Effects of cis-9,trans-11 and trans-10,cis-12 Conjugated Linoleic Acid, Linoleic Acid, Phytanic Acid and the Combination of Various Fatty Acids on Proliferation and Cytokine Expression of Bovine Peripheral Blood Mononuclear Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Cell Culture Conditions and Cell Proliferation Assays
2.3. RNA Isolation and cDNA Synthesis
2.4. Quantitative Real Time PCR
| Gene | Forward (for) and reverse (rev) primer sequences (5′→3′) | concentration (nM) | Bp a | Reference |
|---|---|---|---|---|
| IL-4 | for GCC ACA CGT GCT TGA ACA AA | 900 | 63 | [24] |
| rev TGC TTG CCA AGC TGT TGA GA | 50 | |||
| IL-10 | for CCT TGT CGG AAA TGA TCC AGT TTT | 300 | 67 | [24] |
| rev TCA GGC CCG TGG TTC TCA | 300 | |||
| IL-12 | for TGG TCG TTT CCT GGT TTT CC | 300 | 205 | novel design (Accession No. NM 174356.1) |
| rev GTT TTG CCA GAG CCC AAG AC | 300 | |||
| INF-γ | for TTC AGA GCC AAA TTG TCT CCT TC | 300 | 205 | [25] modified form |
| rev AGT TCA TTT ATG GCT TTG CGC TG | 50 | |||
| TNF-α | for CTT CTG CCT GCT GCA CTT CG | 300 | 156 | [26] |
| rev GAG TTG ATG TCG GCT ACA ACG | 300 | |||
| PPAR-γ | for AAG AAT ATC CCC GGC TTT GT | 300 | 200 | novel design (Accession No. NM 181024) |
| rev TTG GGC TCC ATA AAG TCA CC | 300 |
2.5. Calculations and Statistics
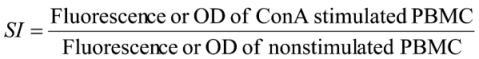
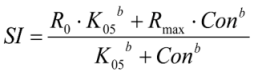
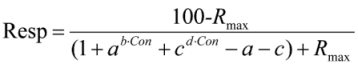
 ), linoleic acid (
), linoleic acid (  ), cis-9,trans-11 (
), cis-9,trans-11 (  ), trans-10,cis-12 (
), trans-10,cis-12 (  ) and phytanic acid (□) on concanavalin A stimulated proliferation of bovine peripheral blood mononuclear cells (n = 3) in the alamar blue assay (means ± standard deviation). * containing 29.8% palmitic acid, 6.7% palmitoleic acid, 17.4% stearic acid and 46.1% oleic acid according to Rukkwamsuk et al. [22]. a–d: different letters indicate significant differences within the same fatty acid, *, #, $ indicate significant differences between fatty acids at the same concentration, p < 0.05, Tukey test.
) and phytanic acid (□) on concanavalin A stimulated proliferation of bovine peripheral blood mononuclear cells (n = 3) in the alamar blue assay (means ± standard deviation). * containing 29.8% palmitic acid, 6.7% palmitoleic acid, 17.4% stearic acid and 46.1% oleic acid according to Rukkwamsuk et al. [22]. a–d: different letters indicate significant differences within the same fatty acid, *, #, $ indicate significant differences between fatty acids at the same concentration, p < 0.05, Tukey test.
 ), linoleic acid (
), linoleic acid (  ), cis-9,trans-11 (
), cis-9,trans-11 (  ), trans-10,cis-12 (
), trans-10,cis-12 (  ) and phytanic acid (□) on concanavalin A stimulated proliferation of bovine peripheral blood mononuclear cells (n = 3) in the alamar blue assay (means ± standard deviation). * containing 29.8% palmitic acid, 6.7% palmitoleic acid, 17.4% stearic acid and 46.1% oleic acid according to Rukkwamsuk et al. [22]. a–d: different letters indicate significant differences within the same fatty acid, *, #, $ indicate significant differences between fatty acids at the same concentration, p < 0.05, Tukey test.
