Do Fat Supplements Increase Physical Performance?
Abstract
:1. Introduction
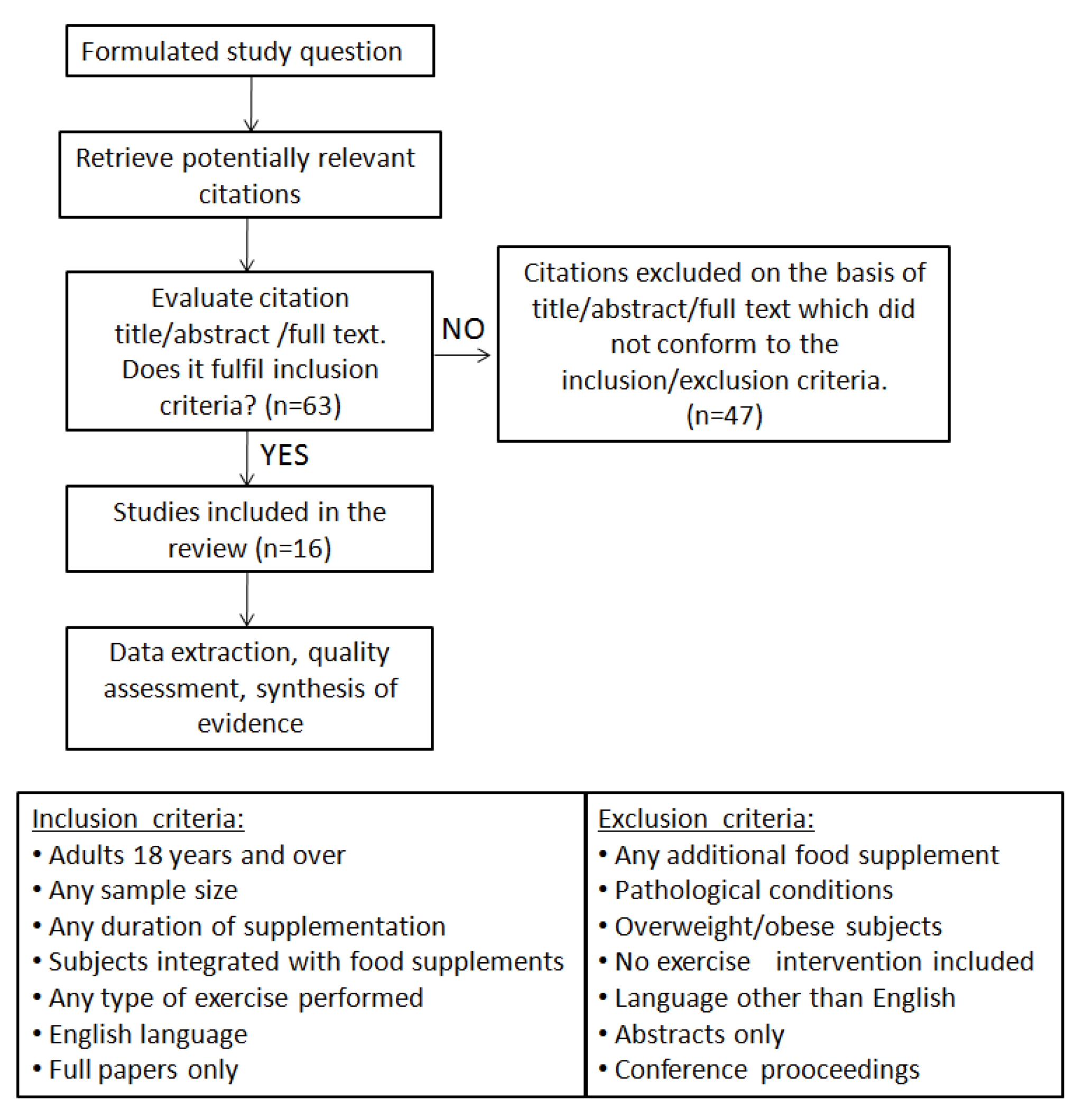
2. Association of Exercise and Fat Supplements
| Reference | Study Design | Participants (N, sex) | Exercise interventions | Time (fish oil) | Main outcome |
|---|---|---|---|---|---|
| Oostenbrug et al. [15] | D–R–P | Cyclist (24, M) | Acute aerobic bout (60 min time trial) | 3 weeks (6 g/day) | • No effect: endurance performance |
| Buckley et al. [16] | D–R–P | Australian rules football players (25, M) | Acute aerobic bout to exhaustion | 5 weeks (6 g/day) | • No effect: endurance performance, recovery; |
| • Improve: CV function | |||||
| Raastad et al. [17] | D–R–P | Soccer players (28, M) | Routine training (not supervised) | 10 weeks (5.2 g/day) | • No effect: maximal aerobic power, anaerobic power, performance |
| Peoples et al. [18] | D–R–P | Cyclist (16, M) | Acute aerobic bout (50% of peak workload) | 8 weeks (8 g/day) | • No effect: endurance performance; |
| • Reduce: whole-body and myocardial O2 demand | |||||
| Brilla et al. [19] | D–R | Sedentary (32, M) | 60 min (3 day/week) aerobic exercise | 10 weeks (4 g/day) | • No effect: Body composition; |
| • Improve: VO2max, VAT | |||||
| Guezennec et al. [20] | D–R–P | Healthy (14, M) | Acute aerobic bout (60 min 70% of VO2max) | 6 weeks (6 g/day) | • Improve: VO2max, RBC deformability |
| Ernst et al. [21] | D | Healthy (14, M) | Acute aerobic bout | 3 weeks (2.8 g/day) | • Reduce: inflammatory acute-phase response |
| Toft et al. [22] | D–R | Runners (20, M) | Marathon | 6 weeks (2.8 g/day) | • No effect: inflammatory acute-phase response |
| Lenn et al. [23] | D–R–P | Healthy (22, M) | 50 Maximal eccentric elbow flexion contractions | 30 days (1.8 g/day) | • No effect: inflammatory acute-phase response |
