Pharmacokinetics of High-Dose Weekly Oral Vitamin D3 Supplementation during the Third Trimester of Pregnancy in Dhaka, Bangladesh
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants
2.2. Study Design and Interventions
2.3. Data Collection Procedures
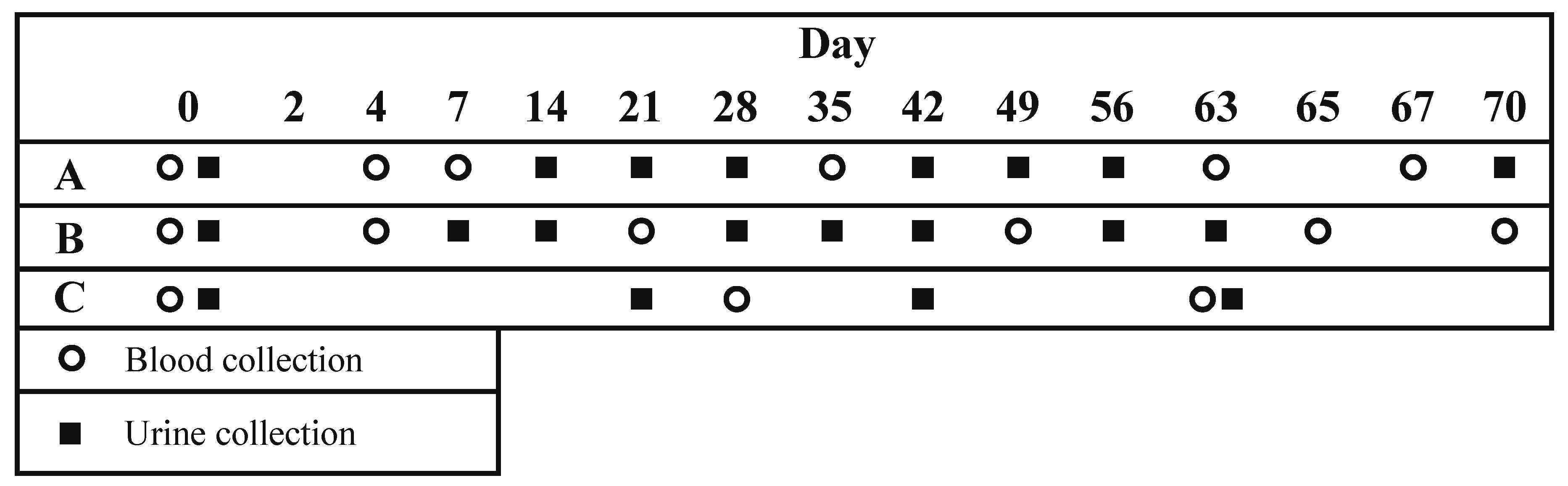
2.4. Specimen Collection and Biochemical Analyses
2.5. Safety Monitoring
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics and Retention.

| NH | PH | PL | p | ||
|---|---|---|---|---|---|
| # Enrolled | 16 | 14 | 14 | ||
| Age (years), Mean (±SD) | 24.6 (±4.5) | 22.2 (±3.1) | 22.1 (±4.8) | 0.190 | |
| Gestational age at enrollment (weeks) | |||||
| Mean (±SD) | - | 28.4 (±1.2) | 28.5 (±1.3) | 0.760 | |
| Range | - | 26.1-30.6 | 27-30.7 | ||
| Married | 12 (75%) | 14 (100%) | 13 (93%) | 0.110 | |
| Education level attained | |||||
| None | 2 (13%) | 4 (29%) | 3 (21%) | 0.285 | |
| Primary | 10 (63%) | 9 (64%) | 11 (79%) | ||
| Secondary or higher | 4 (25%) | 1 (7%) | 0 | ||
| Height (cm), mean (±SD) | 152.1 (±4.7) | 150.7 (±4.7) | 148.9 (±4.5) | 0.179 | |
| Non-pregnant | Pregnant | Pregnant | ||||
|---|---|---|---|---|---|---|
| NH | PH | PL | ||||
| # Enrolled | 16 | 14 | 14 | |||
| Vitamin D3 regimen | ||||||
| Loading dose | 70,000 IU | 70,000 IU | 0 | |||
| Weekly doses | 35,000 IU | 35,000 IU | 14,000 IU | |||
| Duration of supplementation | 10 weeks | 27–30 weeks gestation until delivery | 27–30 weeks gestation until delivery | |||
| Dates of enrollment | 17 Aug–6 Sep 2009 | 3–16 Feb 2010 | 3–16 Feb 2010 | |||
| Participants with [25(OH)D] measured during 10th week (days 63 to 70), n (%) | 14 (88%) | 10 (71%) | 9 (64%) | p value 2 | ||
| Number of specimens per participant, Median | 6 | 6 | 3 | PH vs. NH | PH vs. PL | |
| Baseline [25(OH)D] | ||||||
| Mean [95% CI] | 57 [47,69] | 35 [30,42] | 31 [26,38] | <0.001 | 0.341 | |
| Range (min, max) | 27, 93 | 21, 55 | 20, 57 | |||
| Attained [25(OH)D] in 10th week | ||||||
| Mean [95% CI] | 139 [121,160] | 98 [89,109] | 76 [61,95] | <0.001 | 0.038 | |
| Range (min, max) | 85, 238 | 71, 153 | 36, 119 | |||
| ∆[25(OH)D] in 10th week | ||||||