) and phytanic acid (□) on concanavalin A stimulated proliferation of bovine peripheral blood mononuclear cells (n = 3) in the alamar blue assay (means ± standard deviation). * containing 29.8% palmitic acid, 6.7% palmitoleic acid, 17.4% stearic acid and 46.1% oleic acid according to Rukkwamsuk et al. [22]. a–d: different letters indicate significant differences within the same fatty acid, *, #, $ indicate significant differences between fatty acids at the same concentration, p < 0.05, Tukey test.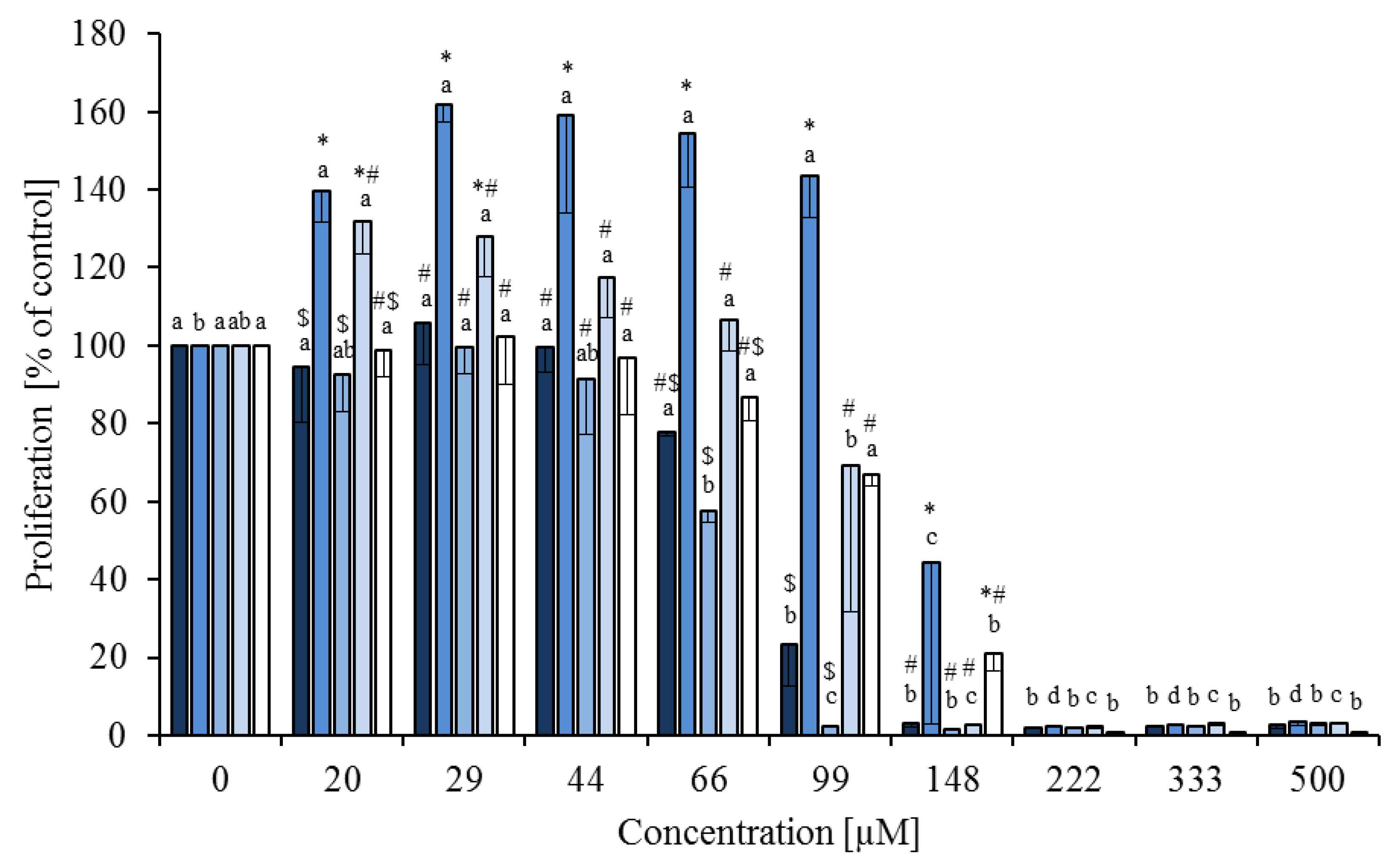
3. Results
3.1. Dose Response Studies
 ), linoleic acid (
), linoleic acid (  ), cis-9,trans-11 (
), cis-9,trans-11 (  ), trans-10,cis-12 (
), trans-10,cis-12 (  ) and phytanic acid (□) on concanavalin A stimulated proliferation of bovine peripheral blood mononuclear cells (n = 3) in the BrdU assay (means ± standard deviation). * containing 29.8% palmitic acid, 6.7% palmitoleic acid, 17.4% stearic acid and 46.1% oleic acid according to Rukkwamsuk et al. [22]. a–c: different letters indicate significant differences within the same fatty acid, *, #, $ indicate significant differences between fatty acids at the same concentration, p < 0.05, Tukey test
) and phytanic acid (□) on concanavalin A stimulated proliferation of bovine peripheral blood mononuclear cells (n = 3) in the BrdU assay (means ± standard deviation). * containing 29.8% palmitic acid, 6.7% palmitoleic acid, 17.4% stearic acid and 46.1% oleic acid according to Rukkwamsuk et al. [22]. a–c: different letters indicate significant differences within the same fatty acid, *, #, $ indicate significant differences between fatty acids at the same concentration, p < 0.05, Tukey test
 ), linoleic acid (
), linoleic acid (  ), cis-9,trans-11 (
), cis-9,trans-11 (  ), trans-10,cis-12 (
), trans-10,cis-12 (  ) and phytanic acid (□) on concanavalin A stimulated proliferation of bovine peripheral blood mononuclear cells (n = 3) in the BrdU assay (means ± standard deviation). * containing 29.8% palmitic acid, 6.7% palmitoleic acid, 17.4% stearic acid and 46.1% oleic acid according to Rukkwamsuk et al. [22]. a–c: different letters indicate significant differences within the same fatty acid, *, #, $ indicate significant differences between fatty acids at the same concentration, p < 0.05, Tukey test
) and phytanic acid (□) on concanavalin A stimulated proliferation of bovine peripheral blood mononuclear cells (n = 3) in the BrdU assay (means ± standard deviation). * containing 29.8% palmitic acid, 6.7% palmitoleic acid, 17.4% stearic acid and 46.1% oleic acid according to Rukkwamsuk et al. [22]. a–c: different letters indicate significant differences within the same fatty acid, *, #, $ indicate significant differences between fatty acids at the same concentration, p < 0.05, Tukey test
3.2. Fatty Acid Combinations
 ), cis-9,trans-11 (
), cis-9,trans-11 (  ) or trans-10,cis-12 (
) or trans-10,cis-12 (  ) or all 3 of them (each 13.3%,
) or all 3 of them (each 13.3%,  ) on concanavalin A stimulated proliferation of bovine peripheral blood mononuclear cells (PBMC) (n = 3) in the alamar blue assay (means ± standard deviation). * containing 29.8% palmitic acid, 6.7% palmitoleic acid, 17.4% stearic acid and 46.1% oleic acid according to Rukkwamsuk et al. [22]. Med-Con = PBMC incubated without fatty acids and DMSO. a–c: different letters indicate significant differences within the same fatty acid, p < 0.05, Tukey test.
