Vitamin D2 Supplementation Amplifies Eccentric Exercise-Induced Muscle Damage in NASCAR Pit Crew Athletes
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Research Design
- 1
- Leg-back dynamometer strength test: With arms straight and knees slightly bent, subjects grasped a bar that was attached to a platform via a chain and dynamometer (Lafayette Instruments, Lafayette, IN, USA), and then lifted up with maximal effort for several seconds. The test was repeated three times, with the highest score recorded;
- 2
- Hand-grip dynamometer strength test: The hand-grip dynamometer (Lafayette Instruments, Lafayette, IN, USA) was adjusted to hand size (with the middle of the fingers on the handle). The subject assumed a slightly bent forward position with the right hand hanging down and forward, and then gripped maximally for 2–3 s. The best of three trials was recorded;
- 3
- Body weight bench press to exhaustion: Subjects bench pressed a weighted bar equal to body weight as many times as possible (to a metronome set at 60 beats/min or 30 lifts/min) until fatigue. The bar touched a small foam block on the chest lightly in the down position, and lifted upwards until the arms were straight in the up position;
- 4
- Vertical jump: Subjects first stood erect with the feet flat on the floor and reached as high as possible with both arms and hands (standing reach height). Subjects then squatted down and jumped as high as possible with one arm and hand, and tapped the measuring device (jump height) (Vertec vertical jump apparatus, Questtek Corp, Northridge, CA, USA). This was repeated three times, with the best score recorded as the difference between the jump and standing reach heights;
