1. Introduction
The National Kidney Foundation Task Force on Cardiovascular Disease recommends that chronic kidney disease (CKD) patients be considered among the highest risk group for developing cardiovascular (CV) disease [
1]. In fact, CV disease is more prevalent in patients with CKD than in the general population [
2] and it is the leading cause of death in patients with end-stage renal disease [
1]. It has been reported that nearly 30% of deaths among patients with CKD are to be attributed to cardiovascular causes [
3].
CKD associated hyperparathyroidism and mineral metabolism disorders, such as hyperphosphatemia, have been significantly correlated with vascular and visceral calcifications, and consequently with increased risk of CV disease [
4].
In addition to vascular problems, left ventricular hypertrophy, subclinical systolic dysfunction, and diastolic dysfunction have been consistently observed in subjects with CKD [
5]. It is conjectured that abnormal diastolic function is an independent predictor of decreased aerobic capacity during the early stages of CKD [
6].
Nevertheless, even though numerous factors have been implicated in the aetiology of the cardiac abnormalities observed in patients with CKD, the exact aetiology of the observed cardiac changes still remains unclear. A number of cardiac pathologies, including heart failure, are associated with alterations in myocardial energy metabolism [
7] as well as with the activation of different intracellular signal transduction pathways.
Vitamin D
3 (in its active form,
i.e., calcitriol or 1,25-dihydroxyvitamin D
3), as many other direct-acting positive inotropic agents, stimulates cyclic AMP (cAMP) formation [
8], a major mediator of the amplitude and time course of cardiac contraction [
8]. This cAMP-mediated inotropic effect occurs through activation of a variety of cAMP-dependent protein kinases that are capable of phosphorylating a series of proteins that affect the energetic status of the cells and alter the flux of calcium in the sarcoplasm [
8].
In fact, calcium release and energetic systems are strictly and subtly modulated by different molecules, and an imbalance of sympathetic and parasympathetic drive to the heart represents an important risk factor for cardiac death in patients with renal insufficiency [
9].
Since vitamin D
3, together with dietary restrictions and phosphate binders, represents the primary medication to treat secondary hyperparathyroidism and the associated calcium and phosphate metabolic alterations in CKD, interest in the role of vitamin D
3 axis in the cardiovascular system recently increased. Thus, the vitamin D axis, which plays a critical role in the development of CKD, includes vitamin D, the polymorphic vitamin D receptor (VDR), and the vitamin D-binding protein (Gc-globulin) [
10]. That is the precursor of a potent macrophage activating factor (GcMAF), which has potent effects on the immune system and angiogenesis [
11].
In parallel, with this interest for beneficial effects [
12], however, other studies warned that an excess of vitamin D
3 increases the risk of hypercalcemia and vascular calcifications thus increasing the risk for CV and reducing survival in patients with CKD [
13,
14].
In this study we investigated the effect of vitamin D
3 as well as of one of its non-hypercalcemic analogs, paricalcitol, on cultured cardiomyocytes. Paricalcitol (19-nor-1,25-dihydroxyvitamin D
2) is a vitamin D
3 analog, acting as a selective VDR activator; for this reason it might provide a vitamin D
3-like protective efficacy without the hypercalcemic and hypophosphatemic side effects of vitamin D
3, thus representing a potentially useful tool against the cardio-renal syndrome [
12,
15]. In particular, to better evaluate the modifications of the intracellular energy status as well as the modifications in signal transduction, in this study we compared the effects of vitamin D
3, to those of paricalcitol in a myoblastic cell line H9c2, not completely differentiated showing electrophysiological and biochemical properties of cardiac muscle tissue [
16]. Proliferation, mitochondrial activity, morphological alterations, calcification, cAMP pathway activation, and response to acetylcholine were thus investigated.
2. Experimental Section
2.1. Pharmacological Agents
Vitamin D3 and Paricalcitol were respectively obtained from Sigma Aldrich, Milano, Italy and from Abbott, Roma, Italy. Due to differences in test compounds, two different treatment vehicles were used during the study: 100% ethanol (for vitamin D3) and 30% polyethylene glycol/20% ethanol in water (for paricalcitol). Preliminary experiments did not show significant differences among different concentrations of vehicles in cell responses (not shown). Therefore, the different vehicle concentrations data were pooled for the analysis in this report. Vitamin D binding protein-derived macrophage activating factor (GcMAF) was obtained from Immuno Biotech Ltd. (Guernsey, Channel Islands). β-Glycerol-phosphate (β-GP), ethanol, polyethylene glycol, and acetylcholine were obtained from Sigma Aldrich, Milano, Italy.