) on concanavalin A stimulated proliferation of bovine peripheral blood mononuclear cells (PBMC) (n = 3) in the alamar blue assay (means ± standard deviation). * containing 29.8% palmitic acid, 6.7% palmitoleic acid, 17.4% stearic acid and 46.1% oleic acid according to Rukkwamsuk et al. [22]. Med-Con = PBMC incubated without fatty acids and DMSO. a–c: different letters indicate significant differences within the same fatty acid, p < 0.05, Tukey test.
 ), cis-9,trans-11 (
), cis-9,trans-11 (  ) or trans-10,cis-12 (
) or trans-10,cis-12 (  ) or all 3 of them (each 13.3%,
) or all 3 of them (each 13.3%,  ) on concanavalin A stimulated proliferation of bovine peripheral blood mononuclear cells (PBMC) (n = 3) in the alamar blue assay (means ± standard deviation). * containing 29.8% palmitic acid, 6.7% palmitoleic acid, 17.4% stearic acid and 46.1% oleic acid according to Rukkwamsuk et al. [22]. Med-Con = PBMC incubated without fatty acids and DMSO. a–c: different letters indicate significant differences within the same fatty acid, p < 0.05, Tukey test.
) on concanavalin A stimulated proliferation of bovine peripheral blood mononuclear cells (PBMC) (n = 3) in the alamar blue assay (means ± standard deviation). * containing 29.8% palmitic acid, 6.7% palmitoleic acid, 17.4% stearic acid and 46.1% oleic acid according to Rukkwamsuk et al. [22]. Med-Con = PBMC incubated without fatty acids and DMSO. a–c: different letters indicate significant differences within the same fatty acid, p < 0.05, Tukey test.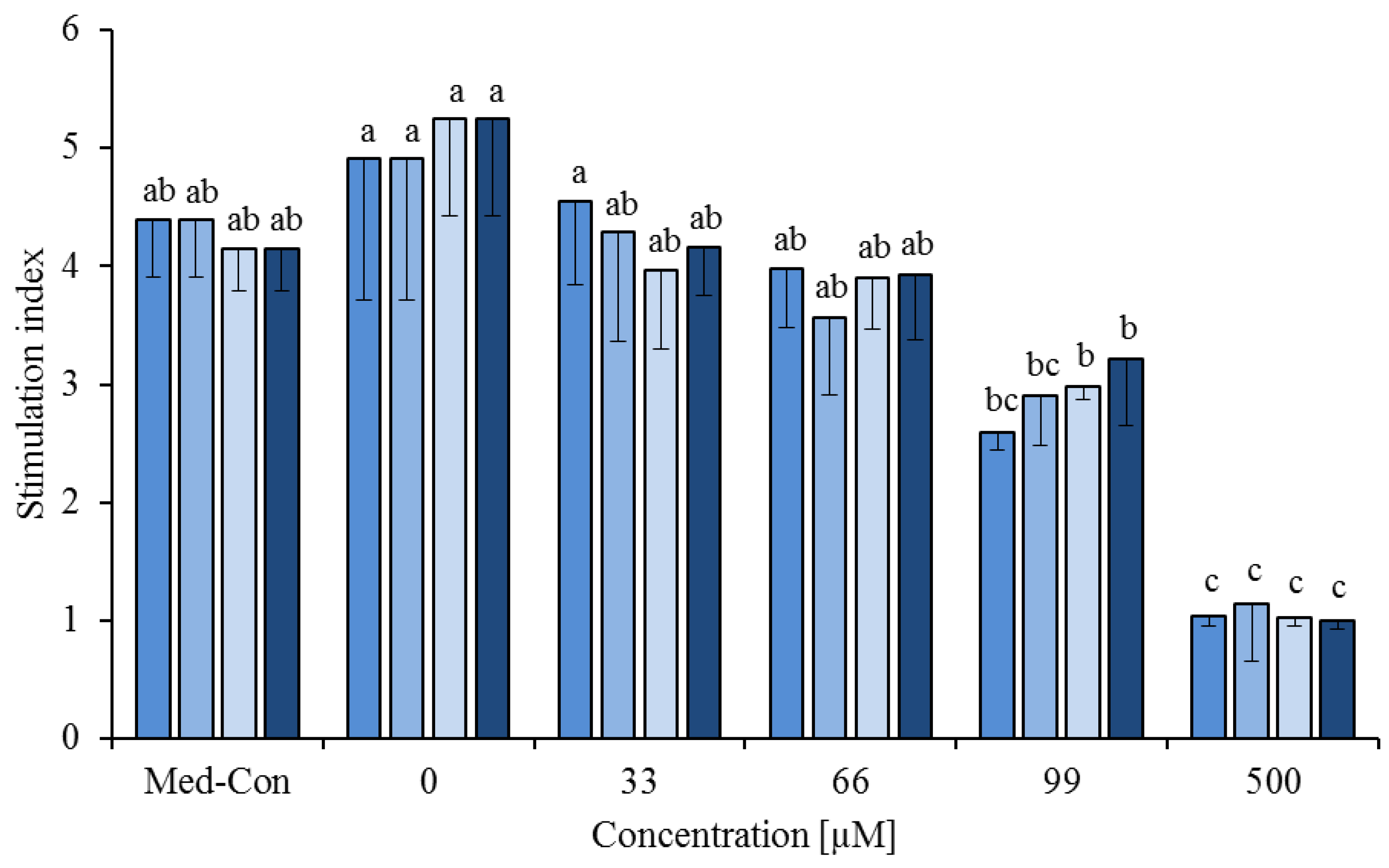
 ) or phytanic acid (20%) and trans-10,cis-12 (20%,
) or phytanic acid (20%) and trans-10,cis-12 (20%,  ) on concanavalin A stimulated proliferation of bovine peripheral blood mononuclear cells (PBMC) (n = 3) in the alamar blue assay (means ± standard deviation). * containing 29.8% palmitic acid, 6.7% palmitoleic acid, 17.4% stearic acid and 46.1% oleic acid according to Rukkwamsuk et al. [22]. Med-Con = PBMC incubated without fatty acids and DMSO. a–c: different letters indicate significant differences within the same fatty acid, p < 0.05, Tukey test.