| Reference | Study Design | Participants (N, sex) | Exercise interventions | Time (CLA) | Main outcome |
|---|---|---|---|---|---|
| Zambell et at.[14] | D–R–P | Healthy (17, F) | Acute aerobic bout (walking) | 64 days (3 g/day) | • No effect: energy expenditure, RER, Fat oxidation |
| Kreider et al. [24] | D–R–P | Bodybuilders (23, M) | Resistance training(not supervised) | 4 weeks (6 g/day) | • No effect: Body composition, bone density, strength |
| Lambert et al. [25] | D–R–P | Physically active (25, M; 37, F) | Routine training (not supervised) | 12 weeks (3.9 g/day) | • No effect: Body composition, RER |
| Macaluso et al. [26] | D–R–P–C | Physically active (10, M) | Resistance training + Acute resistance bout | 3 weeks (6 g/day) | • No effect: Body composition; |
| • Slight increase total testosterone | |||||
| Thom et al. [27] | D–R–P | Physically active (10, M; 10, F) | 90 min (3 day/week)Strenuous exercise | 12 weeks (1.8 g/day) | • Improve: Body composition, endurance performance |
| Colakoglu et al. [28] | D–R–P–C | Healthy (44, F) | 30 min (3 day/week)Aerobic exercise | 6 weeks (3.6 g/day) | • Improve: Body composition, endurance performance |
| Pinkoski et al. [29] | D–R–P | Healthy (17, F) | 90 min (3 day/week)Resistance exercise | 7 weeks (5 g/day) | • Improve: Body composition |
3. Testosterone Biosynthesis
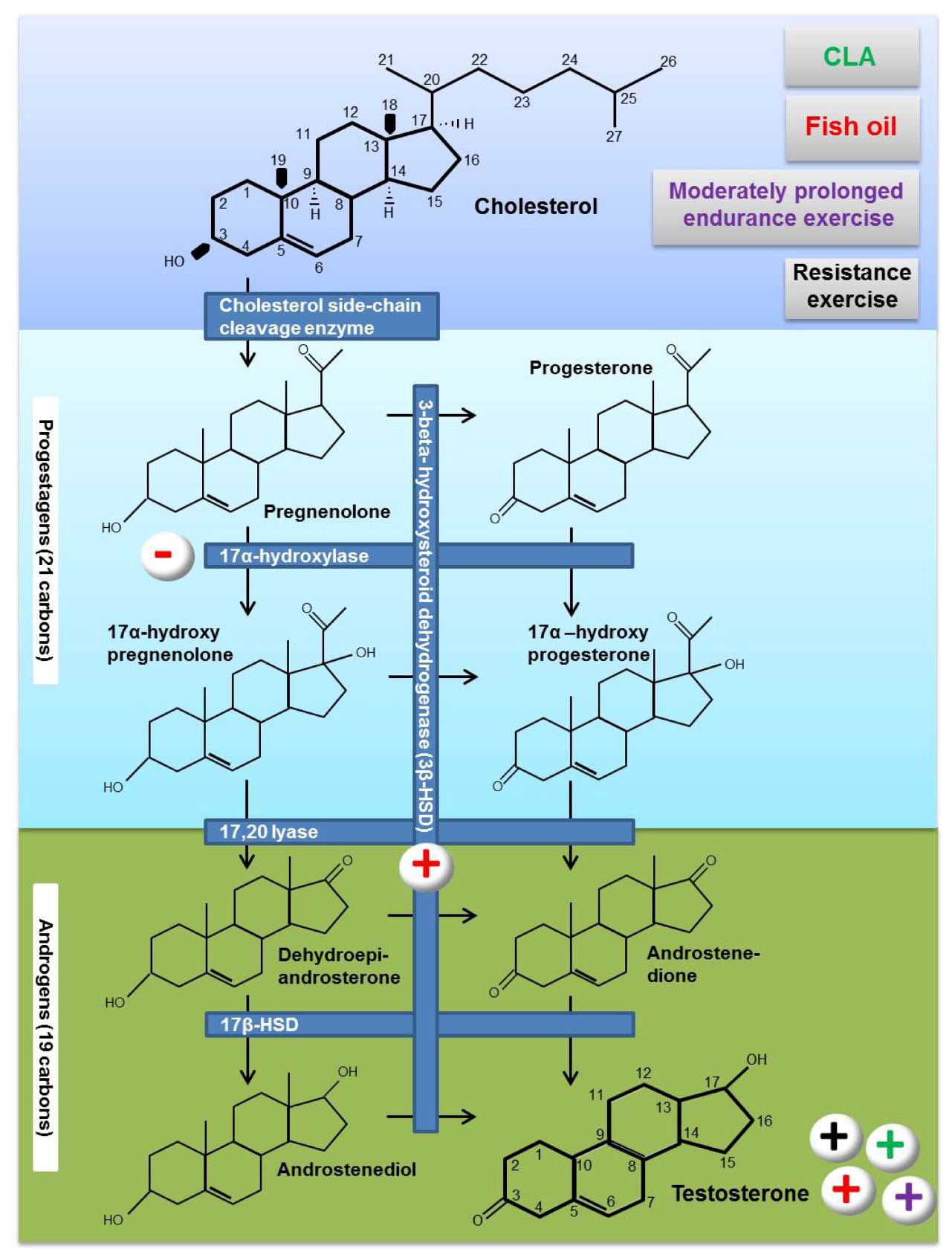
4. Effects of Fat Supplementation on Testosterone Biosynthesis
5. Cellular Mechanisms Responsible for the Effect of Testosterone on Skeletal Muscle and Physical Performance
6. Implications
Abbreviations
| CLA | conjugated linoleic acid |
| DHA | docosahexaenoic acid |
| EPA | eicosapentaenoic acid |
| c9 | cis-9 |
| t11 | trans-11 |
| t10 | trans-10 |
| c12 | cis-12 |
| RBC | red blood cells |
