Selenium Toxicity from a Misformulated Dietary Supplement, Adverse Health Effects, and the Temporal Response in the Nail Biologic Monitor
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects and Nail Samples
| Parameter | Number (%) | Age Mean (s.d.) Median |
|---|---|---|
| Number of subjects who enrolled and provided at least 1 biomonitor sample | 97 | 56.6 (18.5) 58 |
| Females | 63 (64.9) | 57.6 (17.1) 58 |
| Males | 34 (35.1) | 55.1 (20.9) 60 |
| Died (as of July 2011) all females | 3 | 85.0 (6.6) 86 |
| Number of subjects who returned follow-up questionnaire at 2.50 ± 0.14 years | 73 | 57.1 (17.9) 59 |
| Females | 48 (65.8) | 56.8 (16.6) 58 |
| Males | 25 (34.2) | 57.7 (20.4) 61 |
| States (all subjects with ≥1 biomonitor sample) | 97 | |
| Alabama | 5 (5.2) | 67.2 (17.3) 67 |
| Florida | 41 (42.3) | 59.6 (17.0) 61 |
| Georgia | 36 (37.1) | 55.1 (15.2) 55.5 |
| North Carolina | 7 (7.2) | 30.1 (25.0) 34 |
| Tennessee | 8 (8.2) | 64.0 (16.0) 57.5 |
| States (subjects remaining at 2.5 years follow-up) | 73 | |
| Alabama | 4 (5.5) | 61.3 (12.7) 65 |
| Florida | 32 (43.8) | 61.3 (16.1) 62 |
| Georgia | 23 (31.5) | 57.5 (12.8) 56 |
| North Carolina | 7 (9.6) | 30.1 (25.0) 34 |
| Tennessee | 7 (9.6) | 60.9 (14.4) 53 |
2.2. Sample Collection and Preparation
2.2.1. Nail Samples
2.2.2. TBF Product Sample Analysis
2.2.2.1. Total Selenium
2.2.2.2. Dissolved Selenium
2.2.2.3. Bound Selenium
2.3. Neutron Activation Analysis
2.4. Follow-Up Questionnaire
2.5. Statistical Analyses
3. Results
3.1. TBF Supplement Products and Selenium Exposure