| Mean [95% CI] | 76 [61,96] | 57 [44,73] | 36 [22,61] | 0.082 | 0.128 | |
| Range on days 63 to 70 | 28, 160 | 19, 130 | 7, 75 | |||
| ∆[25(OH)D] at days 63 to 70 per daily vitamin D3 dose (nmol/L/mcg) | ||||||
| Mean [95% CI] | 0.61 [0.48, 0.79] | 0.46 [0.34,0.61] | 0.73 [0.38,1.38] | 0.220 | 0.081 | |
| Area under the ∆[25(OH)D]-time curve (nmol·d/L) to day 63/65 (AUC63) 3 | 3500 [2886,4245] | 2925 [2331,3670] | 1678 [923,3053] | 0.383 | 0.020 | |
| Participants with mean [25(OH)D] | ||||||
| ≥ 50 nmol/L in 10th week, #/n (%)4 | 14/14 (100%) | 10/10 (100%) | 8/9 (89%) | 1.000 | 0.166 | |
| Participants with mean [25(OH)D] | ||||||
| ≥ 80 nmol/L in 10th week, #/n (%) 4 | 14/14 (100%) | 9/10 (90%) | 5/9 (56%) | 0.152 | 0.127 | |
| PTH | ||||||
| Baseline (n = 28), mean [95% CI] | - | 2.10 [1.26,3.52] | 1.53 [0.94,2.49] | |||
| Final (n = 22), mean [95% CI] | - | 1.63 [1.01,2.66] | 2.49 [1.61,3.85] | - | 0.011 5 | |
| Cord serum [25(OH)D] (n = 23) | ||||||
| Mean [95% CI] | - | 117 [99,137] | 98 [84,115] | - | 0.074 | |
| Range (min, max) | - | 74, 168 | 53, 124 | |||
| Cord [25(OH)D] ≥ 50 nmol/L, # /n (%) | - | 12/12 (100%) | 11/11 (100%) | - | 1.000 | |
| Cord [25(OH)D] ≥ 80 nmol/L, #/n (%) | - | 11/12 (92%) | 10/11 (91%) | - | 0.949 | |
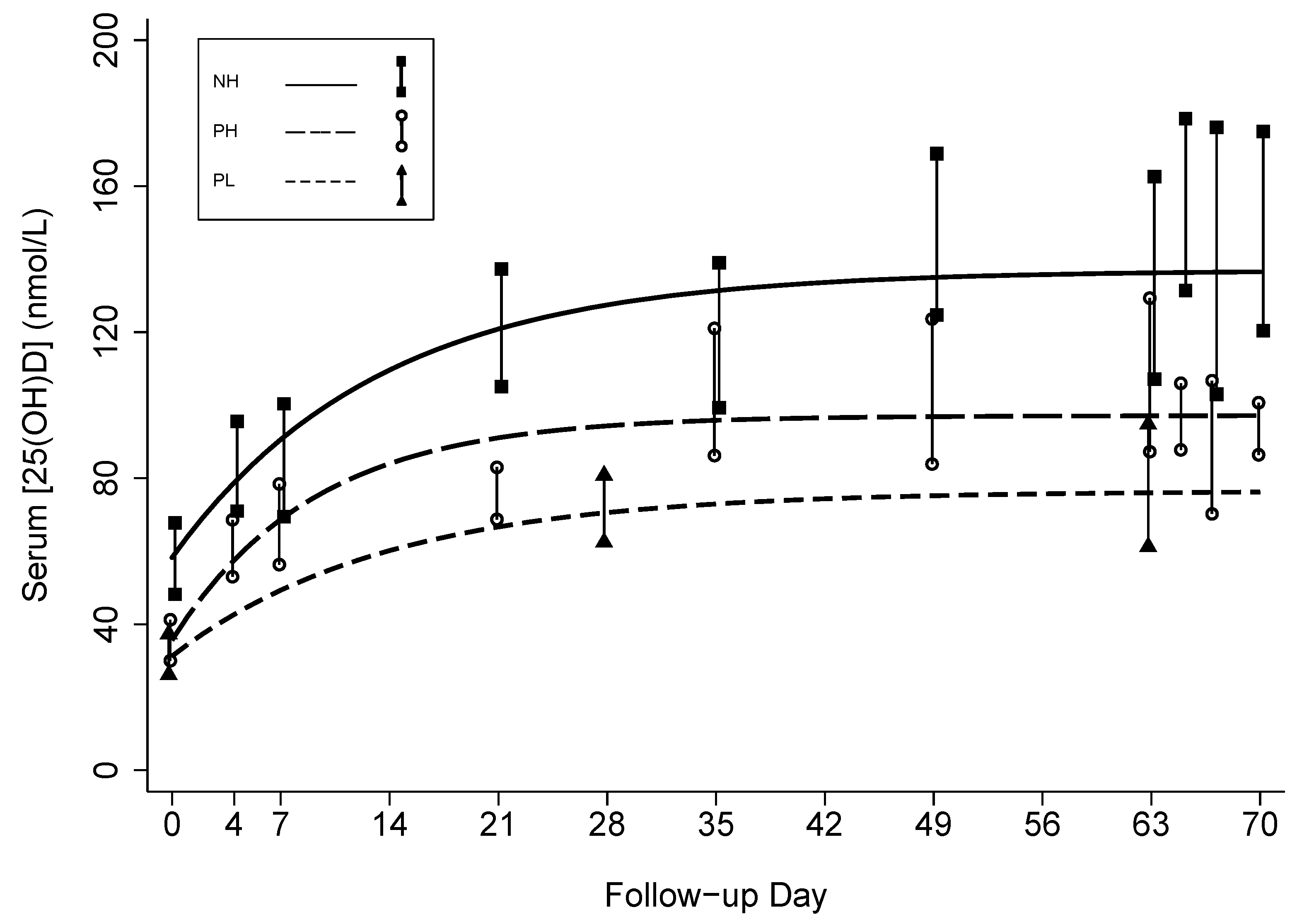
3.2. Effect of Prenatal Vitamin D3 Supplementation on Vitamin D Status
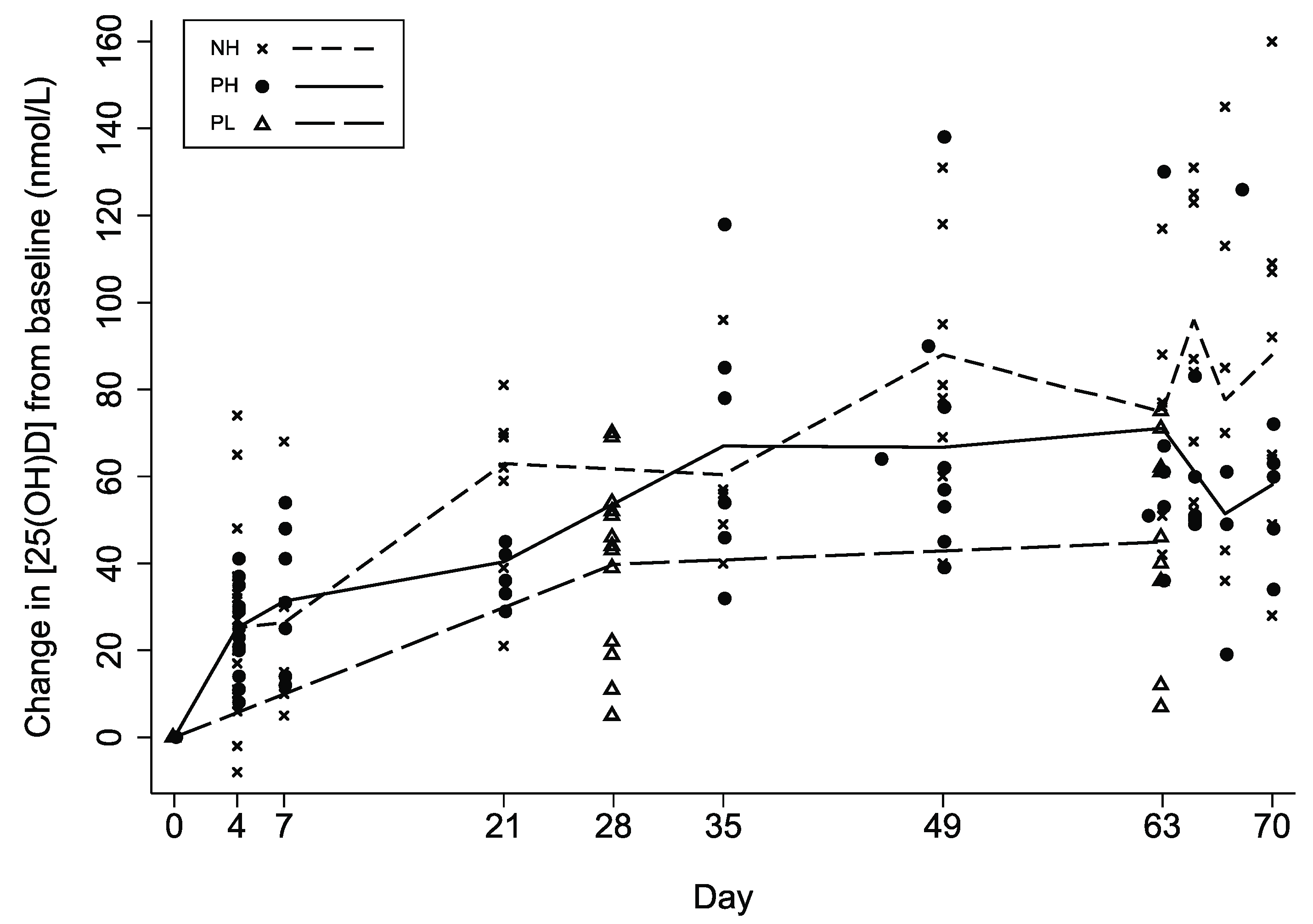
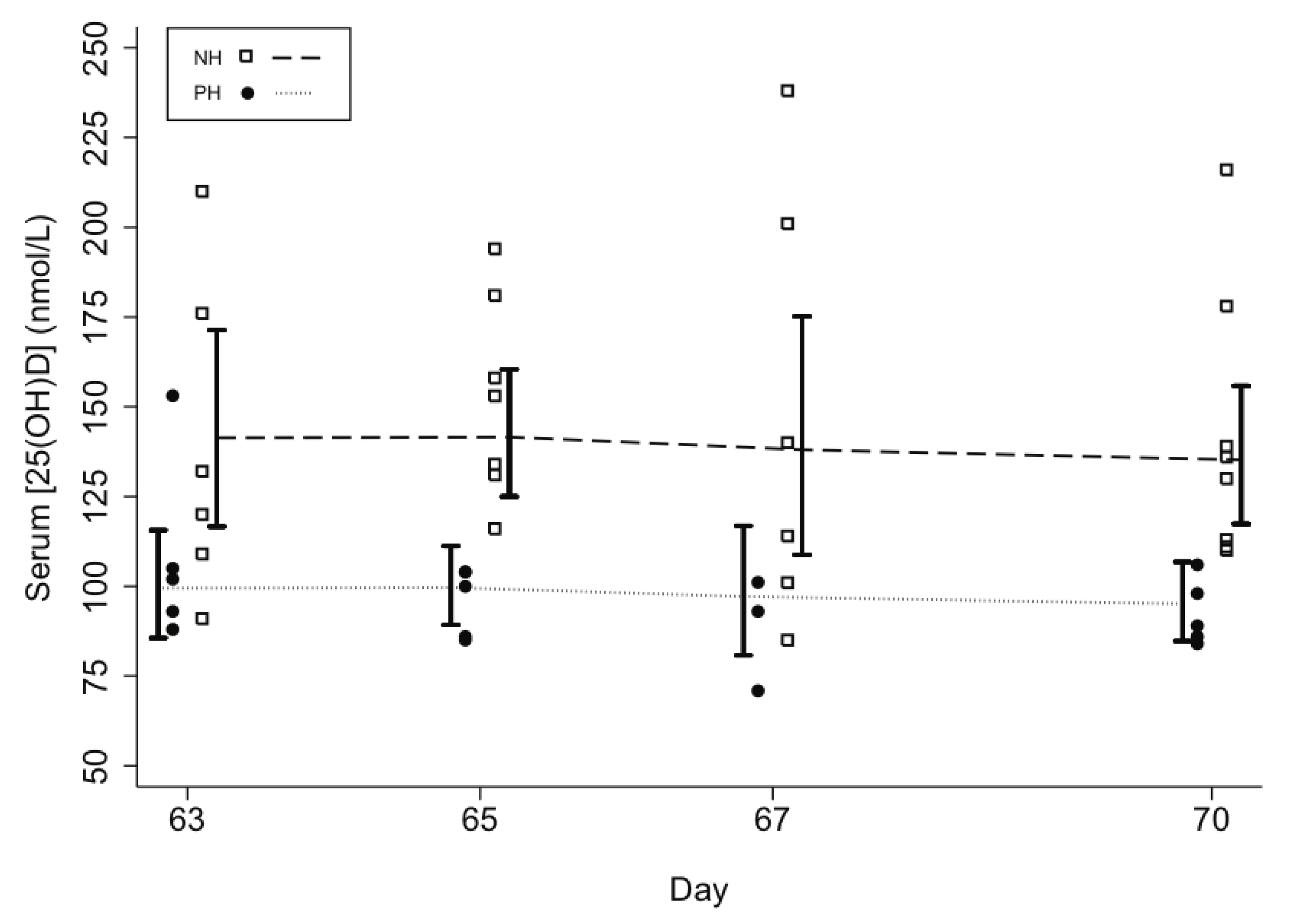
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||
|---|---|---|---|---|---|---|
| Non-pregnant (NH) | Pregnant, higher-dose (PH) | Pregnant, lower-dose (PL) | Pregnant (PL & PH) | Higher dose (NH & PH) | ||
| Number of participants | 16 | 14 | 14 | 28 | 29 | |
| Number of specimens | 89 | 75 | 36 | 111 | 162 | |
| Baseline [25(OH)D] | nmol/L | 58 [48,69] | 36 [29,42] | 31 [25,38] | 31 [25,37] | 57 [47,67] |
| ∆[25(OH)D] at steady-state (a) | nmol/L | 79 [60,97] | 62 [48,75] | 45 [23,67] | 43 [29,57] | 77 [62,93] |
| ∆[25(OH)D] at steady-state per daily dose equivalent | nmol/L/mcg D3 per day | 0.63 [0.48, 0.78] | 0.49 [0.38, 0.60] | 0.90 [0.47,1.34] | - | - |