) on concanavalin A stimulated proliferation of bovine peripheral blood mononuclear cells (PBMC) (n = 3) in the alamar blue assay (means ± standard deviation). * containing 29.8% palmitic acid, 6.7% palmitoleic acid, 17.4% stearic acid and 46.1% oleic acid according to Rukkwamsuk et al. [22]. Med-Con = PBMC incubated without fatty acids and DMSO. a–c: different letters indicate significant differences within the same fatty acid, p < 0.05, Tukey test.
 ) or phytanic acid (20%) and trans-10,cis-12 (20%,
) or phytanic acid (20%) and trans-10,cis-12 (20%,  ) on concanavalin A stimulated proliferation of bovine peripheral blood mononuclear cells (PBMC) (n = 3) in the alamar blue assay (means ± standard deviation). * containing 29.8% palmitic acid, 6.7% palmitoleic acid, 17.4% stearic acid and 46.1% oleic acid according to Rukkwamsuk et al. [22]. Med-Con = PBMC incubated without fatty acids and DMSO. a–c: different letters indicate significant differences within the same fatty acid, p < 0.05, Tukey test.
) on concanavalin A stimulated proliferation of bovine peripheral blood mononuclear cells (PBMC) (n = 3) in the alamar blue assay (means ± standard deviation). * containing 29.8% palmitic acid, 6.7% palmitoleic acid, 17.4% stearic acid and 46.1% oleic acid according to Rukkwamsuk et al. [22]. Med-Con = PBMC incubated without fatty acids and DMSO. a–c: different letters indicate significant differences within the same fatty acid, p < 0.05, Tukey test.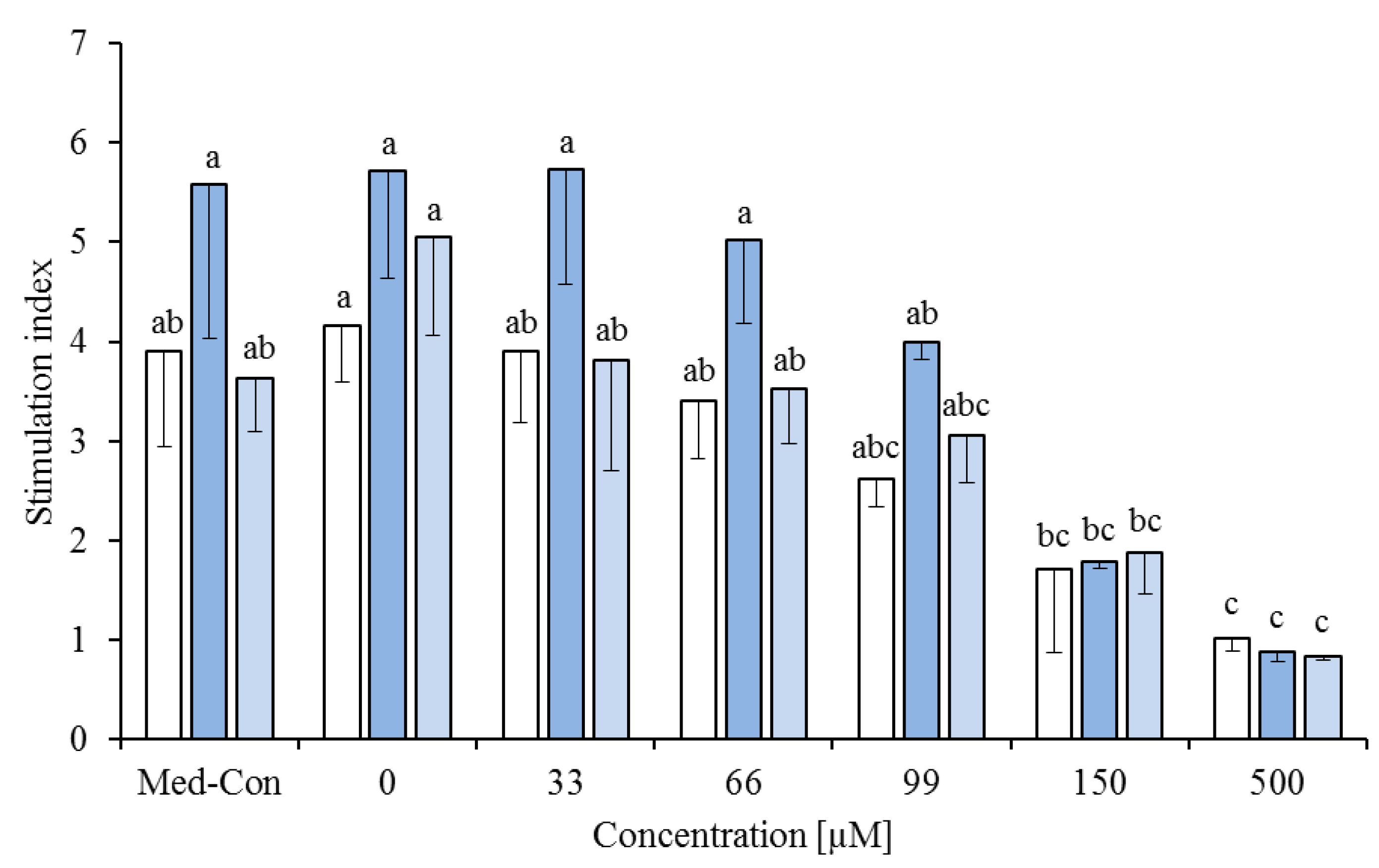
3.3. Cytokine and PPAR-γ mRNA Expression
| Treatment | ConA | IL-4 | IL-10 | TNF-α | IFN-γ | PPAR-γ |
|---|---|---|---|---|---|---|
| DMSO | – | 26,852 ± 3032 | 6201±1083 | 1039 ± 419 | 5181 ± 5759 | 4881 ± 790 |
| + | 22,984 ± 4567 | 3808 ± 1765 | 4969 ± 1640 | 109,082 ± 56,582 a | 3514 ± 1022 a,b | |
| Medium | – | 28,825 ± 5887 | 6700 ± 1848 | 900 ± 237 | 3617 ± 3273 | 4978 ± 565 |
| + | 26,714 ± 3416 | 3719 ± 827 * | 8509 ± 4635 * | 295,989 ±167,635 b,* | 3907 ± 694 a,b | |
| IC50 LA | – | 23,941 ± 4335 | 5312 ± 994 | 370 ± 145 | 613 ± 570 | 4019 ± 739 |
| + | 34,703 ± 7345 | 4146 ± 1362 | 10,194 ± 5630 * | 242,302 ± 126,184 a,b,* | 3454 ± 365 a,b | |
| 77 µM LA | – | 25,845 ± 1695 | 5635 ± 401 | 506 ± 179 | 1809 ± 1570 | 4547 ± 467 |
| + | 28,348 ± 15,180 | 3520 ± 1019 | 9683 ± 7214 | 204,490 ± 159,761 a,b | 2820 ± 635 a | |
| IC50 cis-9,trans-11 | – | 32,230 ± 4300 | 7245 ± 866 | 588 ± 87 | 743 ± 648 | 5507 ± 1203 |
| + | 32,640 ± 1566 | 5504 ± 1085 | 8957 ± 4128 | 97,534 ± 16,261 a,b | 4641 ± 859 a,b | |
| 77 µM cis-9,trans-11 | – | 29,684 ± 3774 | 6917 ± 617 | 511 ± 58 | 430 ± 319 | 5254 ± 712 |
| + | 31,867 ± 7269 | 5252 ± 349 | 8046 ± 4588 | 68,327 ± 28,935 a | 5288 ± 439 b | |
| p-value | 0.182 | <0.001 | <0.001 | <0.001 | <0.001 |
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Kepler, C.R.; Hirons, K.P.; McNeill, J.J.; Tove, S.B. Intermediates and products of the biohydrogenation of linoleic acid by Butyrinvibrio fibrisolvens. J. Biol. Chem. 1966, 241, 1350–1354. [Google Scholar]
- Fritsche, J.; Steinhart, H. Amounts of conjugated linoleic acid (CLA) in German foods and evaluation of daily intake. Z. Lebensm. Unters. F. A 1998, 206, 77–82. [Google Scholar] [CrossRef]