| Se Content (μg) Per 1-Ounce Dose 1 | Se Content (μg/Dose) by Lot | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample ID | Lot # 2,3 | n | average | s.d. | %rsdm | n | average | s.d. | %rsdm |
| 1 | 4016801 | 5 | 32,397 | 853 | 2.63 | 2 | 32,184 | 301 | 0.93 |
| 2 | 4016801 | 5 | 31,972 | 318 | 0.99 | ||||
| 3 | 4016802 | 5 | 25,177 | 181 | 0.72 | 7 | 25,278 | 111 | 0.44 |
| 4 | 4016802 | 5 | 25,209 | 165 | 0.65 | ||||
| 5 | 4016802 | 5 | 25,225 | 345 | 1.37 | ||||
| 6 | 4016802 | 5 | 25,263 | 514 | 2.04 | ||||
| 7 | 4016802 | 5 | 25,343 | 482 | 1.90 | ||||
| 8 | 4016802 | 5 | 25,230 | 127 | 0.50 | ||||
| 9 | 4016802 | 5 | 25,501 | 248 | 0.97 | ||||
| 10 | 4024801 | 5 | 24,383 | 236 | 0.97 | 3 | 24,748 | 325 | 1.31 |
| 11 | 4024801 | 5 | 25,006 | 299 | 1.20 | ||||
| 12 | 4024801 | 5 | 24,857 | 328 | 1.32 | ||||
| 13 | 4031801 | 5 | 26,090 | 264 | 1.01 | 7 | 25,850 | 164 | 0.64 |
| 14 | 4031801 | 5 | 25,667 | 184 | 0.72 | ||||
| 15 | 4031801 | 5 | 25,944 | 133 | 0.51 | ||||
| 16 | 4031801 | 5 | 25,810 | 258 | 1.00 | ||||
| 17 | 4031801 | 5 | 25,624 | 326 | 1.27 | ||||
| 18 | 4031801 | 5 | 25,942 | 452 | 1.74 | ||||
| 19 | 4031801 | 5 | 25,874 | 162 | 0.63 | ||||
| 20 | 4031802 | 5 | 22,490 | 122 | 0.54 | 12 | 22,319 | 151 | 0.68 |
| 21 | 4031802 | 5 | 22,489 | 232 | 1.03 | ||||
| 22 | 4031802 | 5 | 22,431 | 201 | 0.90 | ||||
| 23 | 4031802 | 5 | 22,027 | 226 | 1.03 | ||||
| 24 | 4031802 | 5 | 22,194 | 259 | 1.17 | ||||
| 25 | 4031802 | 5 | 22,242 | 414 | 1.86 | ||||
| 26 | 4031802 | 5 | 22,337 | 214 | 0.96 | ||||
| 27 | 4031802 | 4 | 22,187 | 215 | 0.97 | ||||
| 28 | 4031802 | 3 | 22,388 | 91 | 0.41 | ||||