| Steady-state [25(OH)D] ([25(OH)D]t0 + a) | nmol/L | 137 [116,157] | 97 [87,108] | 76 [54,98] | 74 [61,87] | 134 [117,151] |
| Decay rate (k) | days−1 | 0.08 [0.03,0.12] | 0.11 [0.07,0.15] | 0.07 [−0.01,0.16] | 0.11 [0.07, 0.15] | 0.09 [0.06, 0.12] |
| Group (g) | 0 (Ref) | - | - | - | PW-C | NP-H |
| 1 | - | - | - | PW-H | PW-H | |
| Difference in [25(OH)D] between groups at baseline (β) | nmol/L | - | - | - | 4 [−4,13] | −21 [−33,−9] |
| Difference in ∆[25(OH)D] between groups at steady-state (d) | nmol/L | - | - | - | 19 [1,37] | −15 [−34,5] |
| p = 0.044 | p = 0.131 | |||||
| Adjusted R2 | 0.55 | 0.71 | 0.63 | 0.72 | 0.69 |
3.3. Ancillary Biochemical Parameters
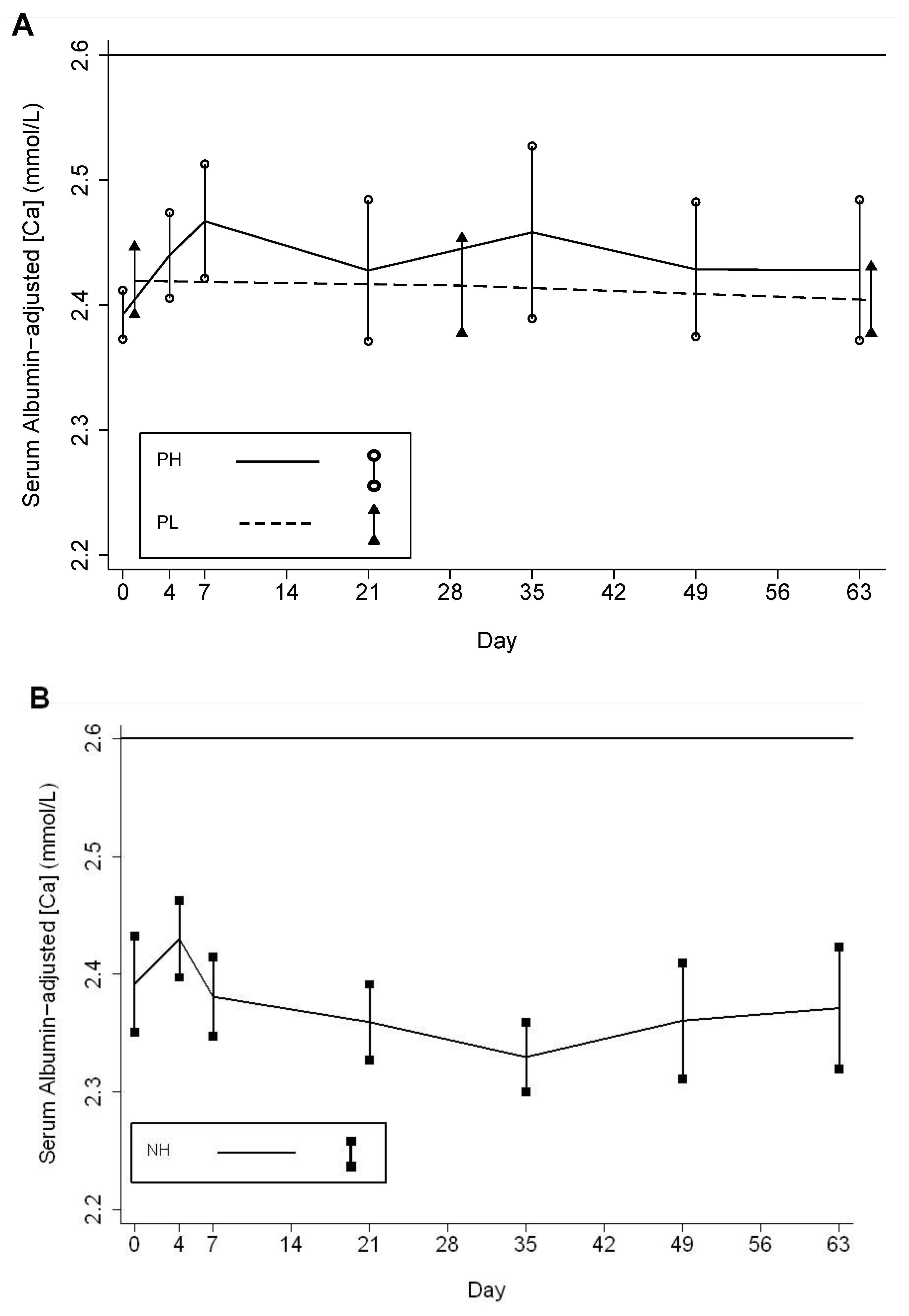
| n 1 | Albumin-adjusted serum calcium concentration (mmol/L) | # Episodes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | p value, | p value, | |||||||||
| Minimum, Maximum | >2.60 mmol/L | ||||||||||
| PH vs. NH 2 | PH vs. PL 2 | ||||||||||
| Follow-up period | NH | PH | PL | NH | PH | PL | NH | PH | PL | ||
| Baseline | 16 | 14 | 14 | 2.39 ± 0.08 | 2.39 ±0.04 | 2.42 ±0.05 | - | - | 0 | 0 | 0 |
| 2.22, 2.5 | 2.3, 2.45 | 2.35, 2.52 | |||||||||
| 1st to 5th week | 31 | 27 | 14 | 2.40 ± 0.07 | 2.45 ±0.07 3 | 2.42 ±0.07 | 0.020 | 0.012 | 0 | 1 4 | 0 |
| 2.25, 2.6 | 2.32, 2.61 | 2.33, 2.55 | |||||||||
| 6th to 10th week | 43 | 33 | 12 | 2.38 ± 0.07 | 2.44 ±0.07 5 | 2.42 ±0.05 | 0.009 | 0.055 | 0 | 0 | 0 |
| 2.2, 2.52 | 2.27, 2.57 | 2.33, 2.52 | |||||||||
| Total | 88 | 74 | 40 | 2.39 ± 0.07 | 2.43 ±0.07 | 2.42 ±0.06 | 0.991 | 0.104 | 0 | 1 | 0 |
| 2.2, 2.6 | 2.27, 2.61 | 2.33, 2.55 | |||||||||
| Cord Serum | - | 12 | 11 | - | 2.69 ± 0.12 | 2.73 ± 0.13 | - | 0.414 | 0 | 0 | 0 |
| 2.37, 2.82 | 2.56, 2.94 | ||||||||||
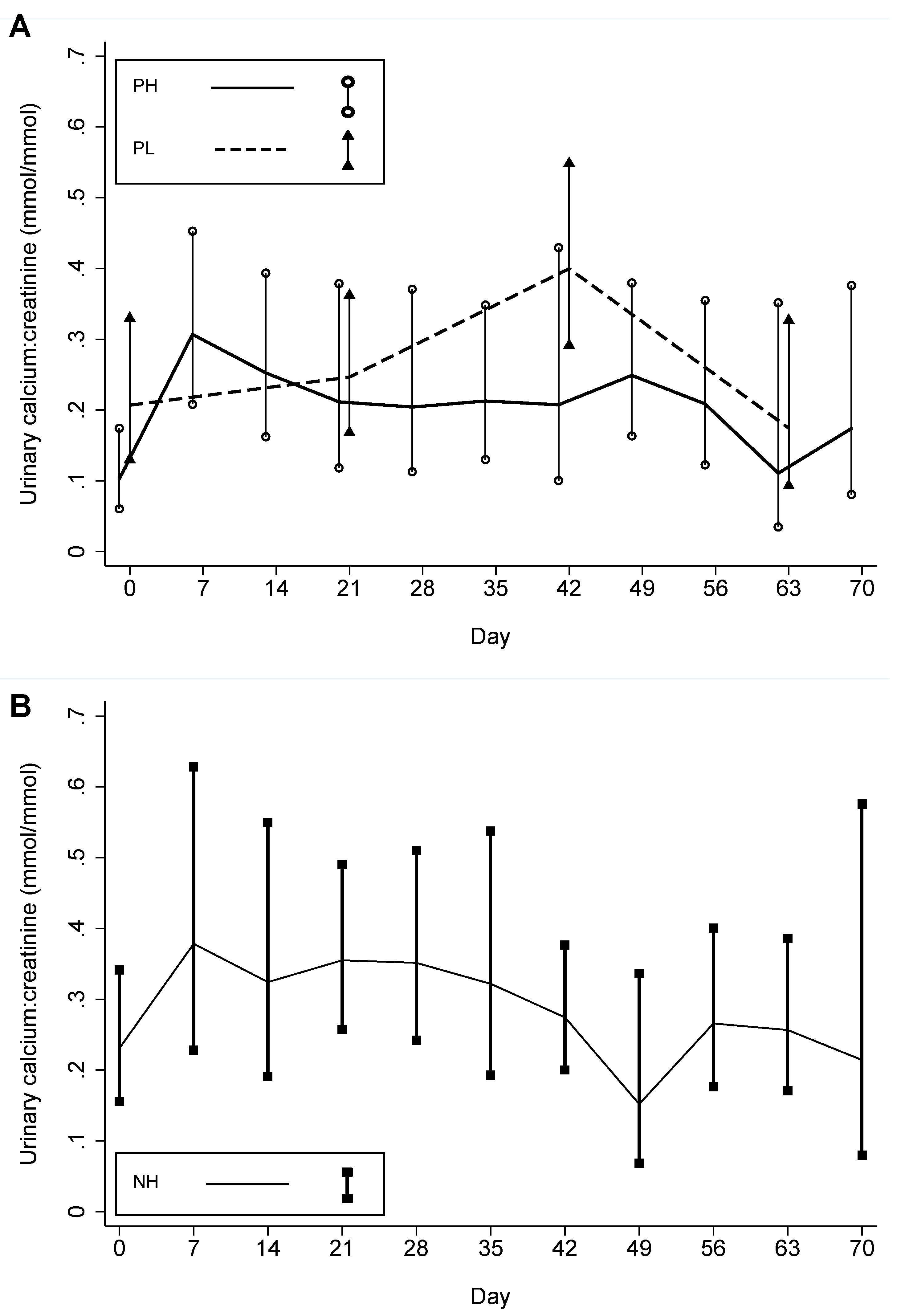
| n 1 | Urinary calcium-creatinine ratio (mmol/mmol) | # Episodes >1.0 mmol/mmol (# Participants ever having >1.0 mmol/mmol) | |||||||||
| Follow-up period | Mean 2Minimum, Maximum | p valuePH vs. NH 3 | p valuePH vs. PL 3 | ||||||||
| NH | PH | PL | NH | PH | PL | NH | PH | PL | |||
| Baseline | 16 | 14 | 14 | 0.23 | 0.10 | 0.21 | - | - | 0 | 0 | 0 |