- 5
- Wingate anaerobic power cycling test: The Lode cycle ergometer (Lode B.V., Groningen, The Netherlands) was adjusted to the body mass of the subject (7 W per kilogram), and then subjects cycled at maximal speed for 30 s. The peak and total wattage power output was recorded and adjusted to body mass.
2.3. Eccentric Exercise
2.4. Supplement
2.5. Mushroom Vitamin D2 Supplement Analysis
2.6. Analytical Measures
2.7. Statistics
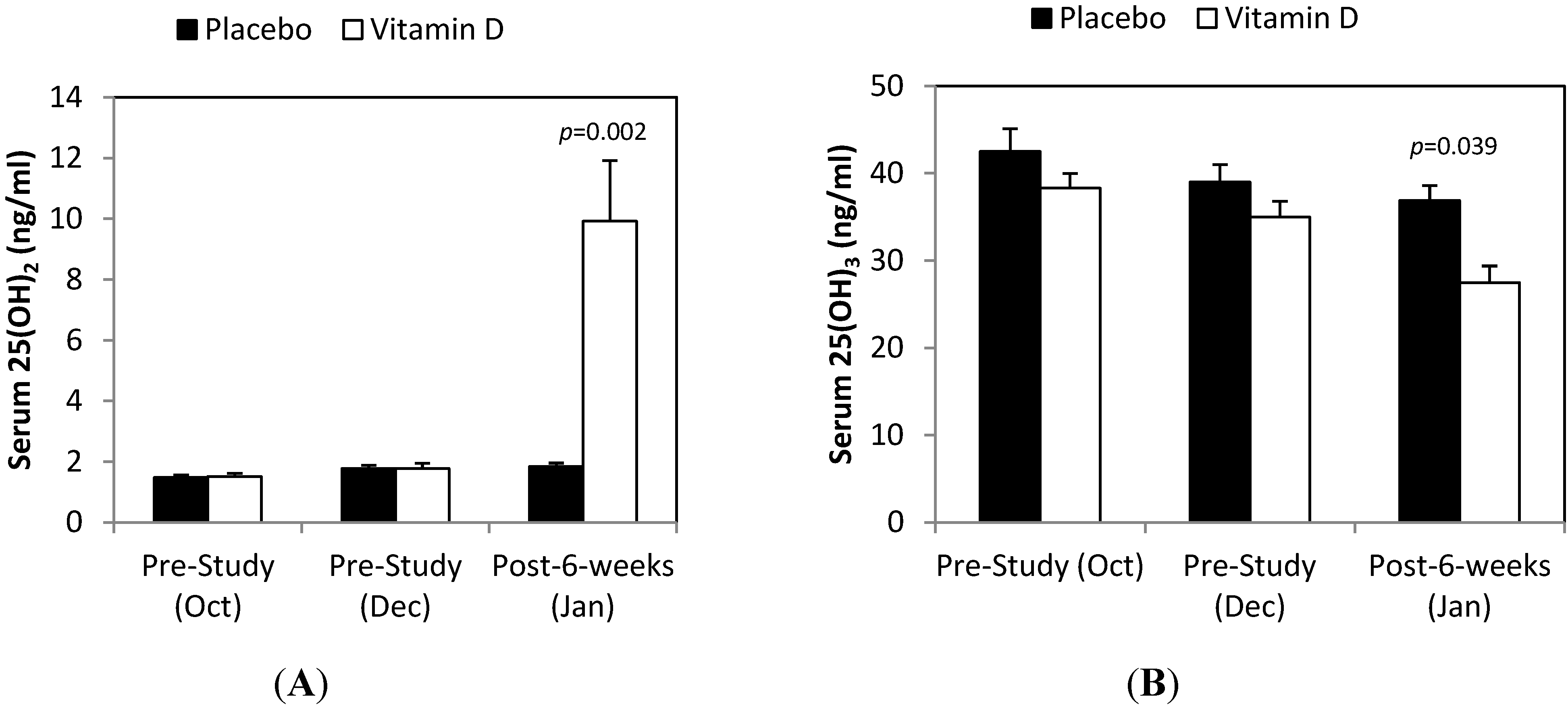
3. Results
| Variable | Placebo (n = 15) | Vitamin D (n = 13) | p-Value |
|---|---|---|---|
| Age (years) | 27.3 ± 0.9 | 27.1 ± 1.5 | 0.880 |
| Height (m) | 1.86 ± 0.02 | 1.85 ± 0.02 | 0.792 |
| Body mass (kg) | 97.7 ± 3.7 | 102 ± 5.8 | 0.486 |
| Body fat (%) | 13.8 ± 0.9 | 14.8 ± 1.5 | 0.534 |
| Variable | Placebo (N = 15) | Vitamin D (N = 13) | Interaction Effect p-Value |
|---|---|---|---|
| Leg-Back Dynamometer (kg) | |||
| Pre-Study | 187 ± 6.4 | 190 ± 6.1 | 0.133 |
| 6-weeks | 218 ± 8.6 | 200 ± 5.7 | |
| Hand Grip Dynamometer (kg) | |||
| Pre-Study | 47.4 ± 2.1 | 50.2 ± 2.0 | 0.208 |
| 6-weeks | 48.3 ± 2.4 | 53.7 ± 2.1 | |
| Bench Press (reps, body mass) | |||
| Pre-Study | 17.4 ± 1.6 | 15.7 ± 1.4 | 0.083 |
| 6-weeks | 16.5 ± 1.5 | 17.3 ± 1.4 | |
| Vertical Jump (inches) | |||
| Pre-Study | 29.3 ± 0.6 | 29.8 ± 1.2 | 0.286 |
| 6-weeks | 29.2 ± 0.5 | 30.4 ± 1.3 | |
| Wingate, Peak Power (W/kg) | |||
| Pre-Study | 16.6 ± 0.6 | 15.7 ± 0.9 | 0.967 |
| 6-weeks | 16.7 ± 0.7 | 15.8 ± 1.1 | |
| Wingate, Anaerobic Capacity (W/kg) | |||
| Pre-Study | 9.11 ± 0.2 | 8.71 ± 0.3 | 0.723 |
| 6-weeks | 9.01 ± 0.2 | 8.56 ± 0.3 |
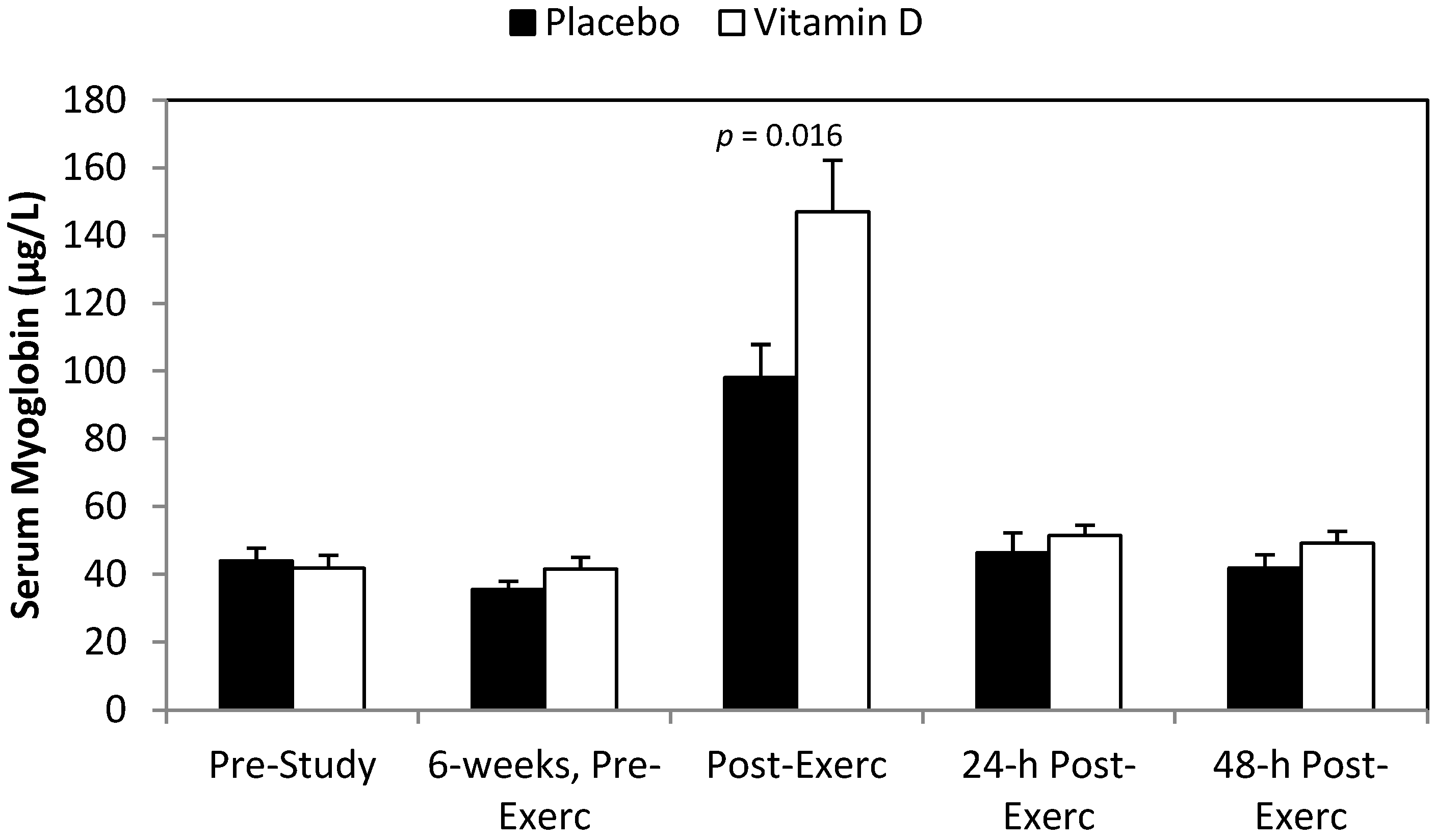
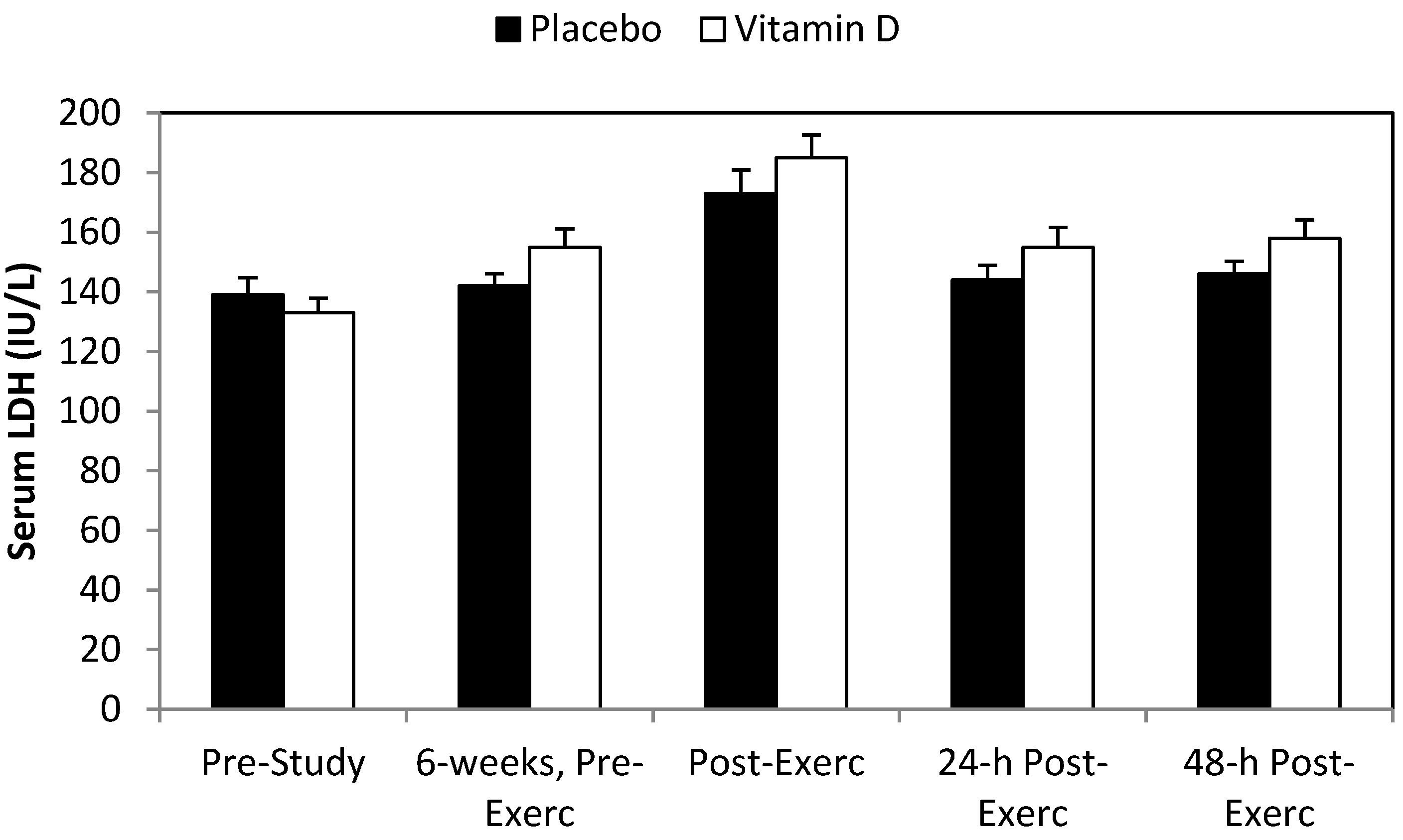