2.2. Cell Cultures
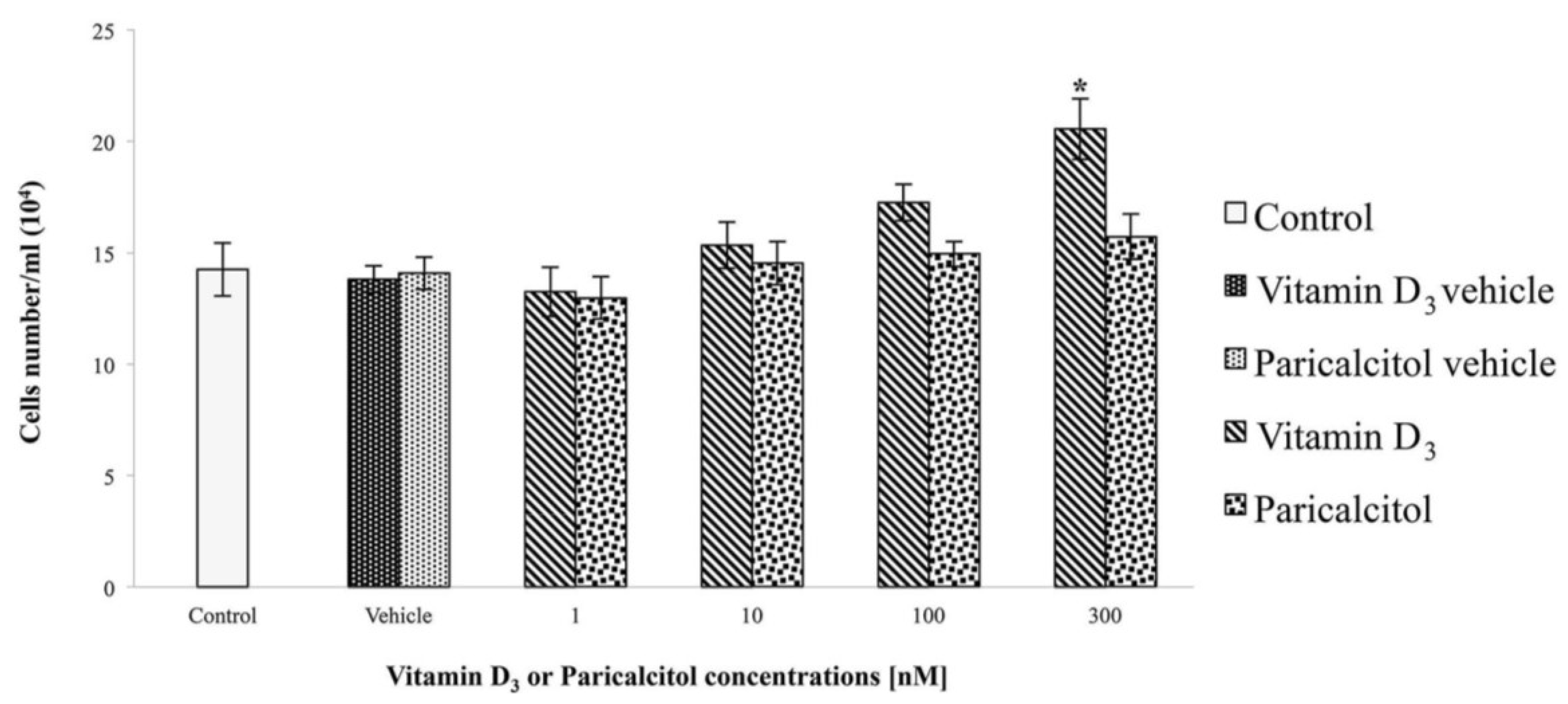
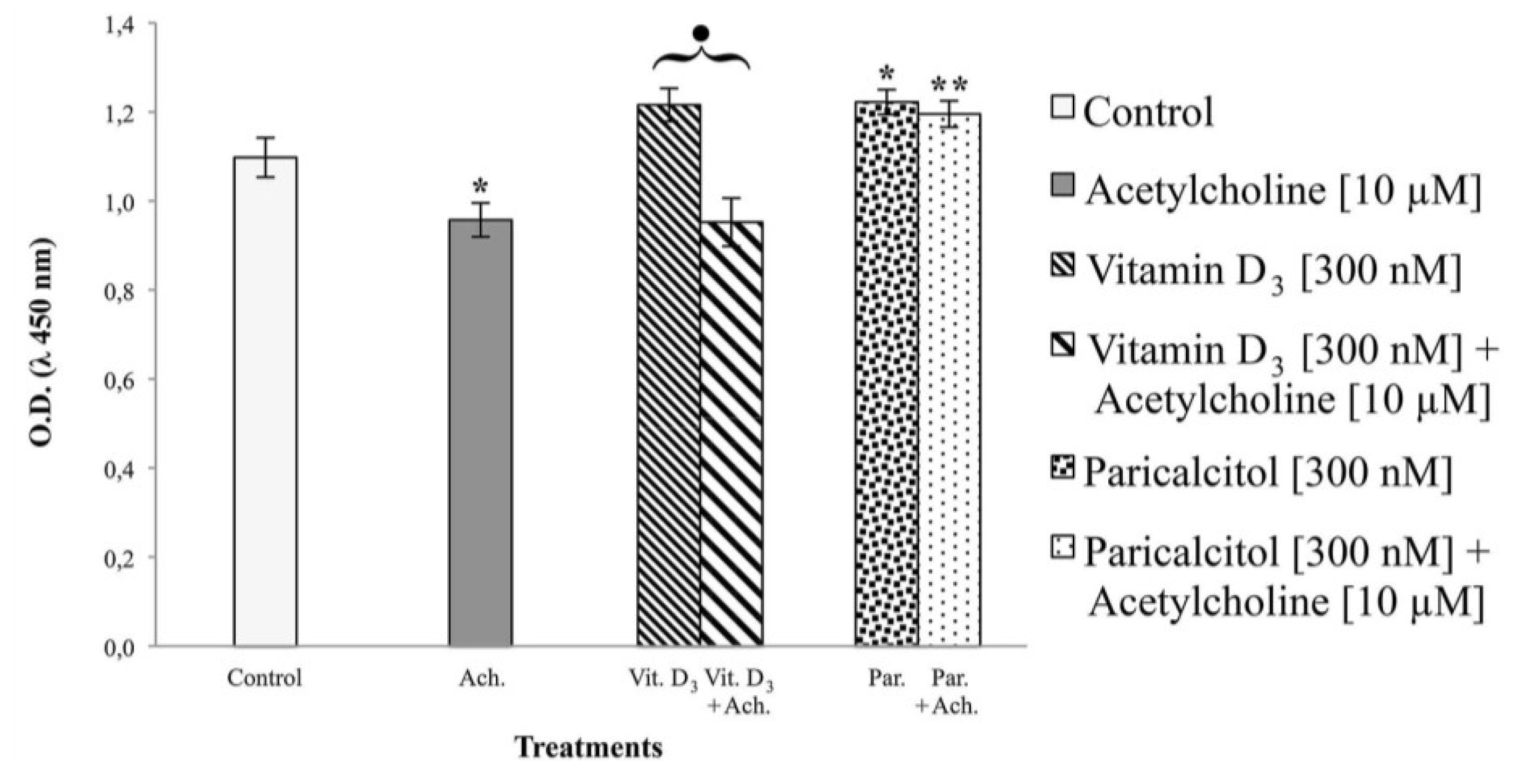
H9c2 cells, immortalized ventricular myocytes derived from rat (Rattus norvegicus) embryos, were obtained from the “Istituto Zooprofilattico della Lombardia e dell’Emilia Romagna”, Brescia, Italy. H9c2 is a myoblast cell line not yet completely differentiated into non-proliferating myocytes/myotubes and with electrophysiological and biochemical properties of both skeletal and cardiac muscle tissue. Cell were grown in a monolayer culture at 37 °C in a 5% CO2 humidified atmosphere in Dulbecco’s modified eagle’s medium (DMEM) supplemented with 10% foetal bovine serum (FBS). The medium was renewed every two to three days, when the cells reached sub-confluence (70%–80%). Vitamin D3 and paricalcitol were added to the cells at the following concentrations: [1 nM], [10 nM], [100 nM], and [300 nM]. Treatment with acetylcholine was performed with and without vitamin D3 and paricalcitol [300 nM] at the concentration of [10 μM] for 1 h.
2.3. Cell Viability and Proliferation
The effects of vitamin D
3 and paricalcitol on H9c2 cell lines, were evaluated by the cell viability assay using the 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulf-ophenyl)-2
H tetrazolium monosodium salt (WST-8) reagent (Sigma Aldrich, Milano, Italy), according to the manufacturer’s protocol. This test is based on a colorimetric conversion of a tetrazolium salt (WST-8) to water-soluble formazan products as previously described [
17]. H9c2 cells were plated at density of 2 × 10
4 per well in a 96-well plate with fresh growth medium for one day. Then, vitamin D
3 and paricalcitol were diluted to appropriate concentration and added to the culture medium for 48 h. After the exposure-time, WST-8 solution was added to each well and the micro-plate incubated for 4 h at 37 °C. Cell viability was measured by a micro-plate reader (
Multiscan FC/FL6111900 model, Thermo Fisher Scientific, M-Medical, Milano, Italy) at 450 nm after incubation-time. To directly evaluate cell proliferation, a cell count by a haemocytometer (Housser Scientific, Horsham, PA, USA) was also performed. Also the evaluation of cardiomyocytes response to acetylcholine in the presence of vitamin D
3 and paricalcitol was evaluated by cell viability assay. In each experiment, at least seven wells were used for each experimental point.