| 0.04, 0.58 | 0.01, 0.44 | 0.06, 0.91 | (0) | (0) | (0) | ||||||
| 1st to 5th weeks | 49 | 36 | 12 | 0.36 4 | 0.24 5 | 0.24 | 0.164 | 0.105 | 3 | 0 | 0 |
| 0.04, 1.47 | 0.02, 0.95 | 0.07, 0.64 | (2) | (0) | (0) | ||||||
| 6th week to end | 62 | 53 | 33 | 0.26 | 0.18 6 | 0.30 | 0.164 | 0.500 | 0 | 0 | 2 |
| 0.03, 0.91 | 0.01, 0.96 | 0.05, 1.05 | (0) | (0) | (1) | ||||||
| Total | 127 | 103 | 59 | 0.29 | 0.19 | 0.26 | 0.014 | 0.047 | 3 | 0 | 2 |
| 0.03, 1.47 | 0.01, 0.96 | 0.05, 1.05 | (2) | (0) | (1) | ||||||
3.4. Clinical Outcomes
| PH | PL | p value (for between-group difference) | ||
|---|---|---|---|---|
| n | 12 | 12 | ||
| Gestational age at birth, weeks (by LMP) 1 | ||||
| Mean (±SD) | 39.2 (±2.3) | 38.5 (±2.7) | 0.512 | |
| Range | 33.6–42.3 | 32.7–43.2 | ||
| Preterm, # (%) | 1 (8%) | 3 (25%) | 0.590 | |
| Birth weight (g) | ||||
| Mean (±SD) 2 | 2774 (±456) | 2604 (±379) | 0.332 | |
| Range | 2210–4000 | 2020–3120 | ||
| # (%) SGA 3 | 9 (75%) | 8 (67%) | 1.000 | |
| # (%) LBW | 2 (17%) | 4 (33%) | 0.640 | |
| Delivery mode, # (%) Cesarean section 4 | 6 (50%) | 6 (50%) | 1.000 | |
| Sex, # (%) female | 6 (50%) | 6 (50%) | 1.000 | |
| Live births 5, # (%) | 12 (100%) | 12 (100%) | - | |
| Alive at 1 month of age, # (%) | 12 (100%) | 12 (100%) | - | |
4. Discussion
5. Conclusions
Appendix
Non-Linear Modeling of Change in 25[(OH)D] over Time
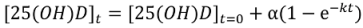
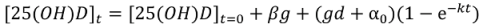

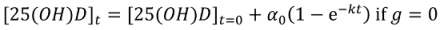
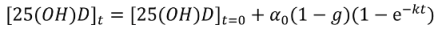
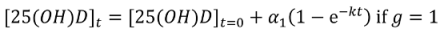
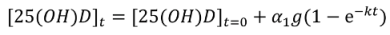
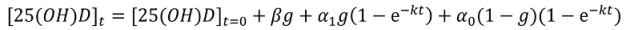
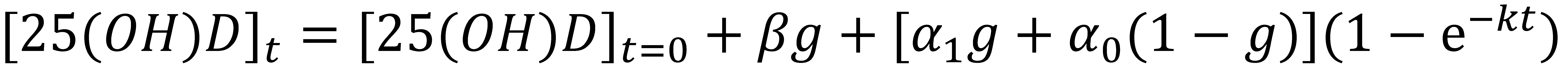
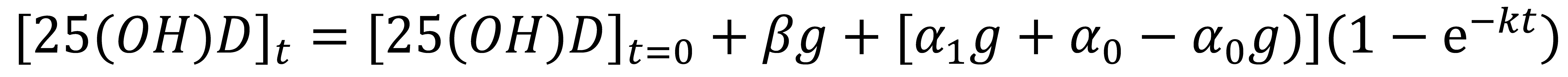
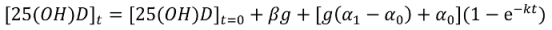
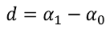
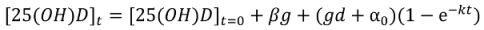
Conflict of Interest
Acknowledgments
References
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine. In Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- De-Regil, L.M.; Palacios, C.; Ansary, A.; Kulier, R.; Pena-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2012, 2. [Google Scholar] [CrossRef]
