X.; Moehs, C.P. The Effects of Reduced Gluten Barley Diet on Humoral and Cell-Mediated Systemic Immune Responses of Gluten-Sensitive Rhesus Macaques. Nutrients 2015, 7, 1657-1671. https://doi.org/10.3390/nu7031657
Sestak K, Thwin H, Dufour J, Aye PP, Liu DX, Moehs CP. The Effects of Reduced Gluten Barley Diet on Humoral and Cell-Mediated Systemic Immune Responses of Gluten-Sensitive Rhesus Macaques. Nutrients. 2015; 7(3):1657-1671. https://doi.org/10.3390/nu7031657
Chicago/Turabian StyleSestak, Karol, Hazel Thwin, Jason Dufour, Pyone P. Aye, David X. Liu, and Charles P. Moehs. 2015. "The Effects of Reduced Gluten Barley Diet on Humoral and Cell-Mediated Systemic Immune Responses of Gluten-Sensitive Rhesus Macaques" Nutrients 7, no. 3: 1657-1671. https://doi.org/10.3390/nu7031657