| 29 | 4031802 | 5 | 22,447 | 286 | 1.28 | ||||
| 30 | 4031802 | 5 | 22,170 | 652 | 2.94 | ||||
| 31 | 4031802 | 5 | 22,427 | 196 | 0.88 | ||||
| 32 | 4031803 | 2 | 25,890 | 258 | 1.00 | 5 | 25,983 | 105 | 0.40 |
| 33 | 4031803 | 5 | 25,880 | 327 | 1.26 | ||||
| 34 | 4031803 | 5 | 25,975 | 311 | 1.20 | ||||
| 35 | 4031803 | 5 | 26,042 | 119 | 0.46 | ||||
| 36 | 4031803 | 5 | 26,129 | 194 | 0.74 | ||||
| 37 | Q1 | 5 | 22,249 | 203 | 0.91 | ||||
| 38 | Q2 | 5 | 22,384 | 173 | 0.77 | ||||
| 39 | Q3 | 5 | 25,452 | 201 | 0.79 | ||||
| 40 | Q4 | 5 | 25,628 | 224 | 0.87 | ||||
| 41 | Q5 | 5 | 25,896 | 287 | 1.11 | ||||
| 42 | Q6 | 5 | 26,172 | 280 | 1.07 | ||||
| 43 | Q7 | 5 | 26,234 | 228 | 0.87 | ||||
| 44 | Q8 | 5 | 26,252 | 279 | 1.06 | ||||
| 45 | Q9 | 5 | 32,302 | 165 | 0.51 | ||||
| 46 | Q10 | 4 | 32,872 | 302 | 0.92 | ||||
| 47 | Q11 | 5 | 32,899 | 165 | 0.5 | ||||
| 48 | Q12 | 5 | 33,025 | 197 | 0.6 | ||||
| 49 | Q13 | 5 | 33,084 | 342 | 1.03 | ||||
| 50 | Q14 | 5 | 33,128 | 138 | 0.42 | ||||
| 51 | Q15 | 5 | 33,221 | 392 | 1.18 | ||||
| 52 | Q16 | 5 | 33,894 | 122 | 0.36 | ||||
| 53 | Q17 | 5 | 911 | 16 | 1.76 | ||||
| 54 | Q18 | 5 | 234 | 14 | 6.14 | ||||

3.2. Toenail and Fingernail Results
3.2.1. Temporal Response Measured in Toenail Samples
| Subject Toenail and Fingernail Samples from Which the Post-Exposure Peak and Restored-Baseline Se Concentration were Measured | |||||
|---|---|---|---|---|---|
| Peak Concentrations (μg/g) | Restored-Baseline Concentrations | ||||
| Toenail Se (μg/g) All Samples | Toenail Se (μg/g) Monthly Samples | Toenail Se (μg/g) Onycholysitic Samples | Fingernail Se (μg/g) Onycholysitic Samples | Toenail Se (μg/g) Monthly Samples | |
