Curcumin Protects against Cadmium-Induced Vascular Dysfunction, Hypertension and Tissue Cadmium Accumulation in Mice
Abstract
:1. Introduction
2. Experimental Section
2.1. Animal Model of Cd-Induced Hypertension and Vascular Dysfunction
2.2. Assessments of Haemodynamic and Arterial Pressure Reactivity
2.3. Biochemical Assays
2.3.1. Assays of Lipid Peroxidation and Protein Oxidation
2.3.2. Assay of Nitrate and Nitrite
2.3.3. Assay of O2•− Production
2.3.4. Assay of Glutathione
2.4. Western Blot Analysis
2.5. Assay of Cd Concentration
2.6. Chemicals and Reagents
2.7. Statistical Analysis
3. Results
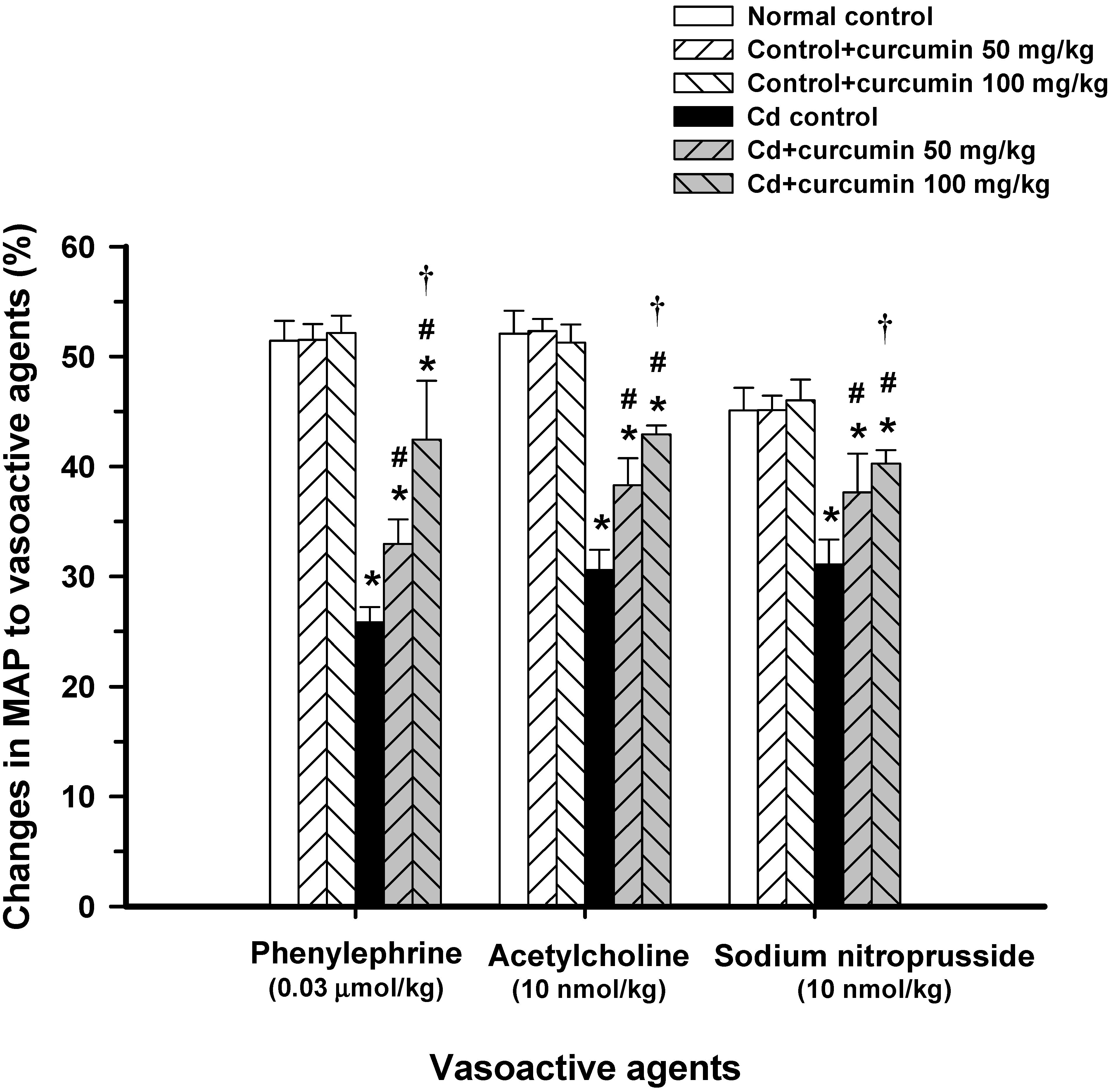
3.1. Effect of Curcumin on Haemodynamic Status and Vascular Reactivity
| Parameters | Normal Control | Normal control + Curcumin (mg/kg) | Cd Control | Cd + Curcumin (mg/kg) | ||
|---|---|---|---|---|---|---|
| 50 | 100 | 50 | 100 | |||
| Systolic pressure (mmHg) | 116 ± 3 | 120 ± 1 | 122 ± 1 | 155 ± 1 * | 130 ± 1 *,# | 129 ± 3 *,# |
| Diastolic pressure (mmHg) | 82 ± 3 | 85 ± 1 | 85 ± 2 | 113 ± 2 * | 102 ± 1 *,# | 91 ± 4 #,† |
| Mean arterial pressure (mmHg) | 96 ± 2 | 97 ± 2 | 95 ± 1 | 136 ± 2 * | 113 ± 1 *,# | 101 ± 4 #,† |
| Heart rate (beats/min) | 330 ± 8 | 337 ± 7 | 340 ± 5 | 340 ± 8 | 331 ± 4 | 340 ± 7 |
3.2. Effect of Curcumin on Oxidant and Antioxidant Status
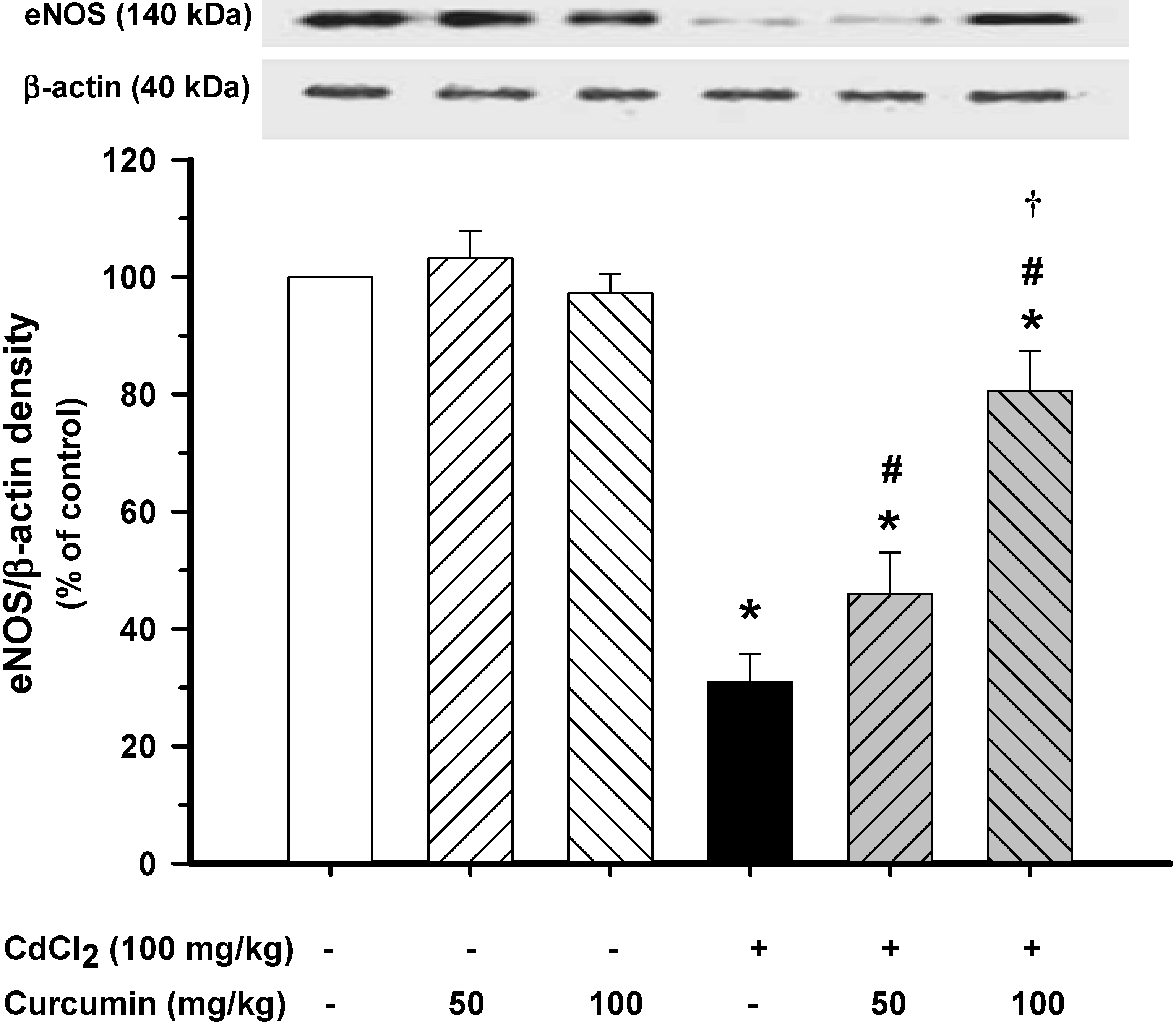
3.3. Effect of Curcumin on Vascular eNOS Protein Expression
3.4. Effect of Curcumin on Cd Concentrations
4. Discussion
| Parameters | Normal Control | Normal control + Curcumin (mg/kg) | Cd Control | Cd + Curcumin (mg/kg) | ||
|---|---|---|---|---|---|---|
| 50 | 100 | 50 | 100 | |||
| Aortic superoxide anion (Counts/mg dry wt./min) | 161.2 ± 15.1 | 160.4 ± 9.5 | 158.7 ± 8.6 | 1202.4 ± 121.9 * | 851.9 ± 73.6 *,# | 711.5 ± 23.0 *,#,† |
| Urinary nitrate/nitrite (nmol/mg creatinine) | 909.7 ± 74.8 | 895.5 ± 57.5 | 920.0 ± 41.7 | 2074.6 ± 102.4 * | 1499.9 ± 43.4 *,# | 1116.5 ± 156.7 #,† |
| Plasma malondialdehyde (μM) | 15.4 ± 0.6 | 15.0 ± 1.3 | 15.9 ± 0.4 | 32.3 ± 2.4 * | 25.6 ± 3.3 *,# | 17.8 ± 1.5 #,† |
| Plasma protein carbonyls (nmol/mg protein) | 1.4 ± 0.09 | 1.4 ± 0.06 | 1.4 ± 0.06 | 3.5 ± 0.4 * | 2.0 ± 0.2 *,# | 1.7 ± 0.4 # |
| Blood GSH (μM) | 825 ± 69 | 811 ± 46 | 801 ± 31 | 270 ± 21 * | 510 ± 40 *,# | 603 ± 32 *,#,† |