- Kay, J.K.; Roche, J.R.; Kolver, E.S.; Thomson, N.A.; Baumgard, L.H. A comparison between feeding systems (pasture and TMR) and the effect of vitamin E supplementation on plasma and milk fatty acid profiles in dairy cows. J. Dairy Res. 2005, 72, 322–332. [Google Scholar] [CrossRef]
- Griinari, J.M.; Corl, B.A.; Lacy, S.H.; Chouinard, P.Y.; Nurmela, K.V.; Bauman, D.E. Conjugated linoleic acid is synthesized endogenously in lactating dairy cows by Delta(9)-desaturase. J. Nutr. 2000, 130, 2285–2291. [Google Scholar]
- Turpeinen, A.M.; Mutanen, M.; Aro, A.; Salminen, I.; Basu, S.; Palmquist, D.L.; Griinari, J.M. Bioconversion of vaccenic acid to conjugated linoleic acid in humans. Am. J. Clin. Nutr. 2002, 76, 504–510. [Google Scholar]
- Baumgard, L.H.; Corl, B.A.; Dwyer, D.A.; Saebo, A.; Bauman, D.E. Identification of the conjugated linoleic acid isomer that inhibits milk fat synthesis. Am. J. Physiol. 2000, 278, R179–R184. [Google Scholar]
- Renner, L.; Pappritz, J.; Kramer, R.; Döll, S.; Jahreis, G.; Dänicke, S. Fatty acid profile and proliferation of bovine blood mononuclear cells after conjugated linoleic acid supplementation. Lipids Health Dis. 2012, 11, 63. [Google Scholar]
- Renner, L.; von Soosten, D.; Sipka, A.; Döll, S.; Beineke, A.; Schuberth, H.J.; Dänicke, S. Effect of trans-10,cis-12 and cis-9,trans-11 conjugated linoleic acid (CLA) on proliferation and cytokine expression of bovine PBMC and splenocytes ex vivo. Arch. Anim. Nutr. 2012, 66, 73–85. [Google Scholar] [CrossRef]
- Hellgren, L.I. Phytanic acid––An overlooked bioactive fatty acid in dairy fat? Ann. N. Y. Acad. Sci. 2010, 1190, 42–49. [Google Scholar] [CrossRef]
- Verhoeven, N.M.; Jakobs, C. Human metabolism of phytanic acid and pristanic acid. Prog. Lipid Res. 2001, 40, 453–466. [Google Scholar] [CrossRef]
- Patton, S.; Benson, A.A. Phytol metabolism in the bovine. Biochim. Biophys. Acta 1966, 125, 22–32. [Google Scholar] [CrossRef]
- Avigan, J. The presence of phytanic acid in normal human and animal plasma. Biochim. Biophys. Acta 1966, 116, 391–394. [Google Scholar] [CrossRef]
- Thompson, G.E.; Christie, W.W. Extraction of plasma triacylglycerols by the mammary gland of the lactating cow. J. Dairy Res. 1991, 58, 251–255. [Google Scholar] [CrossRef]
- Price, A.J.; Allen, N.E.; Appleby, P.N.; Crowe, F.L.; Jenab, M.; Rinaldi, S.; Slimani, N.; Kaaks, R.; Rohrmann, S.; Boeing, H.; et al. Plasma phytanic acid concentration and risk of prostate cancer: Results from the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2010, 91, 1769–1776. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Komen, J.; Ferdinandusse, S. Phytanic acid metabolism in health and disease. Biochim. Biophys. Acta 2011, 1811, 498–507. [Google Scholar] [CrossRef]
- Kitareewan, S.; Burka, L.T.; Tomer, K.B.; Parker, C.E.; Deterding, L.J.; Stevens, R.D.; Forman, B.M.; Mais, D.E.; Heyman, R.A.; McMorris, T.; et al. Phytol metabolites are circulating dietary factors that activate the nuclear receptor RXR. Mol. Biol. Cell 1996, 7, 1153–1166. [Google Scholar]
- Ellinghaus, P.; Wolfrum, C.; Assmann, G.; Spener, F.; Seedorf, U. Phytanic acid activates the peroxisome proliferator-activated receptor α (PPARα) in sterol carrier protein 2-/sterol carrier protein x-deficient mice. J. Biol. Chem. 1999, 274, 2766–2772. [Google Scholar]
- Moya-Camarena, S.Y.; Vanden Heuvel, J.P.; Blanchard, S.G.; Leesnitzer, L.A.; Belury, M.A. Conjugated linoleic acid is a potent naturally occurring ligand and activator of PPAR alpha. J. Lipid Res. 1999, 40, 1426–1433. [Google Scholar]