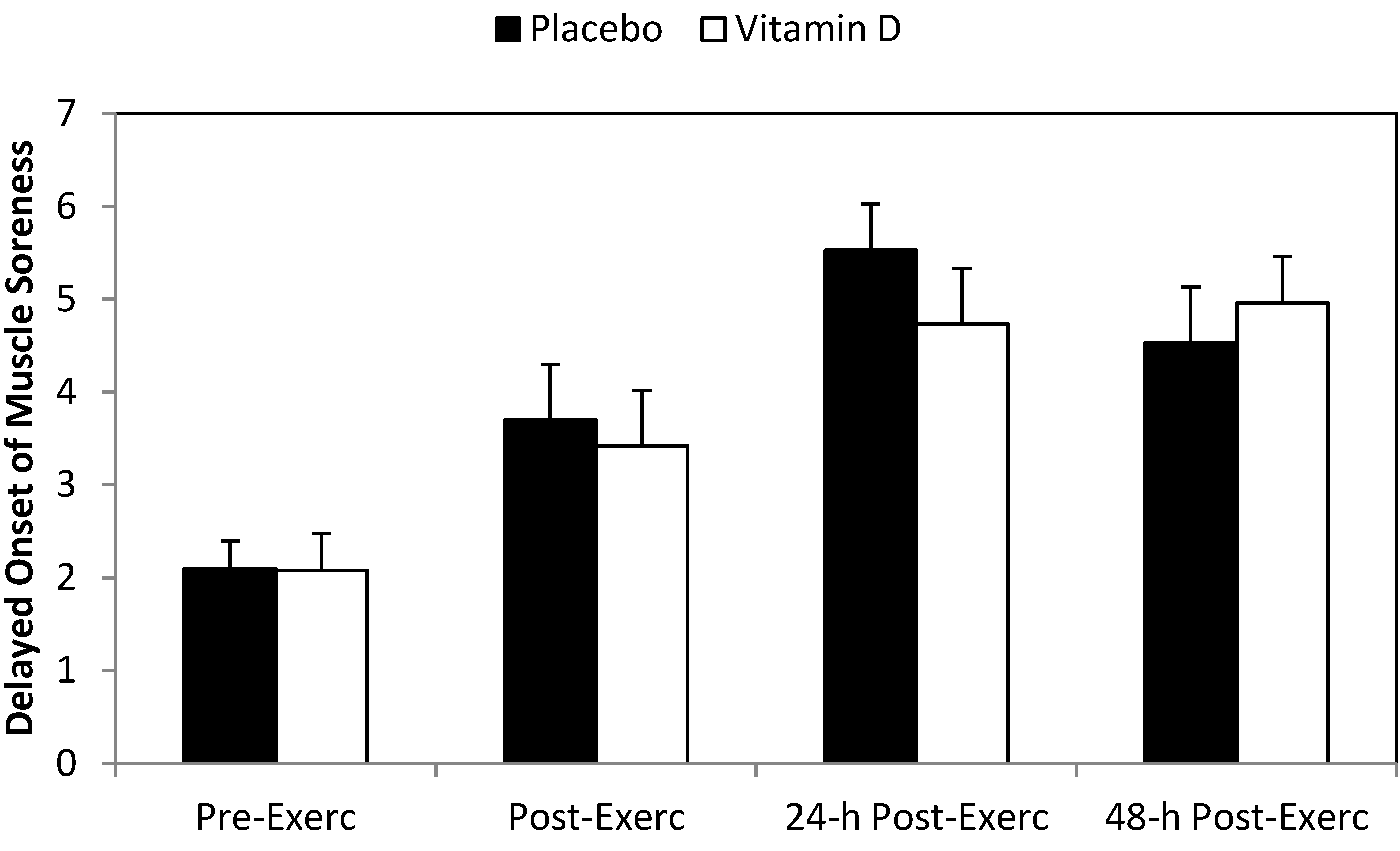
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A. Relevance of vitamin D in muscle health. Rev. Endocrine Metab. Dis. 2012, 13, 71–77. [Google Scholar] [CrossRef]
- Ginde, A.A.; Wolfe, P.; Camargo, C.A., Jr.; Schwartz, R.S. Defining vitamin D status by secondary hyperparathyroidism in the U.S. population. J. Endocrinol. Investig. 2012, 35, 42–48. [Google Scholar]
- Cannell, J.J.; Hollis, B.W.; Sorenson, M.B.; Taft, T.N.; Anderson, J.J. Athletic performance and vitamin D. Med. Sci. Sports Exerc. 2009, 41, 1102–1110. [Google Scholar] [CrossRef]
- Close, G.L.; Russell, J.; Cobley, J.N.; Owens, D.J.; Wilson, G.; Gregson, W.; Fraser, W.D.; Morton, J.P. Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: Implications for skeletal muscle function. J. Sports Sci. 2013, 31, 344–353. [Google Scholar] [CrossRef]
- Constantini, N.W.; Arieli, R.; Chodick, G.; Dubnov-Raz, G. High prevalence of vitamin D insufficiency in athletes and dancers. Clin. J. Sports Med. 2010, 20, 368–371. [Google Scholar]
- Halliday, T.M.; Peterson, N.J.; Thomas, J.J.; Kleppinger, K.; Hollis, B.W.; Larson-Meyer, D.E. Vitamin D status relative to diet, lifestyle, injury, and illness in college athletes. Med. Sci. Sports Exerc. 2011, 43, 335–343. [Google Scholar]
- Magee, P.J.; Pourshahidi, L.K.; Wallace, J. M.W.; Cleary, J.; Conway, J.; Harney, E.; Madigan, S.M. Vitamin D status and supplementation in elite Irish athletes. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 441–448. [Google Scholar]
- Gordon, P.L.; Sakkas, G.K.; Doyle, J.W.; Shubert, T.; Johansen, K.L. Relationship between vitamin D and muscle size and strength in patients on hemodialysis. J. Renal Nutr. 2007, 17, 397–407. [Google Scholar] [CrossRef]