2.4. Cell Morphology
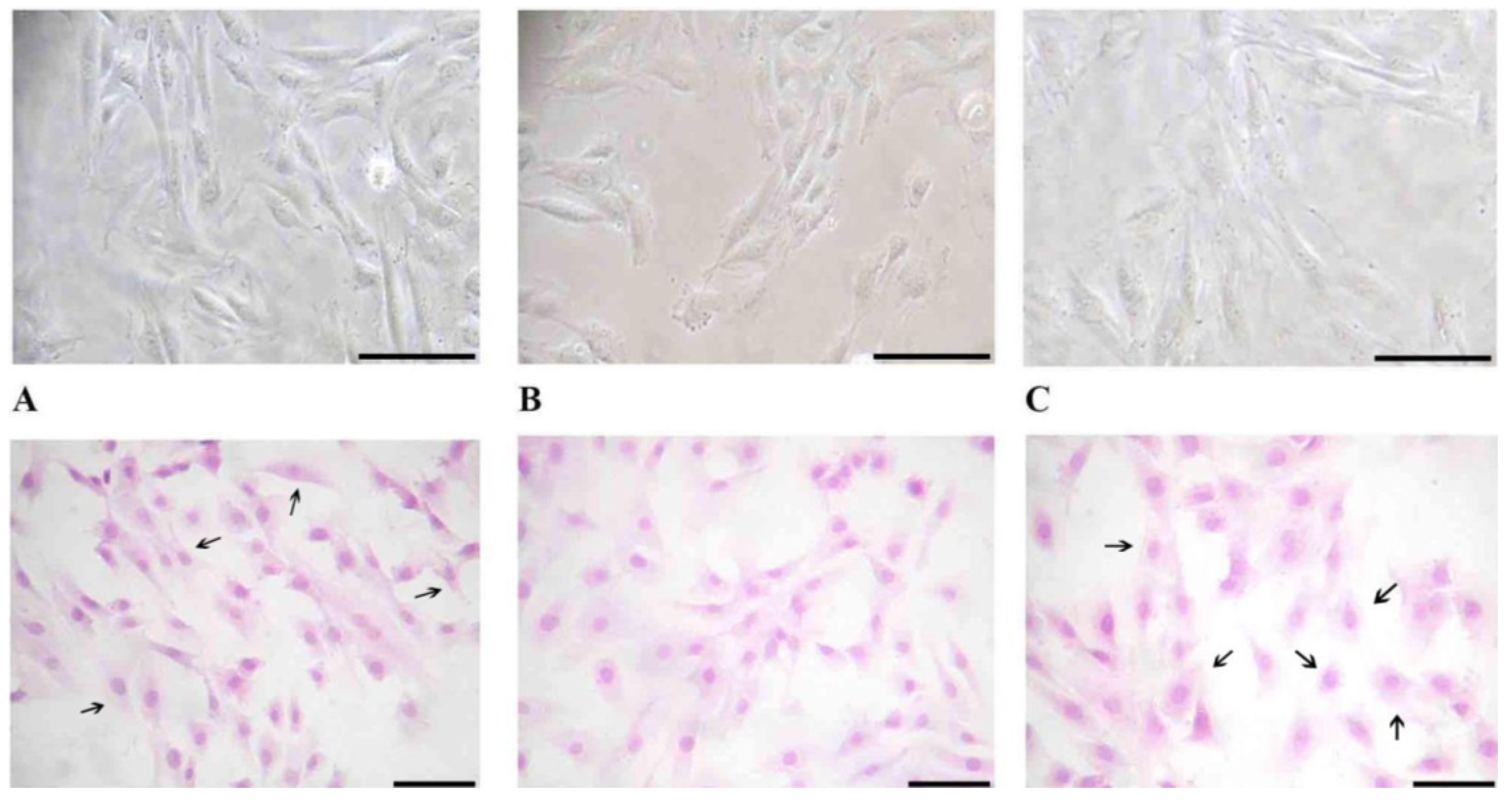
To evaluate changes in H9c2 cell morphology before and after vitamin D3 and paricalcitol exposure, contrast phase microscopy and Haematoxylin-Eosin staining were performed.
Briefly, H9c2 cells were seeded on a cover slip at the density of 10 × 104 cells/cover slip.
Cells were grown in culture medium with different concentrations of vitamin D3 and paricalcitol for 48 h. For the Haematoxylin-Eosin staining, H9c2 cells were fixed with paraformaldehyde 0.5% in [0.1 M] PBS (phosphate buffer saline) and dried for 2 h. Then, cells were stained with Haematoxylin-Eosin dye following a standard protocol.
Cell morphology was evaluated by optical microscope (XDS-2 + M-795 model, Optika Microscope, Milano, Italy) and digital images were captured.