- McCarty, M.F. The chlorophyll metabolite phytanic acid is a natural rexinoid––Potential for treatment and prevention of diabetes. Med. Hypotheses 2001, 56, 217–219. [Google Scholar] [CrossRef]
- Renner, L.; Schwabe, A.; Döll, S.; Höltershinken, M.; Dänicke, S. Effect of rare earth elements on beef cattle growth performance, blood clinical chemical parameters and mitogen stimulated proliferation of bovine peripheral blood mononuclear cells in vitro and ex vivo. Toxicol. Lett. 2011, 201, 277–284. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Rukkwamsuk, T.; Geelen, M.J.H.; Kruip, T.A.M.; Wensing, T. Interrelation of fatty acid composition in adipose tissue, serum, and liver of dairy cows during the development of fatty liver postpartum. J. Dairy Sci. 2000, 83, 52–59. [Google Scholar] [CrossRef]
- Goyarts, T.; Dänicke, S.; Grove, N.; Tiemann, U.; Rothkötter, H.J. Methodical aspects of in vitro proliferation of porcine blood lymphocytes when exposed to deoxynivalenol (DON). Landbauforsch. Völk. 2006, 56, 139–148. [Google Scholar]
- Almeida, P.E.; Weber, P.S.D.; Burton, J.L.; Tempelman, R.J.; Steibel, J.P.; Zanella, A.J. Gene expression profiling of peripheral mononuclear cells in lame dairy cows with foot lesions. Vet. Immunol. Immunopathol. 2007, 120, 234–245. [Google Scholar] [CrossRef]
- Neuvians, T.P.; Schams, D.; Berisha, B.; Pfaffl, M.W. Involvement of pro-inflammatory cytokines, mediators of inflammation, and basic fibroblast growth factor in prostaglandin F-2 alpha-induced luteolysis in bovine corpus luteum. Biol. Reprod. 2004, 70, 473–480. [Google Scholar] [CrossRef]
- Yang, W.; Zerbe, H.; Petzl, W.; Brunner, R.M.; Günther, J.; Draing, C.; von Aulock, S.; Schuberth, H.-J.; Seyfert, H.-M. Bovine TLR2 and TLR4 properly transduce signals from Staphylococcus aureus and E. coli, but S. aureus fails to both activate NF-κB in mammary epithelial cells and to quickly induce TNFα and interleukin-8 (CXCL8) expression in the udder. Mol. Immunol. 2008, 45, 1385–1397. [Google Scholar] [CrossRef]
- Mercer, L.P.; Dodds, S.J.; Smith, D.L. New method for formulation of amino acid concentrations and ratios in diets of rats. J. Nutr. 1987, 117, 1936–1944. [Google Scholar]
- Kehrli, M.E.; Harp, J.A. Immunity in the mammary gland. Vet. Clin. N. Am. 2001, 17, 495–516. [Google Scholar]
- Lacetera, N.; Scalia, D.; Franci, O.; Bernabucci, U.; Ronchi, B.; Nardone, A. Short communication: Effects of nonesterified fatty acids on lymphocyte function in dairy heifers. J. Dairy Sci. 2004, 87, 1012–1014. [Google Scholar] [CrossRef]
- Ster, C.; Loiselle, M.C.; Lacasse, P. Effect of postcalving serum nonesterified fatty acids concentration on the functionality of bovine immune cells. J. Dairy Sci. 2012, 95, 708–717. [Google Scholar] [CrossRef]
- Grummer, R.R.; Mashek, D.G.; Hayirli, A. Dry matter intake and energy balance in the transition period. Vet. Clin. N. Am. 2004, 20, 447–470. [Google Scholar]
- Goff, J.P.; Horst, R.L. Physiological changes at parturition and their relationship to metabolic disorders. J. Dairy Sci. 1997, 80, 1260–1268. [Google Scholar] [CrossRef]
- Contreras, G.A.; Sordillo, L.M. Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp. Immunol. Microb. Infect. Dis. 2011, 34, 281–289. [Google Scholar] [CrossRef]