| n | 30 | 14 | 16 | 10 | 45 |
| Mean 2 | 8.27 | 4.91 | 11.17 | 22.64 | 0.809 |
| Std. Dev. | 4.41 | 2.69 | 3.51 | 8.14 | 0.125 |
| Median | 8.40 | 4.45 | 9.70 | 21.35 | 0.789 |
| Minimum | 1.20 | 1.20 | 7.70 | 13.80 | 0.610 |
| Maximum | 18.30 | 10.10 | 18.30 | 44.10 | 1.213 |
| TBF dose response (see Figure 2A,B) | m = 0.008963 | m = −0.00015 | |||
| b = 1.2436 | b = 0.9049 | ||||
| r = 0.668 | r = 0.340 | ||||
| p = 0.00006 | p = 0.022 | ||||
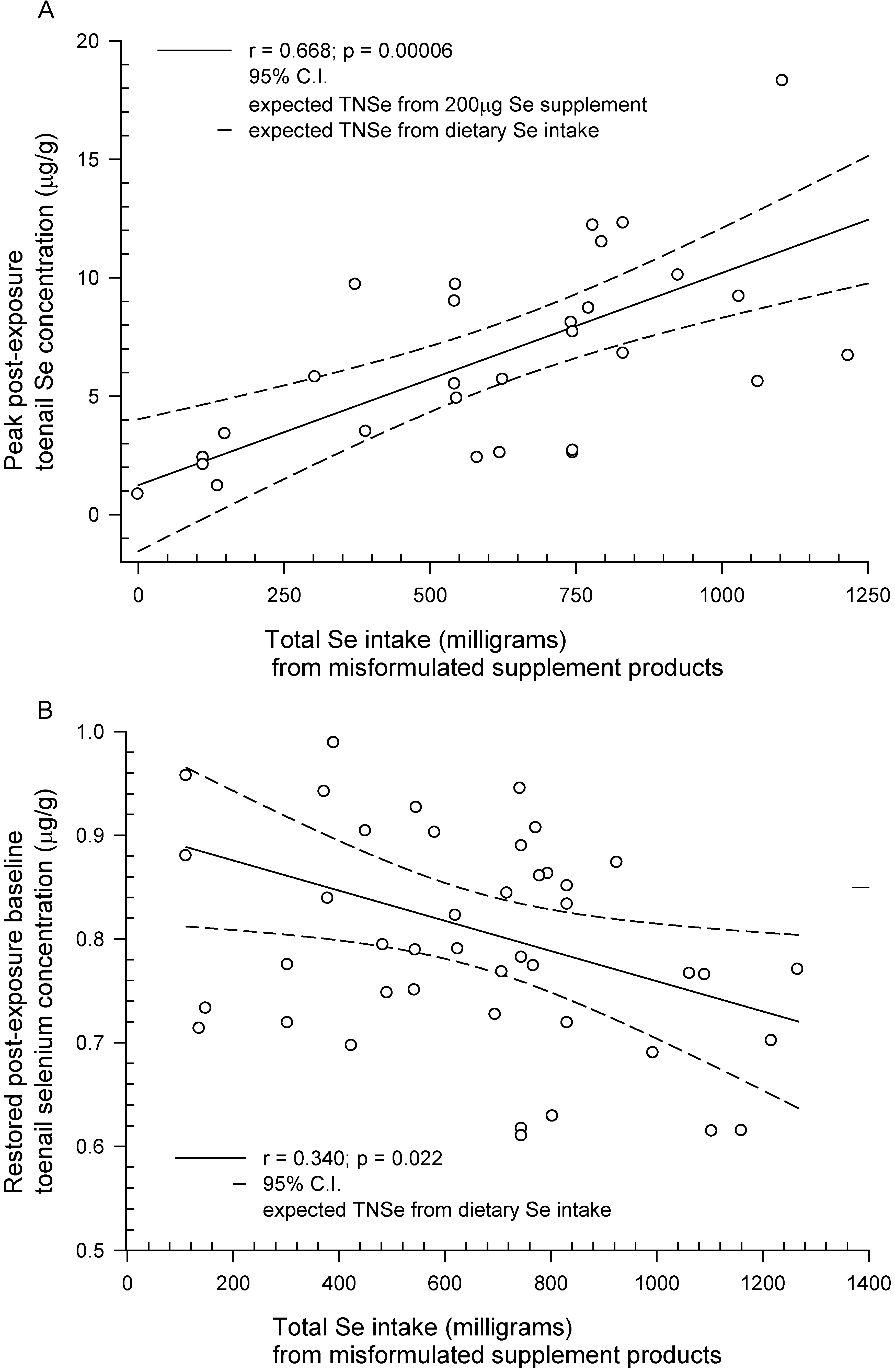
3.2.2. Temporal Response Measured in Onycholysitic Fingernail Samples

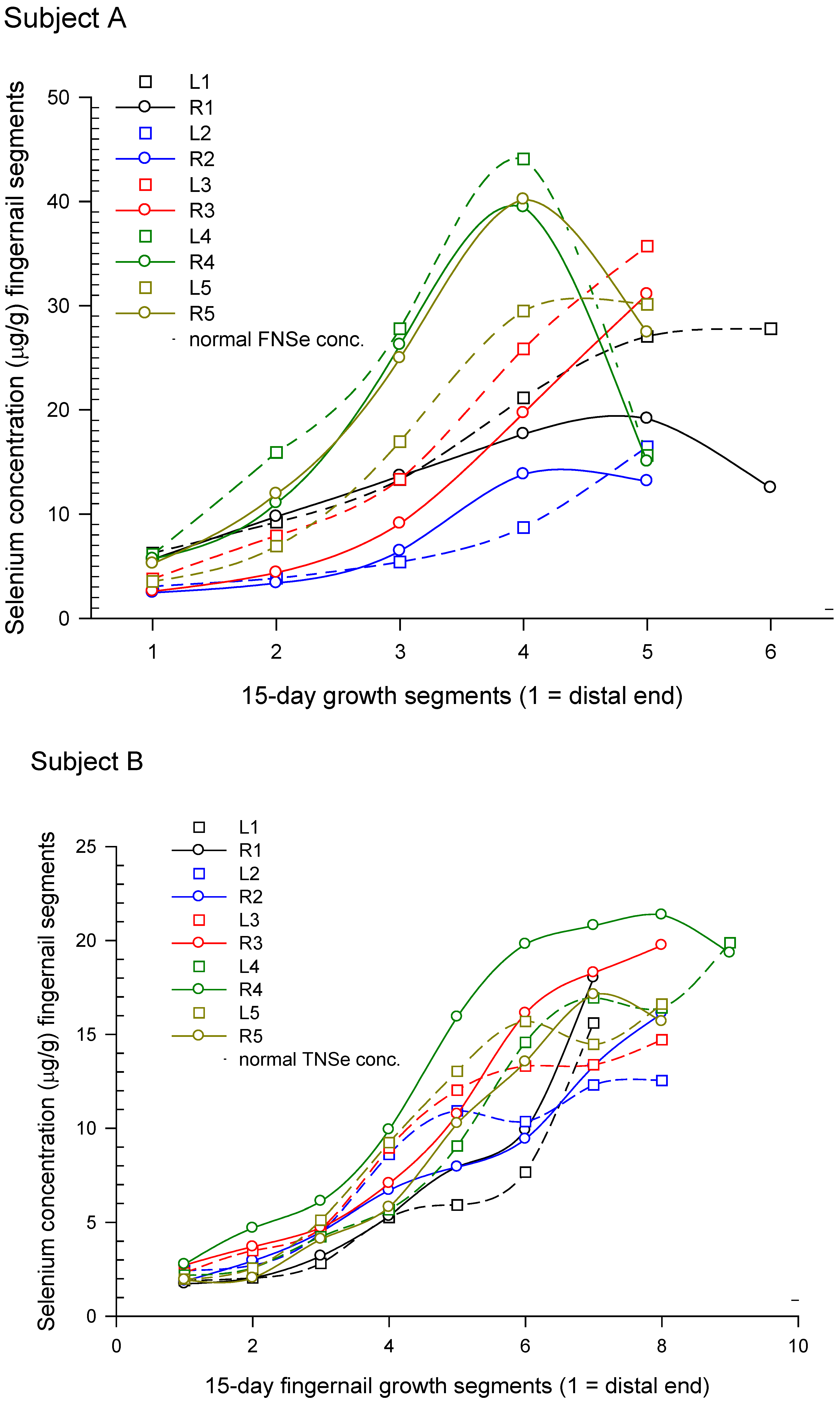
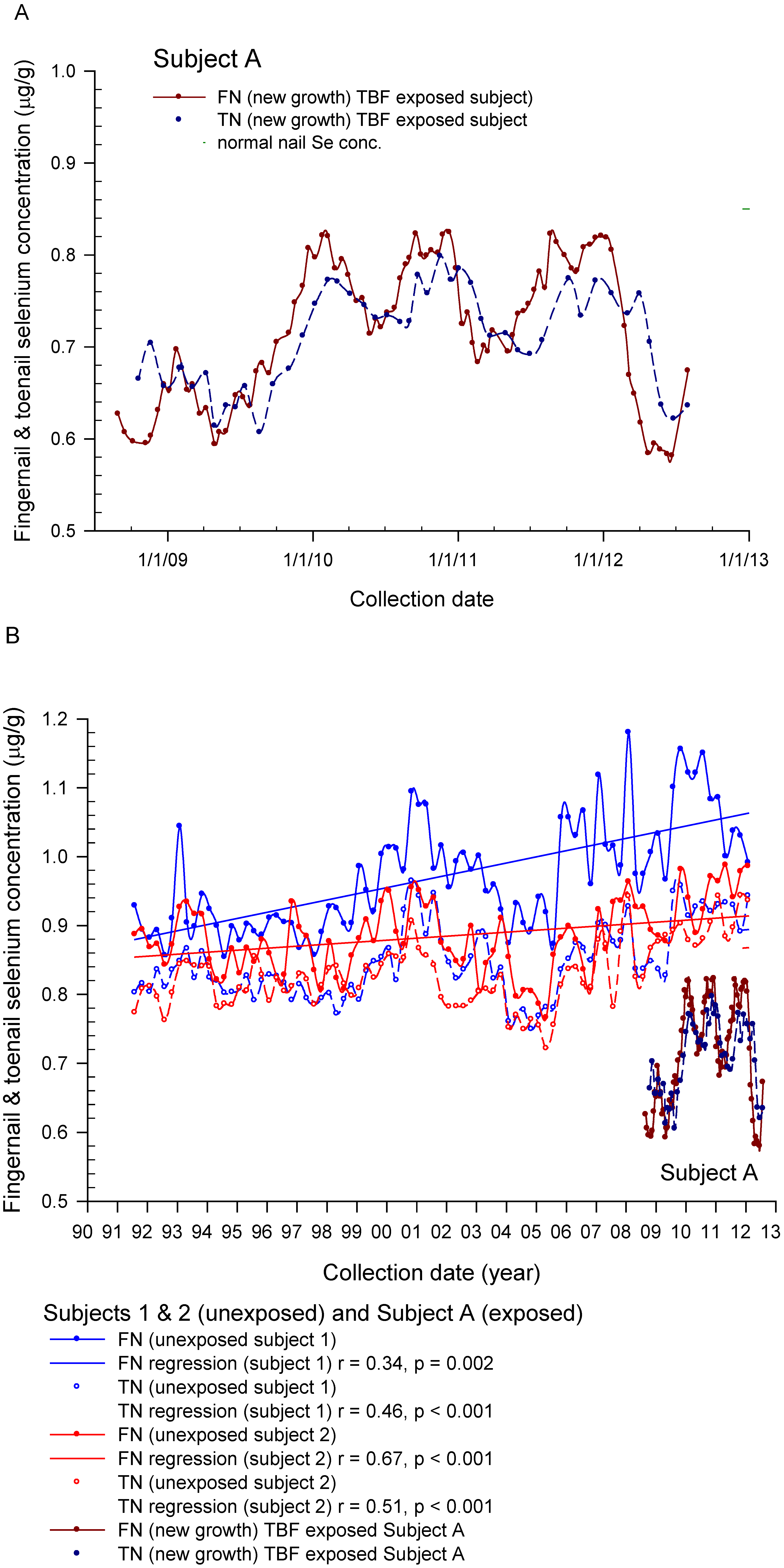
3.3. August 2010 Follow-Up Questionnaire
3.3.1. Selenium Toxicity Symptoms
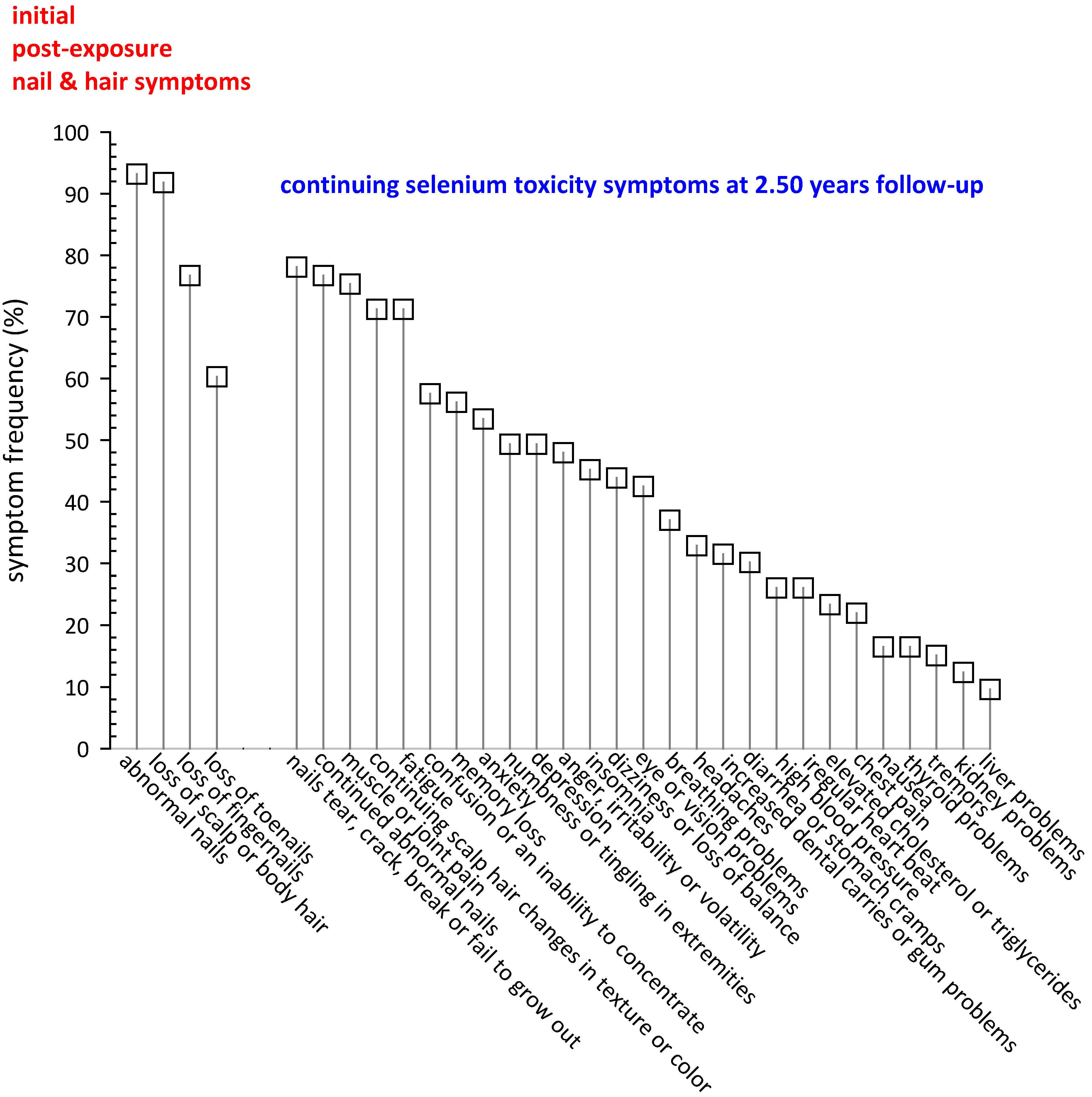
3.3.2. Occupational Impact
4. Discussion
4.1. Other Studies of Selenium Toxicity
4.1.1. Cases of Accidental and Intentional Selenium Poisoning
4.1.2. Selenium Toxicity from Misformulated TBF Products—Previous Studies
4.2. TBF Supplement Products and Selenium Exposure—This Study
4.3. Toenail and Fingernail Biologic Monitors of Selenium Status
4.4. Persistence of Selenium Toxicity Symptoms in the Selenium Supplement Intoxication Study (SSIS)
4.5. Limitations in the SSIS
5. Conclusions and Implications
Ethics Approval
Acknowledgements
Funding
Authors’ Contributions
Subjects’ Contributions
Conflict of Interests
References
- Institute of Medicine, The National Academies, Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids; National Academy Press: Washington, DC, USA, 2000.