| Blood GSH/GSSG | 149 ± 13 | 143.5 ± 11 | 146 ± 11 | 27 ± 3 * | 58 ± 7 *,# | 109 ± 5 *,#,† |
| Treatment | Heart (μg/g Tissue) | Aorta (μg/g Tissue) | Liver (μg/g Tissue) | Kidneys (μg/g Tissue) | Whole Blood (μg/L) |
|---|---|---|---|---|---|
| Normal control | undetectable | 0.023 ± 0.003 | 0.035 ± 0.006 | 0.116 ± 0.006 | 2.20 ± 0.007 |
| Cd control | 0.50 ± 0.039 | 0.24 ± 0.023 * | 11.37 ± 1.53 * | 19.75 ± 2.25 * | 60.40 ± 6.95 * |
| Cd + Curcumin 50 mg/kg | 0.41 ± 0.063 | 0.22 ± 0.005 * | 7.25 ± 0.52 *,# | 15.83 ± 0.81 *,# | 46.46 ± 6.35 *,# |
| Cd + Curcumin 100 mg/kg | 0.24 ± 0.059 #,† | 0.09 ± 0.004 *,#,† | 6.87 ± 0.85 *,# | 12.28 ± 0.79 *,#,† | 23.30 ± 1.59 *,#,† |
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sinha, R.; Anderson, D.E.; McDonald, S.S.; Greenwald, P. Cancer risk and diet in India. J. Postgrad. Med. 2003, 49, 222–228. [Google Scholar]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wang, L.F.; Hsu, Y.C. Curcuminoids suppress the growth of pharynx and nasopharyngeal carcinoma cells through induced apoptosis. J. Agric. Food Chem. 2009, 57, 3765–3770. [Google Scholar] [CrossRef]
- Surh, Y.J.; Chun, K.S. Cancer chemopreventive effects of curcumin. Adv. Exp. Med. Biol. 2007, 595, 149–172. [Google Scholar] [CrossRef]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef]
- Nakmareong, S.; Kukongviriyapan, U.; Pakdeechote, P.; Donpunha, W.; Kukongviriyapan, V.; Kongyingyoes, B.; Sompamit, K.; Phisalaphong, C. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with l-NAME-induced hypertension. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 519–529. [Google Scholar] [CrossRef]
- Sompamit, K.; Kukongviriyapan, U.; Nakmareong, S.; Pannangpetch, P.; Kukongviriyapan, V. Curcumin improves vascular function and alleviates oxidative stress in non-lethal lipopolysaccharide-induced endotoxaemia in mice. Eur. J. Pharmacol. 2009, 616, 192–199. [Google Scholar] [CrossRef]
- Dairam, A.; Limson, J.L.; Watkins, G.M.; Antunes, E.; Daya, S. Curcuminoids, curcumin, and demethoxycurcumin reduce lead-induced memory deficits in male Wistar rats. J. Agric. Food Chem. 2007, 55, 1039–1044. [Google Scholar] [CrossRef]
- Daniel, S.; Limson, J.L.; Dairam, A.; Watkins, G.M.; Daya, S. Through metal binding, curcumin protects against lead- and cadmium-induced lipid peroxidation in rat brain homogenates and against lead-induced tissue damage in rat brain. J. Inorg. Biochem. 2004, 98, 266–275. [Google Scholar] [CrossRef]