- Finkelstein, J.L.; Mehta, S.; Duggan, C.; Manji, K.P.; Mugusi, F.M.; Aboud, S.; Spiegelman, D.; Msamanga, G.I.; Fawzi, W.W. Maternal vitamin D status and child morbidity, anemia, and growth in human immunodeficiency virus-exposed children in Tanzania. Pediatr. Infect. Dis. J. 2012, 31, 171–175. [Google Scholar] [CrossRef]
- Belderbos, M.E.; Houben, M.L.; Wilbrink, B.; Lentjes, E.; Bloemen, E.M.; Kimpen, J.L.; Rovers, M.; Bont, L. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics 2011, 127, e1513–e1520. [Google Scholar] [CrossRef]
- Morales, E.; Guxens, M.; Llop, S.; Rodriguez-Bernal, C.L.; Tardon, A.; Riano, I.; Ibarluzea, J.; Lertxundi, N.; Espada, M.; Rodriguez, A.; et al. Circulating 25-hydroxyvitamin D3 in pregnancy and infant neuropsychological development. Pediatrics 2012, 130, e913–e920. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.; Shao, A.; Dawson-Hughes, B.; Hathcock, J.; Giovannucci, E.; Willett, W. Benefit–risk assessment of vitamin D supplementation. Osteoporosis Int. 2010, 21, 1121–1132. [Google Scholar] [CrossRef] [Green Version]
- Vieth, R. What is the optimal vitamin D status for health? Prog. Biophys. Mol. Biol. 2006, 92, 26–32. [Google Scholar] [CrossRef]
- Hollis, B.W. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J. Nutr. 2005, 135, 317–322. [Google Scholar]
- Roth, D.E. Vitamin D supplementation during pregnancy: Safety considerations in the design and interpretation of clinical trials. J. Perinatol. 2011, 31, 449–459. [Google Scholar] [CrossRef]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; de Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar]
- Islam, M.Z.; Lamberg-Allardt, C.; Karkkainen, M.; Outila, T.; Salamatullah, Q.; Shamim, A.A. Vitamin D deficiency: A concern in premenopausal Bangladeshi women of two socio-economic groups in rural and urban region. Eur. J. Clin. Nutr. 2002, 56, 51–56. [Google Scholar] [CrossRef]
- Roth, D.E.; Shah, M.R.; Black, R.E.; Baqui, A.H. Vitamin D status of infants in northeastern rural Bangladesh: Preliminary observations and a review of potential determinants. J. Health Popul. Nutr. 2010, 28, 458–469. [Google Scholar]