- Xia, Y.; Hill, K.E.; Byrne, D.W.; Xu, J.; Burk, R.F. Effectiveness of selenium supplements in a low-selenium area of China. Am. J. Clin. Nutr. 2005, 81, 829–834. [Google Scholar]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigo, R.; Gladyshev, V.N. Characterization of mammalian selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Karunasinghe, N.; Zhu, S.; Wang, A.H. Selenium and its’ role in the maintenance of genomic stability. Mutat. Res. 2012, 733, 100–110. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Selenoprotein P: An extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu. Rev. Nutr. 2005, 25, 215–235. [Google Scholar] [CrossRef]
- Schwarz, K.; Foltz, C.M. Selenium as an integral part of Factor 3 against dietary necrotic liver degeneration. J. Am. Chem. Soc. 1957, 79, 3292–3293. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Gladyshev, V.N. How selenium has altered our understanding of the genetic code. Mol. Cell. Biol. 2002, 22, 3565–3576. [Google Scholar] [CrossRef]
- Mehta, A.; Rebsch, C.M.; Kinzyi, S.A.; Fletcher, J.E.; Copeland, P.R. Efficiency of mammalian selenocysteine incorporation. J. Biol. Chem. 2004, 279, 37852–37859. [Google Scholar]
- Davis, C.D.; Tsuji, P.A.; Milner, J.A. Selenoproteins and cancer prevention. Annu. Rev. Nutr. 2012, 32, 73–95. [Google Scholar] [CrossRef]
- Mayne, S.T.; Ferrucci, L.M.; Cartmel, B. Lessons learned from randomized clinical trials of micronutrient supplementation for cancer prevention. Annu. Rev. Nutr. 2012, 32, 369–390. [Google Scholar] [CrossRef]
- Tanguy, S.; Grauzam, S.; de Leiris, J.; Boucher, F. Impact of dietary selenium intake on cardiac health: Experimental approaches and human studies. Mol. Nutr. Food Res. 2012, 56, 1106–1121. [Google Scholar] [CrossRef]
- Longnecker, M.P.; Taylor, P.R.; Levander, O.A.; Howe, M.; Veillon, C.; McAdam, P.A.; Patterson, K.Y.; Holden, J.M.; Stampfer, M.J.; Morris, J.S.; et al. Selenium in diet, blood, and toenails in relation to human health in a seleniferous area. Am. J. Clin. Nutr. 1991, 53, 1288–1294. [Google Scholar]
- Yang, G.; Wang, S.; Zhou, R.; Sun, S. Endemic selenium intoxication of humans in China. Am. J. Clin. Nutr. 1983, 37, 872–881. [Google Scholar]
- Reid, M.E.; Stratton, M.S.; Lillico, A.J.; Fakih, M.; Natarajan, R.; Clark, L.C.; Marshall, J.R. A report of high-dose selenium supplementation: Response and toxicities. J. Trace Elem. Med. Biol. 2004, 18, 69–74. [Google Scholar] [CrossRef]
- Bailey, R.L.; Fulgoni, V.L., III; Keast, D.R.; Dwyer, J.T. Dietary supplement use is associated with higher intakes of minerals from food sources. Am. J. Clin. Nutr. 2011, 94, 1376–1381. [Google Scholar] [CrossRef]
- Morris, J.S.; Spate, V.L.; Ngwenyama, R.A. Determinants of selenium in the toenail biomonitor. J. Radioanal. Nucl. Chem. 2006, 269, 283–290. [Google Scholar] [CrossRef]
- Veatch, A.E.; Brockman, J.D.; Spate, V.L.; Robertson, J.D.; Morris, J.S. Selenium and nutrition: The accuracy and variability of the selenium content in commercial supplements. J. Radioanal. Nucl. Chem. 2005, 264, 33–38. [Google Scholar] [CrossRef]
- Helzlsouer, K.; Jacobs, R.; Morris, J.S. Acute selenium intoxication in the United States. Fed. Proc. (FASEB) 1985, 44, 7366. [Google Scholar]
- Epidemiologic Notes and Reports Selenium Intoxication—New York; Morbidity and Mortality Weekly Report; Centers for Disease Control and Prevention: Alanta, GA, USA, 1984; pp. 157–158.