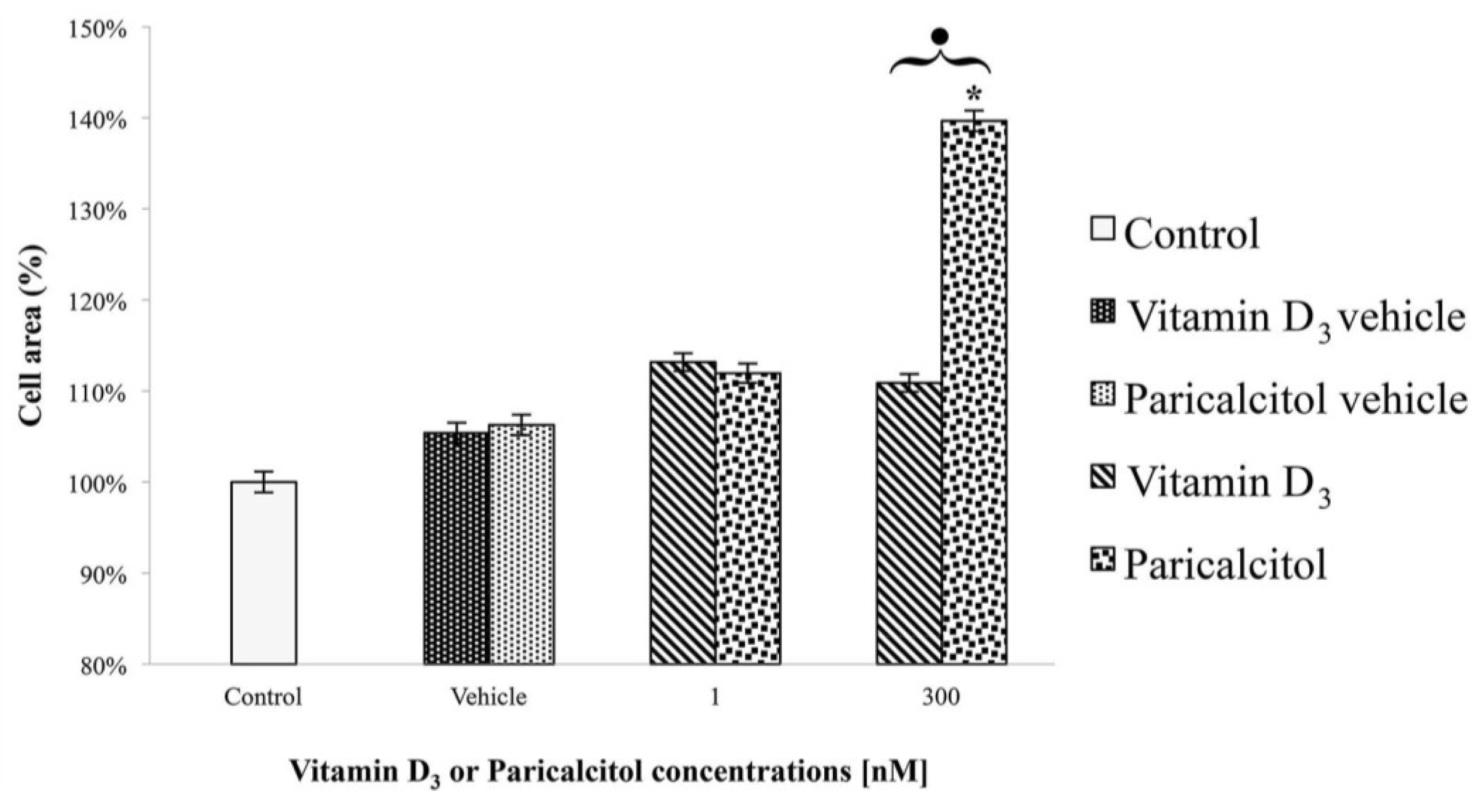
After the treatment at different concentration of vitamin D3 and paricalcitol for 48 h, cell size of H9c2 was measured by Adobe Pohotoshop CS2 software (9.0 version, Adobe System Incorporated 1990–2005; Adobe Systems Inc., San Jose, CA, USA) and Scion Image software (Beta 3b freeware version; based on NIH Image for Macintosh by Wayne Rasband, National Institute of Health, USA and modified for Windows by Scion Corporation; July, the 23rd 1998; Scion Co., Frederick, MD, USA). Images of H9c2 in Haematoxylin-Eosin staining were captured using a digital camera (DIGI-full HD video/photo camera, Optika Microscopes, Milano, Italy). Digital images were adjusted by Adobe Photoshop CS2 to remove from the picture everything (background) except the cells and then H9c2 cells size was measured by Scion Image.
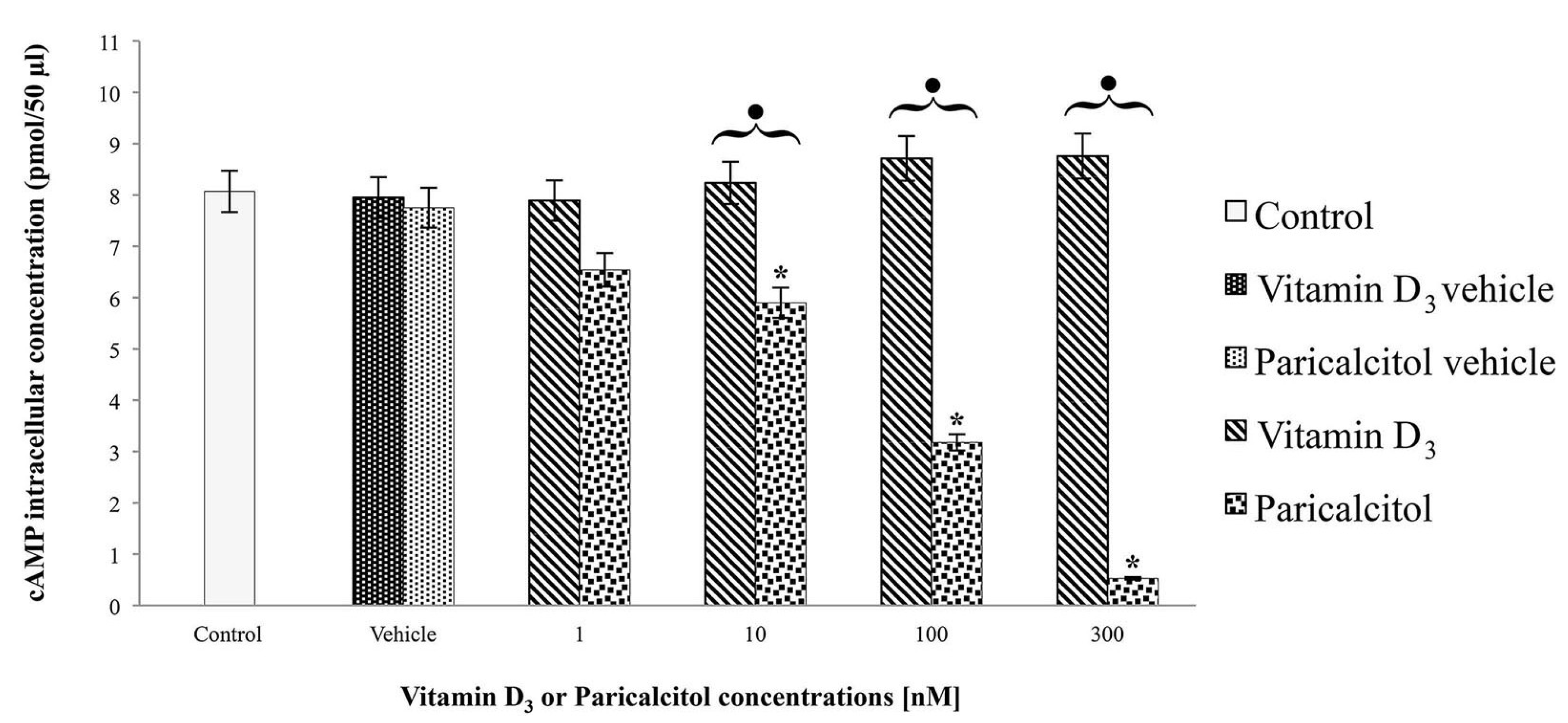
2.5. cAMP Assay
H9c2 cells were cultured at density of 1 × 104 cells per well in a 6-well plate with DMEM supplemented with 10% FBS and exposed at [1 nM], [10 nM], [100 nM], and [300 nM] vitamin D3 and paricalcitol for 48 h. The cAMP intracellular level was evaluated by a direct competitive immunoassay for sensitive and quantitative determination using a cAMP assay kit (Abnova, Heidelberg, Germany) according to the Manufacturer’s instructions. After the treatments, the experiment was blocked adding [0.1 M] HCl. Then, after scraping cells from the substrate, the suspension was centrifuged for 10 min at 2340 rpm. The supernatant was assayed for cAMP concentration by a micro-plate reader (Multiscan FC/FL6111900 model, Thermo Fisher Scientific, M-Medical, Milano, Italy) at 450 nm after incubation-time. cAMP values were normalized to the assayed proteins.
2.6. Analysis of H9c2 Calcification
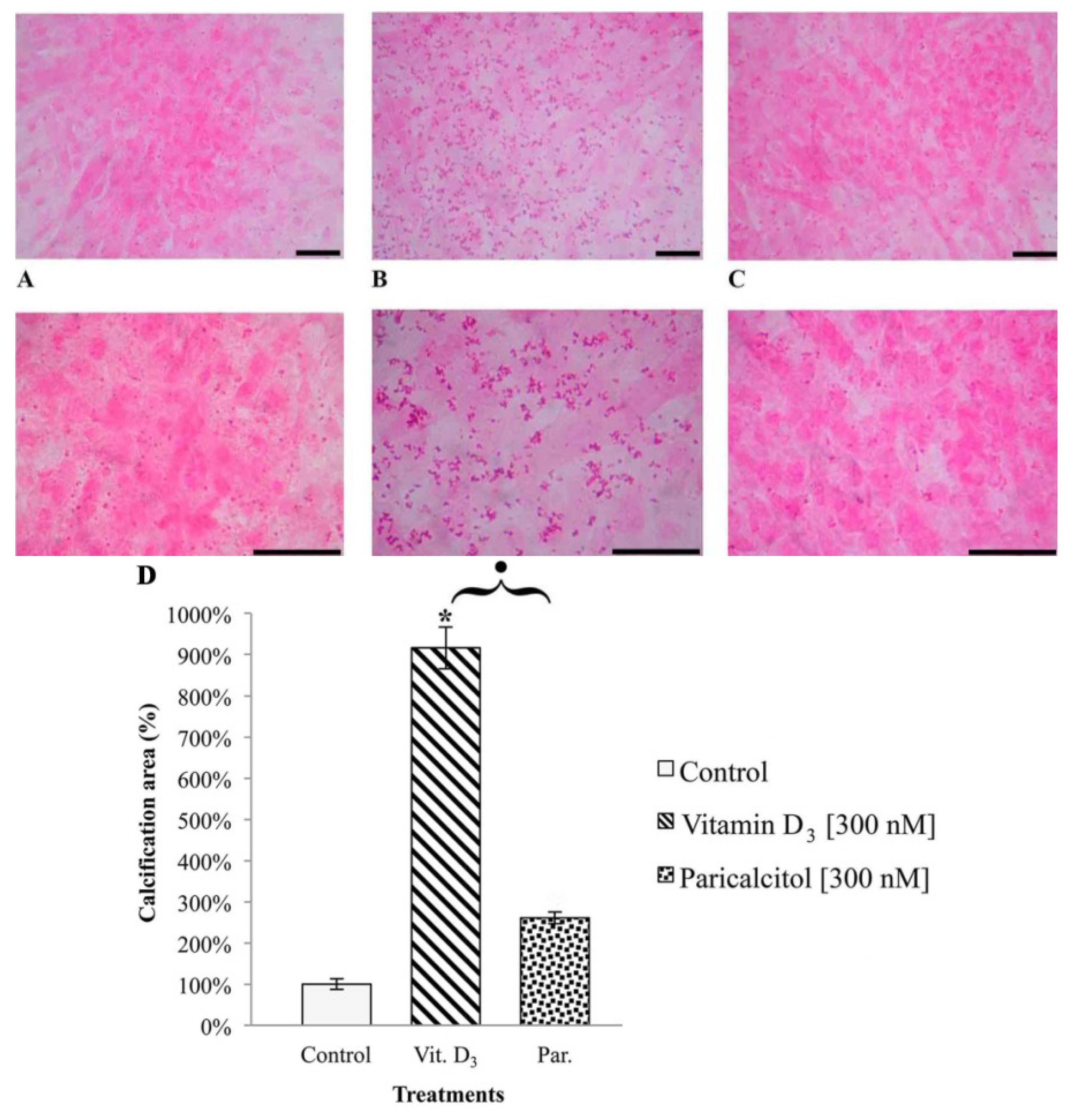