- Obioha, U.E.; Suru, S.M.; Ola-Mudathir, K.F.; Faremi, T.Y. Hepatoprotective potentials of onion and garlic extracts on cadmium-induced oxidative damage in rats. Biol. Trace Elem. Res. 2009, 129, 143–156. [Google Scholar] [CrossRef]
- Roels, H.A.; Hoet, P.; Lison, D. Usefulness of biomarkers of exposure to inorganic mercury, lead, or cadmium in controlling occupational and environmental risks of nephrotoxicity. Ren. Fail. 1999, 21, 251–262. [Google Scholar] [CrossRef]
- Satarug, S.; Nishijo, M.; Ujjin, P.; Vanavanitkun, Y.; Moore, M.R. Cadmium-induced nephropathy in the development of high blood pressure. Toxicol. Lett. 2005, 157, 57–68. [Google Scholar] [CrossRef]
- Eum, K.D.; Lee, M.S.; Paek, D. Cadmium in blood and hypertension. Sci. Total Environ. 2008, 407, 147–153. [Google Scholar] [CrossRef]
- Lee, B.K.; Kim, Y. Association of blood cadmium with hypertension in the Korean general population: Analysis of the 2008–2010 Korean National Health and Nutrition Examination Survey Data. Am. J. Ind. Med. 2012, 55, 1060–1067. [Google Scholar] [CrossRef]
- Lee, M.S.; Park, S.K.; Hu, H.; Lee, S. Cadmium exposure and cardiovascular disease in the 2005 Korea National Health and Nutrition Examination Survey. Environ. Res. 2011, 111, 171–176. [Google Scholar] [CrossRef]
- Olsen, L.; Lind, P.M.; Lind, L. Gender differences for associations between circulating levels of metals and coronary risk in the elderly. Int. J. Hyg. Environ. Health 2012, 215, 411–417. [Google Scholar] [CrossRef]
- Tellez-Plaza, M.; Navas-Acien, A.; Crainiceanu, C.M.; Guallar, E. Cadmium exposure and hypertension in the 1999–2004 National Health and Nutrition Examination Survey (NHANES). Environ. Health Perspect. 2008, 116, 51–56. [Google Scholar]
- Staessen, J.A.; Kuznetsova, T.; Roels, H.A.; Emelianov, D.; Fagard, R. Exposure to cadmium and conventional and ambulatory blood pressures in a prospective population study. Public Health and Environmental Exposure to Cadmium Study Group. Am. J. Hypertens. 2000, 13, 146–156. [Google Scholar] [CrossRef]
- Whittemore, A.S.; DiCiccio, Y.; Provenzano, G. Urinary cadmium and blood pressure: Results from the NHANES II survey. Environ. Health Perspect. 1991, 91, 133–140. [Google Scholar]