- Jones, G. Extrarenal vitamin D activation and interactions between vitamin D2, vitamin D3, and vitamin D analogs. Ann. Rev. Nutr. 2013, 33, 23–44. [Google Scholar] [CrossRef]
- Sato, Y.; Iwamoto, J.; Kanoko, T.; Satoh, K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: A randomized controlled trial. Cerebrovasc. Dis. 2005, 20, 187–192. [Google Scholar] [CrossRef]
- Annweiler, C.; Schott, A.M.; Berrut, G.; Fantino, B.; Beauchet, O. Vitamin D-related changes in physical performance: A systematic review. J. Nutr. Health Aging 2009, 13, 893–898. [Google Scholar] [CrossRef]
- Ardestani, A.; Parker, B.; Mathur, S.; Clarkson, P.; Pescatello, L.S.; Hoffman, H.J.; Polk, D.M.; Thompson, P.D. Relation of vitamin D level to maximal oxygen uptake in adults. Am. J. Cardiol. 2011, 107, 1246–1249. [Google Scholar] [CrossRef]
- Muir, S.W.; Montero-Odasso, M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2011, 59, 2291–2300. [Google Scholar] [CrossRef]
- Stockton, K.A.; Mengersen, K.; Paratz, J.D.; Kandiah, D.; Bennell, K.L. Effect of vitamin D supplementation on muscle strength: A systematic review and meta-analysis. Osteoporos. Int. 2011, 22, 859–871. [Google Scholar]
- Toffanello, E.D.; Perissinotto, E.; Sergi, G.; Zambon, S.; Musacchio, E.; Maggi, S.; Coin, A.; Sartori, L.; Corti, M.C.; Baggio, G.; et al. Vitamin D and physical performance in elderly subjects: The Pro.V.V.A study. PLoS One 2012, 7, e34950. [Google Scholar] [CrossRef]
- Barker, T.; Henriksen, V.T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Schneider, E.D.; Dixon, B.M.; Weaver, L.K. Higher serum 25-hydroxyvitamin D concentrations associate with a faster recovery of skeletal muscle strength after muscular injury. Nutrients 2013, 17, 1253–1275. [Google Scholar]