To evaluate the deposits of calcium in H9c2 after vitamin D3 and Paricalcitol exposure, cells were seeded on cover slip at density 10 × 104 per cover slip and cultured in their culture medium (DMEM + 10% FBS). The medium was substituted with fresh culture medium every two days. After five days from confluence, fresh medium containing different concentration of vitamin D3 and paricalcitol was used for a further seven days. To amplify the calcification effects of vitamin D3 and paricalcitol, the medium was enriched with [4 mM] β-GP (calcification media). Quantification of calcium deposits was performed by von Kossa staining (von Kossa Kit, Bio Optica, Milano, Italy); cells were soaked in a lithium carbonate solution (for 10 min) to prevent false results caused by the presence of uric acid and urates in the samples; then, cells were treated with a silver nitrate solution, left in dark for 1 h, and then a reduction solution was applied for 5 min; then, samples were treated with a sodium sulphite solution for 5 min and with a Mayer’s Carmalum solution to contrast the calcium deposits. The intracellular calcification was evaluated by optical microscope (XDS-2 + M-795 model, Optika Microscope, Milano, Italy) and images of H9c2 cell line in von Kossa staining were captured using a digital camera (DIGI-full HD video/photo camera, Optika Microscopes, Milano, Italy) and then analyzed.
2.7. Statistics
All values are means ± standard error (SE) for at least three determinations. Differences between experimental points were evaluated by Student’s t-test. p was considered statistically significant when p < 0.05.
4. Discussion
Recent observational studies provided support for a possible protective role for vitamin D
3 in the CKD population [
1,
2]. Recent scientific evidence suggests that vitamin D
3 may negatively regulate the renin-angiotensin system, inhibit cardiomyocyte hypertrophy and proliferation, as well as modulate and suppress the inflammatory response to blood vessel injury [
18]. Cardiovascular diseases, especially atherosclerosis, have been reported to be the main causes of dialysis-related morbidity and mortality [