- Clark, R.F.; Strukle, E.; Williams, S.R.; Manoguerra, A.S. Selenium poisoning from a nutritional supplement. J. Am. Med. Assoc. 1996, 275, 1087–1088. [Google Scholar]
- Welsh, S.O.; Holden, J.M.; Wolf, W.R.; Levander, O.A. Selenium in self-selected diets of Maryland residents. J. Am. Diet. Assoc. 1981, 79, 277–285. [Google Scholar]
- Pennington, J.A.; Schoen, S.A. Total diet study: Estimated dietary intakes of nutritional elements, 1982–1991. Int. J. Vitam. Nutr. Res. 1996, 66, 350–362. [Google Scholar]
- Combs, G.F., Jr. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef]
- Burk, R.F.; Norsworthy, B.K.; Hill, K.E.; Motley, A.K.; Byrne, D.W. Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 804–810. [Google Scholar] [CrossRef]
- Chun, O.K.; Floegel, A.; Chung, S.J.; Chung, C.E.; Song, W.O.; Koo, S.I. Estimation of antioxidant intakes from diet and supplements in U.S. adults. J. Nutr. 2010, 140, 317–324. [Google Scholar] [CrossRef]
- Timbo, B.B.; Ross, M.P.; McCarthy, P.V.; Lin, C.T.J. Dietary supplements in a national survey: Prevalence of use and reports of adverse effects. J. Am. Diet Assoc. 2006, 106, 1966–1974. [Google Scholar] [CrossRef]
- Bertrand, G. On the role of trace substances in agriculture. 8th Int. Cong. Appl. Chem. 1912, 28, 30–49. [Google Scholar]
- Mertz, W. Defining Trace Element Deficiencies and Toxicities in Man. In Molybdenum in the Environment; Dekker Publishing: New York, NY, USA, 1976; Volume 1, pp. 267–293. [Google Scholar]
- Mertz, W. Between a rock and a hard place: Dietary and toxicological standards for essential minerals. J. Nutr. 1998, 128, 375S–378S. [Google Scholar]
- Renwick, A.G. Toxicilogy of micronutrients: Adverse effects and uncertainty. J. Nutr. 2006, 136, 493S–501S. [Google Scholar]
- Mulholland, C.A.; Benford, D.J. What is known about the safety of multivitamin-multimineral supplements for the generally healthy population? Theoretical basis for harm. Am. J. Clin. Nutr. 2007, 85, 318S–322S. [Google Scholar]
- Hurst, R.; Hooper, L.; Norat, T.; Lau, R.; Aune, D.; Greenwood, D.C.; Vieira, R.; Collins, R.; Harvey, L.J.; Sterne, J.A.C.; et al. Selenium and prostate cancer: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 96, 111–122. [Google Scholar] [CrossRef]
- Bleys, J.; Navas-Acien, A.; Guallar, E. Serum selenium levels and all-cause cancer and cardiovascular mortality among U.S. adults. Arch. Intern. Med. 2008, 168, 404–410. [Google Scholar] [CrossRef]
- Chaing, E.C.; Shen, S.; Kengeri, S.S.; Xu, H.; Combs, G.F.; Morris, J.S.; Bostwick, D.G.; Waters, D.J. Defining the optimal selenium dose for prostate cancer risk reduction: Insights from the U-shaped relationship between selenium status, DNA damage, and apoptosis. Dose Response 2009, 8, 285–300. [Google Scholar]
- Bleys, J.; Navas-Acien, A.; Lachaustra, M.; Pastor-Barriuso, R.; Menke, A.; Ordovas, J.; Stranges, S.; Guallar, E. Serum selenium and peripheral arterial disease: Results from the National Health and Nutrition Examination Survey, 2003–2004. Am. J. Epidem. 2009, 169, 996–1003. [Google Scholar] [CrossRef]
- Laclaustra, M.; Navas-Acien, A.; Stranges, S.; Ordoval, J.M.; Guallar, E. Serum selenium concentrations and hypertension in the US population. Circ. Cardiovasc. Qual. Outcomes 2009, 2, 369–376. [Google Scholar] [CrossRef]
- Laclaustra, M.; Stranges, S.; Navas-Acien, A.; Ordovas, J.M.; Guallar, E. Serum selenium and serum lipids in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Atherosclerosis 2010, 210, 643–648. [Google Scholar] [CrossRef]
- Stranges, S.; Marshall, J.R.; Trevisian, M.; Natarajan, R.; Donahue, R.P.; Combs, G.F.; Farinaro, E.; Clark, L.C.; Reid, M.E. Effects of selenium supplementation on cardiovascular disease incidence and mortality: Secondary analyses in a randomized trial. Am. J. Epidemiol. 2006, 163, 694–699. [Google Scholar] [CrossRef]
- Navas-Acien, A.; Bleys, J.; Guallar, E. Selenium intake and cardiovascular risk: What is new? Curr. Opin. Lipidol. 2008, 19, 43–49. [Google Scholar] [CrossRef]
- Stranges, S.; Navas-Acien, A.; Rayman, M.P.; Guallar, E. Selenium status and cardiometabolic health: State of the evidence. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 754–760. [Google Scholar] [Green Version]
- Laclaustra, M.; Navas-Acien, A.; Stranges, S.; Ordovas, J.M.; Guallar, E. Serum selenium concentration and diabetes in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Environ. Health Persp. 2009, 117, 1409–1413. [Google Scholar]
- Park, K.; Rimm, E.B.; Siscovick, D.S.; Spiegelman, D.; Manson, J.E.; Morris, J.S.; Hu, F.B.; Mozaffarian, D. Toenail selenium and incidence of type 2 diabetes in US men and women. Diabetes Care 2012, 35, 1545–1551. [Google Scholar]
- FDA warns consumers about “Total Body Formula” and “Total Body Mega Formula”. U.S. Food and Drug Administration Web site. 27 March 2008. Available online: http://www.fda.gov/newsevents/newsroom/pressannouncements/2008/ucm116873.htm (accessed on 27 August 2012).
- FDA finds hazardous levels of selenium in samples of “Total Body Formula” and “Total Body Mega Formula”. U.S. Food and Drug Administration Web site. 9 April 2008. Available online: http://www.fda.gov/newsevents/newsroom/pressannouncements/2008/ucm116878.htm (accessed on 27 August 2012).
- CDC alert on adverse effects associated with consuming “Total Body Formula” and “Total Body Mega Formula”. Centers for Disease Control and Prevention Web site. 14 April 2008. Available online: http://emergency.cdc.gov/han/han00274.asp (accessed on 27 August 2012).
- FDA completes final analysis of “Total Body Formula” and “Total Body Mega Formula” products. Testing reveals high chromium levels in addition to selenium. U.S. Food and Drug Administration Web site. 1 May 2008. Available online: http://www.fda.gov/newsevents/newsroom/pressannouncements/2008/ucm116892.htm (accessed on 27 August 2012).