- Nwokocha, C.R.; Baker, A.; Douglas, D.; McCalla, G.; Nwokocha, M.; Brown, P.D. Apocynin ameliorates cadmium-induced hypertension through elevation of endothelium nitric oxide synthase. Cardiovasc. Toxicol. 2013, 13, 357–363. [Google Scholar] [CrossRef]
- Sompamit, K.; Kukongviriyapan, U.; Donpunha, W.; Nakmareong, S.; Kukongviriyapan, V. Reversal of cadmium-induced vascular dysfunction and oxidative stress by meso-2,3-dimercaptosuccinic acid in mice. Toxicol. Lett. 2010, 198, 77–82. [Google Scholar] [CrossRef]
- Yoopan, N.; Watcharasit, P.; Wongsawatkul, O.; Piyachaturawat, P.; Satayavivad, J. Attenuation of eNOS expression in cadmium-induced hypertensive rats. Toxicol. Lett. 2008, 176, 157–161. [Google Scholar] [CrossRef]
- Oner, G.; Senturk, U.K.; Izgut-Uysal, N. The role of cadmium in the peroxidative response of kidney to stress. Biol. Trace Elem. Res. 1995, 48, 111–117. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Edwards, J.R.; Nebert, D.W.; Woods, J.M.; Barchowsky, A.; Atchison, W.D. The vascular system as a target of metal toxicity. Toxicol. Sci. 2008, 102, 207–218. [Google Scholar]
- Wang, Y.; Fang, J.; Leonard, S.S.; Rao, K.M. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic. Biol. Med. 2004, 36, 1434–1443. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Thijssen, S.; Maringwa, J.; Faes, C.; Lambrichts, I.; van Kerkhove, E. Chronic exposure of mice to environmentally relevant, low doses of cadmium leads to early renal damage, not predicted by blood or urine cadmium levels. Toxicology 2007, 229, 145–156. [Google Scholar] [CrossRef]
- Naowaboot, J.; Pannangpetch, P.; Kukongviriyapan, V.; Kongyingyoes, B.; Kukongviriyapan, U. Antihyperglycemic, antioxidant and antiglycation activities of mulberry leaf extract in streptozotocin-induced chronic diabetic rats. Plant Foods Hum. Nutr. 2009, 64, 116–121. [Google Scholar] [CrossRef]
- Somparn, N.; Kukongviriyapan, U.; Tassaneeyakul, W.; Jetsrisuparb, A.; Kukongviriyapan, V. Modification of CYP2E1 and CYP3A4 activities in haemoglobin E-beta thalassemia patients. Eur. J. Clin. Pharmacol. 2007, 63, 43–50. [Google Scholar]
- Kukongviriyapan, V.; Somparn, N.; Senggunprai, L.; Prawan, A.; Kukongviriyapan, U.; Jetsrisuparb, A. Endothelial dysfunction and oxidant status in pediatric patients with hemoglobin E-beta thalassemia. Pediatr. Cardiol. 2008, 29, 130–135. [Google Scholar] [CrossRef]