- Grimaldi, A.S.; Parker, B.A.; Capizzi, J.A.; Clarkson, P.M.; Pescatello, L.S.; White, M.C.; Thompson, P.D. 25(OH) Vitamin D is associated with greater muscle strength in healthy men and women. Med. Sci. Sports Exerc. 2013, 45, 157–162. [Google Scholar] [CrossRef]
- Janssen, H.C.; Emmelot-Vonk, M.H.; Verhaar, H.J.; van der Schouw, Y.T. Vitamin D and muscle function: Is there a threshold in the relation? J. Am. Med. Dir. Assoc. 2013, 14, 627.e13–627.e18. [Google Scholar]
- Moran, D.S.; McClung, J.P.; Kohen, T.; Lieberman, H.R. Vitamin D and physical performance. Sports Med. 2013, 43, 601–611. [Google Scholar] [CrossRef]
- Wyon, M.A.; Koutedakis, Y.; Wolman, R.; Nevill, A.M.; Allen, N. The influence of winter vitamin D supplementation on muscle function and injury occurrence in elite ballet dancers: A controlled study. J. Sci. Med. Sport 2013, 17, 8–12. [Google Scholar]
- Choi, M.; Park, H.; Cho, S.; Lee, M. Vitamin D3 supplementation modulates inflammatory responses from the muscle damage induced by high-intensity exercise in SD rats. Cytokine 2013, 63, 27–35. [Google Scholar] [CrossRef]
- Smith, L.L.; Brunetz, M.H.; Chenier, T.C.; McCammon, M.R.; Houmard, J.A.; Franklin, M.E.; Israel, R.G. The effects of static and ballistic stretching on delayed onset muscle soreness and creatine kinase. Res. Q. Exerc. Sport 1993, 64, 103–107. [Google Scholar] [CrossRef]
- Shanely, R.A.; Nieman, D.C.; Knab, A.M.; Gillitt, N.D.; Meaney, M.P.; Jin, F.; Sha, W.; Cialdella-Kam, L. Influence of vitamin D mushroom powder supplementation on exercise-induced muscle damage in vitamin D insufficient high school athletes. J. Sport Sci. 2013, in press. [Google Scholar]