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef]
- Morris, J.S.; Stampfer, M.J.; Willett, W.C. Dietary selenium in humans, toenails as an indicator. Biol. Trace Elem. Res. 1983, 5, 529–537. [Google Scholar] [CrossRef]
- Morris, J.S.; Ngwenyama, R.A.; Guthrie, J.M.; Brockman, J.D.; Spate, V.L.; Robertson, J.D. Quality control in the neutron activation analysis of biological markers for selenium in epidemiological investigations. J. Radioanal. Nucl. Chem. 2008, 276, 7–13. [Google Scholar] [CrossRef]
- Kerdel-Vegas, F. The depilatory and cytotoxic action of “Coco de Mono” (Lecythis ollaria) and its relationship to chronic selenosis. Econ. Bot. 1966, 20, 187–195. [Google Scholar] [CrossRef]
- Parekh, P.P.; Khan, A.R.; Torres, M.A.; Kitto, M.E. Concentrations of selenium, barium, and radium in Brazil nuts. J. Food Comp. Anal. 2008, 21, 332–335. [Google Scholar] [CrossRef]
- Thomson, C.D.; Chisholm, A.; McLachlan, S.K.; Campbell, J.M. Brazil nuts: An effective way to improve selenium status. Am. J. Clin. Nutr. 2008, 87, 379–384. [Google Scholar]
- Yang, J. Brazil nuts and associated health benefits: A review. Food Sci. Technol. 2009, 42, 1573–1580. [Google Scholar]
- Müller, D.; Desel, H. Acute selenium poisoning by paradise nuts (Lecythis ollaria). Hum. Exp. Toxicol. 2010, 29, 431–434. [Google Scholar] [CrossRef]
- Schellmann, B.; Raithel, H.J.; Schaller, K.H. Acute fatal selenium poisoning. Toxicological and occupational medical aspects. Arch. Toxicol. 1986, 59, 61–63. [Google Scholar] [CrossRef]
- Koppel, C.; Baudisch, H.; Beyer, K.H.; Kloppel, I.; Schneider, V. Fatal poisoning with selenium dioxide. J. Toxicol. Clin. Toxicol. 1986, 24, 21–35. [Google Scholar] [CrossRef]
- Vinceti, M.; Wei, E.T.; Malagoli, C.; Bergomi, M.; Vivoli, G. Adverse health effects of selenium in humans. Rev. Environ. Health 2001, 16, 233–251. [Google Scholar]
- Kise, Y.; Yoshimura, S.; Akieda, K.; Umezawa, K.; Okada, K.; Yoshitake, N.; Shiramizu, H.; Yamamoto, I.; Inokuchi, S. Acute oral selenium intoxication with ten times the lethal dose resulting in deep gastric ulcer. J. Emerg. Med. 2004, 26, 183–187. [Google Scholar] [CrossRef]
- Nuttall, K.L. Review: Evaluating selenium poisoning. Ann. Clin. Lab. Sci. 2006, 36, 409–420. [Google Scholar]
- Spiller, H.A.; Pfiefer, E. Case report: Two fatal cases of selenium toxicity. Forensic Sci. Int. 2007, 171, 67–72. [Google Scholar] [CrossRef]
- Williams, R. Acute selenium toxicity: Australia’s second fatality. Pathology 2007, 39, 289–290. [Google Scholar] [CrossRef]
- Sutter, M.E.; Thomas, J.D.; Brown, J.; Morgan, B. Selenium toxicity: A case of selenosis caused by a nutritional supplement. Ann. Intern. Med. 2008, 148, 970–971. [Google Scholar]
- Melstrom, P.C. Investigation of selenosis associated with a dietary supplement. Georgia Epidemiol. Rep. 2008, 24, 1–4. [Google Scholar]
- Lopez, R.E.; Knable, A.L.; Burruss, J.B. Ingestion of a dietary supplement resulting in selenium toxicity. J. Am. Acad. Dermatol. 2010, 63, 168–169. [Google Scholar] [CrossRef]
- Aldosary, B.M.; Sutter, M.E.; Schwartz, M.; Morgan, B.W. Case series of selenium toxicity from a nutritional supplement. Clin. Toxicol. 2012, 50, 57–64. [Google Scholar] [CrossRef]
- Ashar, B.H. The Dietary Supplement Health and Education Act: Time for a reassessment. Arch. Intern. Med. 2010, 170, 261–263. [Google Scholar] [CrossRef]
- Hunter, D.J.; Morris, J.S.; Chute, C.G.; Kushner, E.; Colditz, G.A.; Stampfer, M.J.; Speizer, F.E.; Willett, W.C. Predictors of selenium concentration in human toenails. Am. J. Epidemiol. 1990, 132, 114–122. [Google Scholar]
- Van den Brandt, P.A.; Goldbohm, R.A.; van’t Veer, P.; Bode, P.; Hermus, R.J.; Strumans, F. Predictors of toenail selenium levels in men and women. Cancer Epidemiol. Biomarkers Prevent. 1993, 2, 107–112. [Google Scholar]
- Garland, M.; Morris, J.S.; Rosner, B.A.; Stampfer, M.J.; Spate, V.L.; Baskett, C.J.; Willett, W.C.; Hunter, D.J. Toenail trace element levels as biomarkers: Reproducibility over a 6-year period. Cancer Epidemiol. Biomarkers Prev. 1993, 2, 493–497. [Google Scholar]
- Satia, J.A.; King, I.B.; Morris, J.S.; Stratton, K.; White, E. Toenail and plasma levels as biomarkers of selenium exposure. Ann. Epidemiol. 2006, 16, 53–58. [Google Scholar] [CrossRef]
- Swanson, C.A.; Longnecker, M.P.; Veillon, C.; Howe, M.; Levander, O.A.; Taylor, P.R.; McAdam, P.A.; Brown, C.C.; Stampfer, M.J.; Willett, W.C. Selenium intake, age, gender, and smoking in relation to indices of selenium status of adults residing in a seleniferous area. Am. J. Clin. Nutr. 1990, 52, 858–862. [Google Scholar]
- Longnecker, M.P.; Stampfer, M.J.; Morris, J.S.; Spate, V.; Baskett, C.; Mason, M.; Willett, W.C. A 1-y trial of the effect of high-selenium bread on selenium concentrations in blood and toenails. Am. J. Clin. Nutr. 1993, 57, 408–413. [Google Scholar]