- Luangaram, S.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P. Protective effects of quercetin against phenylhydrazine-induced vascular dysfunction and oxidative stress in rats. Food Chem. Toxicol. 2007, 45, 448–455. [Google Scholar] [CrossRef]
- Morales, A.I.; Vicente-Sanchez, C.; Sandoval, J.M.; Egido, J.; Mayoral, P.; Arevalo, M.A.; Fernandez-Tagarro, M.; Lopez-Novoa, J.M.; Perez-Barriocanal, F. Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem. Toxicol. 2006, 44, 2092–2100. [Google Scholar] [CrossRef]
- Evans, H.; Giglio, J. Interferences in inductivity coupled plasma mass spectrometry. J. Anal. At. Spectrom. 1993, 8, 1–18. [Google Scholar] [CrossRef]
- Oparil, S.; Zaman, M.A.; Calhoun, D.A. Pathogenesis of hypertension. Ann. Intern. Med. 2003, 139, 761–776. [Google Scholar] [CrossRef]
- Skoczynska, A.; Martynowicz, H. The impact of subchronic cadmium poisoning on the vascular effect of nitric oxide in rats. Hum. Exp. Toxicol. 2005, 24, 353–361. [Google Scholar] [CrossRef]
- Wakabayashi, I.; Sakamoto, K.; Hatake, K. Inhibitory effects of cadmium ion on extracellular Ca2+-independent contraction of rat aorta. Eur. J. Pharmacol. 1995, 293, 133–140. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Watanabe, S.; Kaji, T. Promotion of cultured vascular smooth muscle cell proliferation by low levels of cadmium. Toxicol. Lett. 1998, 94, 175–180. [Google Scholar] [CrossRef]
- Moskovitz, J.; Yim, M.B.; Chock, P.B. Free radicals and disease. Arch. Biochem. Biophys. 2002, 397, 354–359. [Google Scholar] [CrossRef]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. 1996, 271, C1424–C1437. [Google Scholar]
- Landmesser, U.; Dikalov, S.; Price, S.R.; McCann, L.; Fukai, T.; Holland, S.M.; Mitch, W.E.; Harrison, D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Investig. 2003, 111, 1201–1209. [Google Scholar] [CrossRef]
- Dorta, D.J.; Leite, S.; DeMarco, K.C.; Prado, I.M.; Rodrigues, T.; Mingatto, F.E.; Uyemura, S.A.; Santos, A.C.; Curti, C. A proposed sequence of events for cadmium-induced mitochondrial impairment. J. Inorg. Biochem. 2003, 97, 251–257. [Google Scholar] [CrossRef]
- Singh, S.; Aggarwal, B.B. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef]