- Roth, D.E.; Mahmud, A.; Raqib, R.; Black, R.E.; Baqui, A.H. Pharmacokinetics of a single oral dose of vitamin D3 (70,000 IU) in pregnant and non-pregnant women. Nutr. J. 2012, 11. [Google Scholar] [CrossRef]
- Wagner, D.; Hanwell, H.E.; Vieth, R. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin. Biochem. 2009, 42, 1549–1556. [Google Scholar] [CrossRef]
- Carter, G.D.; Berry, J.L.; Gunter, E.; Jones, G.; Jones, J.C.; Makin, H.L.; Sufi, S.; Wheeler, M.J. Proficiency testing of 25-hydroxyvitamin D (25-OHD) assays. J. Steroid. Biochem. Mol. Biol. 2010, 121, 176–179. [Google Scholar] [CrossRef]
- Perkins, S.L.; Livesey, J.F.; Belcher, J. Reference intervals for 21 clinical chemistry analytes in arterial and venous umbilical cord blood. Clin. Chem. 1993, 39, 1041–1044. [Google Scholar]
- Vieth, R.; Chan, P.C.; MacFarlane, G.D. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am. J. Clin. Nutr. 2001, 73, 288–294. [Google Scholar]
- Gordon, A.Y.; Salzman, P. Optimality of the holm procedure among general step-down multiple testing procedures. Stat. Probab. Lett. 2008, 78, 1878–1884. [Google Scholar] [CrossRef]
- Oken, E.; Kleinman, K.P.; Rich-Edwards, J.; Gillman, M.W. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003, 3. [Google Scholar] [CrossRef] [Green Version]
- Delvin, E.E.; Salle, B.L.; Glorieux, F.H.; Adeleine, P.; David, L.S. Vitamin D supplementation during pregnancy: Effect on neonatal calcium homeostasis. J. Pediatr. 1986, 109, 328–334. [Google Scholar] [CrossRef]
- Hollis, B.W.; Johnson, D.; Hulsey, T.C.; Ebeling, M.; Wagner, C.L. Vitamin D supplementation during pregnancy: Double blind, randomized clinical trial of safety and effectiveness. J. Bone Miner Res. 2011, 26, 2341–2357. [Google Scholar]
- Wagner, C.L.; McNeil, R.; Hamilton, S.A.; Winkler, J.; Rodriguez Cook, C.; Warner, G.; Bivens, B.; Davis, D.J.; Smith, P.G.; Murphy, M.; et al. A randomized trial of vitamin D supplementation in 2 community health center networks in south carolina. Am. J. Obstet. Gynecol. 2013, 208, 137.e1–137.e13. [Google Scholar] [CrossRef]