| PUFA | polyunsaturated fatty acids |
| P450ssc | P450 side-chain cleavage enzyme |
| 3β-HSD | 3β-hydroxysteroid dehydrogenase |
| P450c17 | 17α-hydroxylase/17,20-lyase |
| 17β-HSD | 17β-hydroxysteroid dehydrogenase |
| CYP17A1 | cytochrome P450, steroid 17α-hydroxylase/17,20-lyase |
| H295R | human adrenocortical carcinoma cells |
| PGF2α | prostagladin F2-α |
| MUFA | mono-unsaturated fatty acid |
| SFA | saturated fatty acid |
| CYP1A1 | cytochrome P450, family 1, subfamily A, polypeptide 1 |
| MC2R | melanocortin 2 receptor |
| StAR | steroidogenic acute regulatory protein |
| CYP11B1 | cytochrome P450, family 11, subfamily B, polypeptide 1 |
| CYP11B2 | cytochrome P450, family 11, subfamily B, polypeptide 2 |
| CYP19A1 | cytochrome P450, family 19, subfamily A, polypeptide 1 |
| HSD3B2 | hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 2 |
| STAT3 | signal transducer and activator of transcription 3 |
| SOCS3 | suppressor of cytokine signaling 3 |
| COX-2 | cyclooxygenase-2 |
References
- Dwyer, J.T.; Allison, D.B.; Coates, P.M. Dietary supplements in weight reduction. J. Am. Diet. Assoc. 2005, 105, S80–S86. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Aldred, S. Fat supplementation, health, and endurance performance. Nutrition 2004, 20, 678–688. [Google Scholar]
- Sciotto, C.; Mjos, S.A. Trans Isomers of EPA and DHA in Omega-3 Products on the European Market. Lipids 2012, 47, 659–667. [Google Scholar]
- Dewailly, E.E.; Blanchet, C.; Gingras, S.; Lemieux, S.; Sauve, L.; Bergeron, J.; Holub, B.J. Relations between n-3 fatty acid status and cardiovascular disease risk factors among Quebecers. Am. J. Clin. Nutr. 2001, 74, 603–611. [Google Scholar]
- Hill, A.M.; Buckley, J.D.; Murphy, K.J.; Howe, P.R. Combining fish-oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors. Am. J. Clin. Nutr. 2007, 85, 1267–1274. [Google Scholar]