2]. In the development of these vasculopathies, not only are traditional risk factors involved, but also CKD-related biochemical changes such as hypocalcemia, vitamin D deficiency and hyperparathyroidism, which are considered promoting factors. Previous
in vitro studies [
19] have demonstrated that endothelial cells stimulated with low Ca
2+, high advanced glycation end products (AGEs), and parathyroid hormone (PTH), responded by increasing the expression and production of pro-inflammatory and atherosclerotic parameters such as interleukin 6 (IL6), nuclear factor kappa B (NFκB), and endothelial nitric oxide synthase (eNOS). These CKD-related biochemical changes are significantly counteracted by vitamin D
3 treatment, which induces a decrease in the elevated IL-6 mRNA expression, positively affects the activity of NFκB, and normalizes the parameters associated with the eNOS system.
On the other hand, other studies highlighted that an excess of vitamin D
3 increases the risk of hypercalcemia and vascular calcification, thus worsening CV risk and reducing survival in patients with CKD [
4,
20]. In fact, vitamin D
3 belongs to the category of general VDR activators, having a wide range of affinity for the components of the vitamin D
3 system, both for the vitamin D
3-binding protein and for the nuclear VDR. Among the VDR activators, selective activators such as Paricalcitol play a major role in controlling mineral metabolism. These selective molecules act more efficiently on parathyroid glands rather than on intestine and bone; this leads both to a lower serum calcium and phosphorus increases and to an improvement of hyperplasia of the parathyroid gland and secondary hyperparathyroidism [
15]. For these reasons, selective VDR activators could provide a vitamin D-like protective efficacy without the hypercalcemic and hypophosphatemic collateral effects, thus representing a potential therapeutic tool against the cardio-renal syndrome.