- Heaney, R.P.; Davies, K.M.; Chen, T.C.; Holick, M.F.; Barger-Lux, M.J. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am. J. Clin. Nutr. 2003, 77, 204–210. [Google Scholar]
- Aloia, J.F.; Patel, M.; Dimaano, R.; Li-Ng, M.; Talwar, S.A.; Mikhail, M.; Pollack, S.; Yeh, J.K. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am. J. Clin. Nutr. 2008, 87, 1952–1958. [Google Scholar]
- Barger-Lux, M.J.; Heaney, R.P.; Dowell, S.; Chen, T.C.; Holick, M.F. Vitamin D and its major metabolites: Serum levels after graded oral dosing in healthy men. Osteoporos. Int. 1998, 8, 222–230. [Google Scholar]
- Wagner, C.L.; Hollis, B.W. Beyond PTH: Assessing vitamin D status during early pregnancy. Clin. Endocrinol. 2011, 75, 285–286. [Google Scholar] [CrossRef]
- Goswami, R.; Gupta, N.; Goswami, D.; Marwaha, R.K.; Tandon, N.; Kochupillai, N. Prevalence and significance of low 25-hydroxyvitamin D concentrations in healthy subjects in delhi. Am. J. Clin. Nutr. 2000, 72, 472–475. [Google Scholar]
- Sachan, A.; Gupta, R.; Das, V.; Agarwal, A.; Awasthi, P.K.; Bhatia, V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am. J. Clin. Nutr. 2005, 81, 1060–1064. [Google Scholar]
- Bhalala, U.; Desai, M.; Parekh, P.; Mokal, R.; Chheda, B. Subclinical hypovitaminosis D among exclusively breastfed young infants. Indian Pediatr. 2007, 44, 897–901. [Google Scholar]
- Doi, M.; Sultana Rekha, R.; Ahmed, S.; Okada, M.; Kumar Roy, A.; El Arifeen, S.; Ekstrom, E.C.; Raqib, R.; Wagatsuma, Y. Association between calcium in cord blood and newborn size in Bangladesh. Br. J. Nutr. 2011, 106, 1398–1407. [Google Scholar] [CrossRef]
- Kovacs, C.S. Vitamin D in pregnancy and lactation: Maternal, fetal, and neonatal outcomes from human and animal studies. Am. J. Clin. Nutr. 2008, 88, 520S–528S. [Google Scholar]
- Papapetrou, P.D. The interrelationship of serum 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D in pregnancy at term: A meta-analysis. Hormones (Athens) 2010, 9, 136–144. [Google Scholar]
- Kovacs, C.S.; Kronenberg, H.M. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr. Rev. 1997, 18, 832–872. [Google Scholar] [CrossRef]