- Huang, M.; Winters, D. Application of ultra-performance liquid chromatography/tandem mass spectrometry for the measurement of vitamin D in foods and nutritional supplements. J. AOAC Int. 2011, 94, 211–223. [Google Scholar]
- Calton, L.J.; Molloy, B.J.; Keevil, B.G.; Cooper, D.P. The analysis of 25-hydroxyvitamin D in serum using semi-automated solid phase extraction and LC/MS/MS. Clin. Chem. 2009, 55, A187–A188. [Google Scholar]
- Urbain, P.; Singler, F.; Ihorst, G.; Biesalski, H.K.; Bertz, H. Bioavailability of vitamin D2 from UV-B-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D: A randomized controlled trial. Eur. J. Clin. Nutr. 2011, 65, 965–971. [Google Scholar] [CrossRef]
- Houghton, L.A.; Vieth, R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am. J. Clin. Nutr. 2006, 84, 694–697. [Google Scholar]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef]
- Biancuzzo, R.M.; Clarke, N.; Reitz, R.E.; Travison, T.G.; Holick, M.F. Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation. J. Clin. Endocrinol. Metab. 2013, 98, 973–979. [Google Scholar] [CrossRef]
- Logan, V.F.; Gray, A.R.; Peddie, M.C.; Harper, M.J.; Houghton, L.A. Long-term vitamin D3 supplementation is more effective than vitamin D2 in maintaining serum 25-hydroxyvitamin D status over the winter months. Br. J. Nutr. 2013, 109, 1082–1088. [Google Scholar] [CrossRef]
- Stephensen, C.B.; Zerofsky, M.; Burnett, D.J.; Lin, Y.P.; Hammock, B.D.; Hall, L.M.; McHugh, T. Ergocalciferol from mushrooms or supplements consumed with a standard meal increases 25-hydroxyergocalciferol but decreases 25-hydroxycholecalciferol in the serum of healthy adults. J. Nutr. 2012, 142, 1246–1252. [Google Scholar] [CrossRef]