From data presented in this study, it emerges that selective activators of the VDR such as Paricalcitol are not only associated with less side effects in comparison to vitamin D
3 but they also can also act directly on cardiomyocytes inducing morphological changes, differentiation, and variations in cell ability to respond to acetylcholine. The most notable differences
in vitro were observed at high doses; this phenomenon could be interpreted considering the peculiar mode interaction between VDR and the genes that it regulates. A differential occupancy of the receptor might in fact be responsible for the difference in the array of genes that are being regulated by VDR [
21].
In fact, one of the most potentially dangerous side effects associated with long term therapy with vitamin D
3, i.e., intracellular calcification, appears to be much less relevant when the selective activator of vitamin D
3 axis paricalcitol is used instead. From our results it appears that vitamin D
3 and its analog paricalcitol activate different signal transduction pathways in H9c2 cells: vitamin D
3 induces cell proliferation associated with an increase of cAMP, whereas paricalcitol induces, at the same concentration, cell differentiation, associated with cAMP decreases. In fact, low levels of intracellular cAMP are necessary to induce muscle cell differentiation of different myogenic cell lines [
16] such as H9c2. It is worth remembering that these cells derive from embryonic rat ventricle; thus, they are not yet completely differentiated into non-proliferating myocytes/myotubes and they maintain the electrophysiological and biochemical properties of both skeletal and cardiac muscle tissue [
16]. Data in literature demonstrate that H9c2 cell differentiation is associated with an increase of myogenin expression, a typical skeletal muscle protein [
16], and with an increase of myotrophin, a protein associated with normal cardiomyocyte development [
22].
Treatment with high concentration of paricalcitol also induces a significant increase in cell size. Cardiomyocyte hypertrophy occurs in response to numerous agonists [
23] and growth factor signaling pathways that induce hypertrophy are intimately interconnected with intracellular pathways that link changes in calcium homeostasis with the reprogramming of cardiac gene expression [
24]. The calcium, calmodulin-dependent protein, phosphatase calcineurin, serves as a point of convergence of these different intracellular pathways [
25] and has been shown to be necessary and sufficient for hypertrophy in response to a wide range of signals
in vivo and
in vitro [
26]. Therefore, cardiomyocyte hypertrophy in response to paricalcitol has to be interpreted as an adaptive response that parallels and accompanies cell differentiation. Future studies will assess whether this adaptive responses will occur also in fully differentiated cardiomyocytes.
Given the recent interest in the role of vitamin D
3 deficiency in CV system and heart functionality and integrity, we investigated the effects on heart diastolic function of the components of the vitamin D
3 axis that are known to be associated with VDR, that are vitamin D
3, paricalcitol, and GcMAF, and preliminary data demonstrated that the components of the vitamin D
3 axis differently affect the diastolic function. Diastolic function has been evaluated by a rapid and non-invasive method, based on ultrasounds and measuring the interval in milliseconds between aortic closure and mitral opening; this interval of time is known as iso-volumetric relaxation time (IVRT) [
27]. Physiologically, it is the time that it takes to pump free calcium out of the myocardium, to produce relaxation of the myofibrils and to allow ventricular filling, and takes all the available free energy in the heart to do so. IVRT is inversely related to the cellular free energy so the higher the IVRT is, the lower the cellular free energy is. From our data emerged that, while vitamin D
3 increased IVRT, paricalcitol significantly decreased it, thus demonstrating a positive inotropic effects on the levels of cellular free energy. In fact, these results can be interpreted as that paricalcitol increased the cellular free energy, a novel positive feature of this analog that had not been described before. The effects of GcMAF were comparable to those obtained with paricalcitol. These results can be interpreted considering that paricalcitol and GcMAF showed similar, although not superimposable, effects at the cellular and organism level in other model systems. In fact, both compounds stimulated cAMP formation in human mononuclear cells with the highest effect on the “bb” genotype of the VDR. Both paricalcitol and GcMAF inhibited the angiogenesis induced by prostaglandin E1 in the chick embryo chorionallantoic membrane [
28]. This unexpected effect of paricalcitol suggests that its advantage over vitamin D
3 might be far more ranging than simply being non-hypercalcemic. In fact, the decrease of cellular free energy in a chronic condition is at the basis of a number of signs and symptoms that are negatively associated with the prognosis. Our observation that paricalcitol and GcMAF show an inotropic effect at variance with vitamin D
3 opens the perspective of administering this molecule in a variety of chronic conditions where the use of vitamin D
3, although potentially beneficial, has been discouraged by the concomitant and potentially harmful side effects of vitamin D
3. Since the majority of the results presented in this study have been conducted
in vitro in a rat myoblast cell line, not yet completely differentiated, the results cannot be directly extrapolated to clinical recommendations. Nevertheless, such a model system could provide novel indications that can be applied to the clinic. Thus, paricalcitol is a molecule that has been safely used for years and this study demonstrates that it shows some novel effects that could be exploited in order to maximize therapeutic effects while, at the same time, minimizing side effects. The stimulation of cardiomyocyte viability and differentiation without inducing intracellular calcification, and the positive inotropic effect in the absence of hypercalcemia, might therefore represent novel fields of application of this molecule.