- Zehnder, D.; Evans, K.N.; Kilby, M.D.; Bulmer, J.N.; Innes, B.A.; Stewart, P.M.; Hewison, M. The ontogeny of 25-hydroxyvitamin D(3) 1alpha-hydroxylase expression in human placenta and decidua. Am. J. Pathol. 2002, 161, 105–114. [Google Scholar] [CrossRef]
- Standing Committee on the Scientific Evaluation of Dietary Reference IntakesFood and Nutrition BoardInstitute of MedicineDRI: Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academy Press: Washington, DC, USA, 1997.
- Hathcock, J.N.; Shao, A.; Vieth, R.; Heaney, R. Risk assessment for vitamin D. Am. J. Clin. Nutr. 2007, 85, 6–18. [Google Scholar]
- Joshi, R. Hypercalcemia due to hypervitaminosis D: Report of seven patients. J. Trop. Pediatr. 2009, 55, 396–398. [Google Scholar] [CrossRef]
- Heaney, R.P.; Recker, R.R.; Grote, J.; Horst, R.L.; Armas, L.A. Vitamin D(3) is more potent than vitamin D(2) in humans. J. Clin. Endocrinol. Metab. 2011, 96, E447–E452. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Roth, D.E.; Mahmud, A.A.; Raqib, R.; Akhtar, E.; Black, R.E.; Baqui, A.H. Pharmacokinetics of High-Dose Weekly Oral Vitamin D3 Supplementation during the Third Trimester of Pregnancy in Dhaka, Bangladesh. Nutrients 2013, 5, 788-810. https://doi.org/10.3390/nu5030788
Roth DE, Mahmud AA, Raqib R, Akhtar E, Black RE, Baqui AH. Pharmacokinetics of High-Dose Weekly Oral Vitamin D3 Supplementation during the Third Trimester of Pregnancy in Dhaka, Bangladesh. Nutrients. 2013; 5(3):788-810. https://doi.org/10.3390/nu5030788
Chicago/Turabian StyleRoth, Daniel E., Abdullah Al Mahmud, Rubhana Raqib, Evana Akhtar, Robert E. Black, and Abdullah H. Baqui. 2013. "Pharmacokinetics of High-Dose Weekly Oral Vitamin D3 Supplementation during the Third Trimester of Pregnancy in Dhaka, Bangladesh" Nutrients 5, no. 3: 788-810. https://doi.org/10.3390/nu5030788
APA StyleRoth, D. E., Mahmud, A. A., Raqib, R., Akhtar, E., Black, R. E., & Baqui, A. H. (2013). Pharmacokinetics of High-Dose Weekly Oral Vitamin D3 Supplementation during the Third Trimester of Pregnancy in Dhaka, Bangladesh. Nutrients, 5(3), 788-810. https://doi.org/10.3390/nu5030788