- Majumder, S.; Muley, A.; Kolluru, G.K.; Saurabh, S.; Tamilarasan, K.P.; Chandrasekhar, S.; Reddy, H.B.; Purohit, S.; Chatterjee, S. Cadmium reduces nitric oxide production by impairing phosphorylation of endothelial nitric oxide synthase. Biochem. Cell Biol. 2008, 86, 1–10. [Google Scholar] [CrossRef]
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef]
- Renugadevi, J.; Prabu, S.M. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp. Toxicol. Pathol. 2010, 62, 171–181. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Lee, H.; Rangasamy, T.; Reddy, S.P.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Investig. 2006, 116, 984–995. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Barregard, L.; Fabricius-Lagging, E.; Lundh, T.; Molne, J.; Wallin, M.; Olausson, M.; Modigh, C.; Sallsten, G. Cadmium, mercury, and lead in kidney cortex of living kidney donors: Impact of different exposure sources. Environ. Res. 2010, 110, 47–54. [Google Scholar] [CrossRef]
- Eybl, V.; Kotyzova, D.; Koutensky, J. Comparative study of natural antioxidants—Curcumin, resveratrol and melatonin—In cadmium-induced oxidative damage in mice. Toxicology 2006, 225, 150–156. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kukongviriyapan, U.; Pannangpetch, P.; Kukongviriyapan, V.; Donpunha, W.; Sompamit, K.; Surawattanawan, P. Curcumin Protects against Cadmium-Induced Vascular Dysfunction, Hypertension and Tissue Cadmium Accumulation in Mice. Nutrients 2014, 6, 1194-1208. https://doi.org/10.3390/nu6031194
Kukongviriyapan U, Pannangpetch P, Kukongviriyapan V, Donpunha W, Sompamit K, Surawattanawan P. Curcumin Protects against Cadmium-Induced Vascular Dysfunction, Hypertension and Tissue Cadmium Accumulation in Mice. Nutrients. 2014; 6(3):1194-1208. https://doi.org/10.3390/nu6031194
Chicago/Turabian StyleKukongviriyapan, Upa, Patchareewan Pannangpetch, Veerapol Kukongviriyapan, Wanida Donpunha, Kwanjit Sompamit, and Praphassorn Surawattanawan. 2014. "Curcumin Protects against Cadmium-Induced Vascular Dysfunction, Hypertension and Tissue Cadmium Accumulation in Mice" Nutrients 6, no. 3: 1194-1208. https://doi.org/10.3390/nu6031194
APA StyleKukongviriyapan, U., Pannangpetch, P., Kukongviriyapan, V., Donpunha, W., Sompamit, K., & Surawattanawan, P. (2014). Curcumin Protects against Cadmium-Induced Vascular Dysfunction, Hypertension and Tissue Cadmium Accumulation in Mice. Nutrients, 6(3), 1194-1208. https://doi.org/10.3390/nu6031194