- Longnecker, M.P.; Stram, D.O.; Taylor, P.R.; Levander, O.A.; Howe, M.; Veillon, C.; McAdam, P.A.; Patterson, K.Y.; Holden, J.M.; Morris, J.S.; et al. Use of selenium concentrations in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology 1996, 7, 384–390. [Google Scholar] [CrossRef]
- Krogh, V.; Pala, V.; Vinceti, M.; Berrino, F.; Ganzi, A.; Micheli, A.; Muti, P.; Vescov, L.; Ferrari, A.; Fortini, K.; Sieri, S.; Vivoli, G. Toenail selenium as a biomarker: Reproducibility over a one-year period and factors influencing reproducibility. J. Trace Elem. Med. Biol. 2003, 17, 31–36. [Google Scholar]
- Garland, M.; Morris, J.S.; Stampfer, M.J.; Colditz, G.A.; Spate, V.L.; Baskett, C.K.; Rosner, B.; Speizer, F.E.; Willett, W.C.; Hunter, D.J. Prospective study of toenail selenium and cancer among women. J. Natl. Cancer Inst. 1995, 87, 497–505. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Willett, W.C.; Morris, J.S.; Stampfer, M.J.; Spiegelman, D.; Rimm, E.B.; Giovannucci, E. Study of prediagnostic selenium level in toenails and the risk of advanced prostate cancer. J. Natl. Cancer Inst. 1998, 90, 1219–1224. [Google Scholar] [CrossRef]
- Park, K.; Rimm, E.; Siscovick, D.; Spiegelman, D.; Morris, J.S.; Mozaffarian, D. Demographic and lifestyle factors and selenium levels in men and women in the U.S. Nutr. Res. Pract. 2011, 5, 357–364. [Google Scholar] [CrossRef]
- Xun, P.; Liu, K.; Morris, J.S.; Daviglus, M.L.; He, K. Longitudinal association between toenail selenium levels and measures of subclinical atherosclerosis: The CARDIA trace element study. Atherosclerosis 2010, 210, 662–667. [Google Scholar] [CrossRef]
- Xun, P.; Liu, K.; Morris, J.S.; Daviglus, M.L.; Stevens, J.; Jacobs, D.R., Jr.; He, K. Associations of toenail selenium levels with inflammatory biomarkers of fibrinogen, high-sensitivity C-reactive protein, and interleukin-6. Am. J. Epidemiol. 2010, 171, 793–800. [Google Scholar] [CrossRef]
- Xun, P.; Hou, N.; Daviglus, M.; Liu, K.; Morris, J.S.; Shikany, J.M.; Sidney, S.; Jacobs, D.R.; He, K. Fish oil, selenium and mercury in relation to incidence of hypertension: A 20-year follow-up study. J. Intern. Med. 2010, 270, 175–186. [Google Scholar]
- Xun, P.; Bujnowski, D.; Liu, K.; Morris, J.S.; Guo, Z.; He, K. Distribution of toenail selenium levels in young adult Caucasians and African Americans in the United States: The CARDIA trace element study. Environ. Res. 2011, 111, 514–519. [Google Scholar] [CrossRef]
- George, G.N.; Singh, S.P.; Myers, G.J.; Watson, G.E.; Pickering, I.J. The chemical forms of mercury in human hair: A study using X-ray absorption spectroscopy. J. Biol. Inorg. Chem. 2010, 15, 709–715. [Google Scholar] [CrossRef]
- Morris, J.S.; Spate, V.L.; Ngwenyama, R.A.; Waters, D.J. Determination of selenium status using the nail biologic monitor in a canine model. J. Radioanal. Nucl. Chem. 2012, 291, 439–444. [Google Scholar] [CrossRef]
- Behne, D.; Alber, D.; Kyriakopoulos, A. Selenium distribution in tissues and monitor materials after long-term selenium supplementation investigated by neutron activation analysis. J. Radioanal. Nucl. Chem. 2009, 281, 31–34. [Google Scholar] [CrossRef]
- Patterson, B.H.; Levander, O.A.; Helzlsouer, K.; McAdam, P.A.; Lewis, S.A.; Taylor, P.R.; Veillon, C.; Zech, A. Human selenite metabolism: A kinetic model. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1989, 257, 556–567. [Google Scholar]
- Gaziano, J.M.; Sesso, H.D.; Christen, W.G.; Bubes, V.; Smith, J.P.; MacFadyen, J.; Schvartz, M.; Manson, J.E.; Glynn, R.J.; Buring, J.E. Multivitamins in the prevention of cancer in men. J. Am. Med. Assoc. 2012, 308, 1871–1880. [Google Scholar] [CrossRef]
- Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010; US Department of Agriculture and US Department of Health and Human Services: Washington, DC, USA, 2010. Available online: http://www.cnpp.usda.gov/DGAs2010-DGACReport.htm (accessed on 30 October 2012).
- NIH State-of-the-Science Panel. National Institutes of Health State-of-the-Science conference statement: Multivitamin/multimineral supplements and chronic disease prevention. Ann. Intern. Med. 2006, 145, 364–371.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Morris, J.S.; Crane, S.B. Selenium Toxicity from a Misformulated Dietary Supplement, Adverse Health Effects, and the Temporal Response in the Nail Biologic Monitor. Nutrients 2013, 5, 1024-1057. https://doi.org/10.3390/nu5041024
Morris JS, Crane SB. Selenium Toxicity from a Misformulated Dietary Supplement, Adverse Health Effects, and the Temporal Response in the Nail Biologic Monitor. Nutrients. 2013; 5(4):1024-1057. https://doi.org/10.3390/nu5041024
Chicago/Turabian StyleMorris, John Steven, and Stacy B. Crane. 2013. "Selenium Toxicity from a Misformulated Dietary Supplement, Adverse Health Effects, and the Temporal Response in the Nail Biologic Monitor" Nutrients 5, no. 4: 1024-1057. https://doi.org/10.3390/nu5041024