- Stratos, I.; Li, Z.; Herlyn, P.; Rotter, R.; Behrendt, A.K.; Mittlmeier, T.; Vollmar, B. Vitamin D increases cellular turnover and functionally restores the skeletal muscle after crush injury in rats. Am. J. Pathol. 2013, 182, 895–904. [Google Scholar] [CrossRef]
- Close, G.L.; Leckey, J.; Patterson, M.; Bradley, W.; Owens, D.J.; Fraser, W.D.; Morton, J.P. The effects of vitamin D3 supplementation on serum total 25[OH]D concentration and physical performance: A randomized dose-response study. Br. J. Sports Med. 2013, 47, 692–696. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nieman, D.C.; Gillitt, N.D.; Shanely, R.A.; Dew, D.; Meaney, M.P.; Luo, B. Vitamin D2 Supplementation Amplifies Eccentric Exercise-Induced Muscle Damage in NASCAR Pit Crew Athletes. Nutrients 2014, 6, 63-75. https://doi.org/10.3390/nu6010063
Nieman DC, Gillitt ND, Shanely RA, Dew D, Meaney MP, Luo B. Vitamin D2 Supplementation Amplifies Eccentric Exercise-Induced Muscle Damage in NASCAR Pit Crew Athletes. Nutrients. 2014; 6(1):63-75. https://doi.org/10.3390/nu6010063
Chicago/Turabian StyleNieman, David C., Nicholas D. Gillitt, R. Andrew Shanely, Dustin Dew, Mary Pat Meaney, and Beibei Luo. 2014. "Vitamin D2 Supplementation Amplifies Eccentric Exercise-Induced Muscle Damage in NASCAR Pit Crew Athletes" Nutrients 6, no. 1: 63-75. https://doi.org/10.3390/nu6010063
APA StyleNieman, D. C., Gillitt, N. D., Shanely, R. A., Dew, D., Meaney, M. P., & Luo, B. (2014). Vitamin D2 Supplementation Amplifies Eccentric Exercise-Induced Muscle Damage in NASCAR Pit Crew Athletes. Nutrients, 6(1), 63-75. https://doi.org/10.3390/nu6010063