- Gonzalez, R.J.; Tarloff, J.B. Evaluation of hepatic subcellular fractions for Alamar blue and MTT reductase activity. Toxicol. In Vitro 2001, 15, 257–259. [Google Scholar] [CrossRef]
- Lacetera, N.; Kuzminsky, G.; Morera, R.; Basirico, L. Fatty acids affect proliferation of peripheral blood mononuclear cells in dairy cows. Ital. J. Anim. Sci. 2007, 6, 434–436. [Google Scholar]
- Thanasak, J.; Muller, K.E.; Dieleman, S.J.; Hoek, A.; Noordhuizen, J.P.; Rutten, V.P. Effects of polyunsaturated fatty acids on the proliferation of mitogen stimulated bovine peripheral blood mononuclear cells. Vet. Immunol. Immunopathol. 2005, 104, 289–295. [Google Scholar] [CrossRef]
- Lacetera, N.; Franci, O.; Scalia, D.; Bernabucci, U.; Ronchi, B.; Nardone, A. Effects on functions of ovine blood mononuclear cells for each of several fatty acids at concentrations found in plasma of healthy and ketotic ewes. Am. J. Vet. Res. 2002, 63, 958–962. [Google Scholar] [CrossRef]
- Ma, D.W.L.; Field, C.J.; Clandinin, M.T. An enriched mixture of trans-10,cis-12-CLA inhibits linoleic acid metabolism and PGE2 synthesis in MDA-MB-231 cells. Nutr. Cancer 2002, 44, 203–212. [Google Scholar] [CrossRef]
- Luongo, D.; Bergamo, P.; Rossi, M. Effects of conjugated linoleic acid on growth and cytokine expression in Jurkat T cells. Immunol. Lett. 2003, 90, 195–201. [Google Scholar] [CrossRef]
- Igarashi, M.; Miyazawa, T. The growth inhibitory effect of conjugated linoleic acid on a human hepatoma cell line, HepG2, is induced by a change in fatty acid metabolism, but not the facilitation of lipid peroxidation in the cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2001, 1530, 162–171. [Google Scholar] [CrossRef]
- Lai, K.L.; Torres-Duarte, A.P.; Vanderhoek, J.Y. 9-trans,11-trans-CLA: Antiproliferative and proapoptotic effects on bovine endothelial cells. Lipids 2005, 40, 1107–1116. [Google Scholar] [CrossRef]
- Tang, X.H.; Suh, M.J.; Li, R.; Gudas, L.J. Cell proliferation inhibition and alterations in retinol esterification induced by phytanic acid and docosahexaenoic acid. J. Lipid Res. 2007, 48, 165–176. [Google Scholar] [CrossRef]
- Xu, J.; Thornburg, T.; Turner, A.R.; Vitolins, M.; Case, D.; Shadle, J.; Hinson, L.; Sun, J.; Liu, W.; Chang, B.; et al. Serum levels of phytanic acid are associated with prostate cancer risk. Prostate 2005, 63, 209–214. [Google Scholar] [CrossRef]
- Sullivan, K.E.; Cutilli, J.; Piliero, L.M.; Ghavimi-Alagha, D.; Starr, S.E.; Campbell, D.E.; Douglas, S.D. Measurement of cytokine secretion, intracellular protein expression, and mRNA in resting and stimulated peripheral blood mononuclear cells. Clin. Diagn. Lab. Immunol. 2000, 7, 920–924. [Google Scholar]
- Wattegedera, S.R.; Watson, D.M.; Hope, J.C.; Kaiser, P.; Sales, J.; McInnes, C.J.; Entrican, G. Relative quantitative kinetics of interferon-gamma and interleukin-10 mRNA and protein production by activated ovine peripheral blood mononuclear cells. Vet. Immunol. Immunopathol. 2010, 136, 34–42. [Google Scholar] [CrossRef]
- Zheng, Z.M.; Specter, S.C.; Lancz, G. Bovine serum albumin preparations enhance in vitro production of tumor necrosis factor alpha by murine macrophages. Immunol. Investig. 1995, 24, 737–756. [Google Scholar] [CrossRef]
- Perdomo, M.C.; Santos, J.E.; Badinga, L. Trans-10,cis-12 conjugated linoleic acid and the PPAR-γ agonist rosiglitazone attenuate lipopolysaccharide-induced TNF-α production by bovine immune cells. Domest. Anim. Endocrinol. 2011, 41, 118–125. [Google Scholar] [CrossRef]
- Aderem, A.; Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 2000, 406, 782–787. [Google Scholar] [CrossRef]
- Changhua, L.; Jindong, Y.; Defa, L.; Lidan, Z.; Shiyan, Q.; Jianjun, X. Conjugated linoleic acid attenuates the production and gene expression of proinflammatory cytokines in weaned pigs challenged with lipopolysaccharide. J. Nutr. 2005, 135, 239–244. [Google Scholar]
- Kim, D.I.; Kim, K.H.; Kang, J.H.; Jung, E.M.; Kim, S.S.; Jeung, E.B.; Yang, M.P. Trans-10,cis-12-conjugated linoleic acid modulates NF-κB activation and TNF-α production in porcine peripheral blood mononuclear cells via a PPARgamma-dependent pathway. Br. J. Nutr. 2011, 105, 1329–1336. [Google Scholar] [CrossRef]
- Loscher, C.E.; Draper, E.; Leavy, O.; Kelleher, D.; Mills, K.H.G.; Roche, H.M. Conjugated linoleic acid suppresses NF-κB activation and IL-12 production in dendritic cells through ERK-mediated IL-10 induction. J. Immunol. 2005, 175, 4990–4998. [Google Scholar]
- Long, F.Y.; Guo, Y.M.; Wang, Z.; Liu, D.; Zhang, B.K.; Yang, X. Conjugated linoleic acids alleviate infectious bursal disease virus-induced immunosuppression in broiler chickens. Poult. Sci. 2011, 90, 1926–1933. [Google Scholar] [CrossRef]
- Hussen, J.; Schuberth, H.J. The Effect of 9-cis:11-trans Conjugated Linoleic Acid (CLA),15d-PGJ2 and GW9662 on the Proliferation of Bovine Peripheral Blood Mononuclear Cells in vitro. In Proceedings of the 65th Conference of the Society of Nutrition Physiology, Göttingen, Germany, 15–17 March 2011; p. 97.
- Kang, J.H.; Lee, G.S.; Jeung, E.B.; Yang, M.P. Trans-10,cis-12-conjugated linoleic acid increases phagocytosis of porcine peripheral blood polymorphonuclear cells in vitro. Br. J. Nutr. 2007, 97, 117–125. [Google Scholar] [CrossRef]
- Brown, J.M.; Boysen, M.S.; Jensen, S.S.; Morrison, R.F.; Storkson, J.; Lea-Currie, R.; Pariza, M.; Mandrup, S.; McIntosh, M.K. Isomer-specific regulation of metabolism and PPARγ signaling by CLA in human preadipocytes. J. Lipid Res. 2003, 44, 1287–1300. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Renner, L.; Kersten, S.; Duevel, A.; Schuberth, H.-J.; Dänicke, S. Effects of cis-9,trans-11 and trans-10,cis-12 Conjugated Linoleic Acid, Linoleic Acid, Phytanic Acid and the Combination of Various Fatty Acids on Proliferation and Cytokine Expression of Bovine Peripheral Blood Mononuclear Cells. Nutrients 2013, 5, 2667-2683. https://doi.org/10.3390/nu5072667
Renner L, Kersten S, Duevel A, Schuberth H-J, Dänicke S. Effects of cis-9,trans-11 and trans-10,cis-12 Conjugated Linoleic Acid, Linoleic Acid, Phytanic Acid and the Combination of Various Fatty Acids on Proliferation and Cytokine Expression of Bovine Peripheral Blood Mononuclear Cells. Nutrients. 2013; 5(7):2667-2683. https://doi.org/10.3390/nu5072667
Chicago/Turabian StyleRenner, Lydia, Susanne Kersten, Anna Duevel, Hans-Joachim Schuberth, and Sven Dänicke. 2013. "Effects of cis-9,trans-11 and trans-10,cis-12 Conjugated Linoleic Acid, Linoleic Acid, Phytanic Acid and the Combination of Various Fatty Acids on Proliferation and Cytokine Expression of Bovine Peripheral Blood Mononuclear Cells" Nutrients 5, no. 7: 2667-2683. https://doi.org/10.3390/nu5072667