- Lee, T.H.; Hoover, R.L.; Williams, J.D.; Sperling, R.I.; Ravalese, J., III; Spur, B.W.; Robinson, D.R.; Corey, E.J.; Lewis, R.A.; Austen, K.F. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N. Engl. J. Med. 1985, 312, 1217–1224. [Google Scholar]
- Andrade, P.M.; Ribeiro, B.G.; Bozza, M.T.; Costa Rosa, L.F.; Tavares do Carmo, M.G. Effects of the fish-oil supplementation on the immune and inflammatory responses in elite swimmers. Prostaglandins Leukot. Essent. Fatty Acids 2007, 77, 139–145. [Google Scholar]
- Hill, A.M.; Worthley, C.; Murphy, K.J.; Buckley, J.D.; Ferrante, A.; Howe, P.R. n-3 Fatty acid supplementation and regular moderate exercise: Differential effects of a combined intervention on neutrophil function. Br. J. Nutr. 2007, 98, 300–309. [Google Scholar] [CrossRef]
- Morris, M.C.; Manson, J.E.; Rosner, B.; Buring, J.E.; Willett, W.C.; Hennekens, C.H. Fish consumption and cardiovascular disease in the physicians’ health study: A prospective study. Am. J. Epidemiol. 1995, 142, 166–175. [Google Scholar]
- O’Shea, M.; Bassaganya-Riera, J.; Mohede, I.C. Immunomodulatory properties of conjugated linoleic acid. Am. J. Clin. Nutr. 2004, 79, 1199S–1206S. [Google Scholar]
- Di Felice, V.; Macaluso, F.; Montalbano, A.; Gammazza, A.M.; Palumbo, D.; Angelone, T.; Bellafiore, M.; Farina, F. Effects of conjugated linoleic acid and endurance training on peripheral blood and bone marrow of trained mice. J. Strength Cond. Res. 2007, 21, 193–198. [Google Scholar] [CrossRef]
- MacDonald, H.B. Conjugated linoleic acid and disease prevention: A review of current knowledge. J. Am. Coll. Nutr. 2000, 19, 111S–118S. [Google Scholar]
- Wang, Y.W.; Jones, P.J. Conjugated linoleic acid and obesity control: Efficacy and mechanisms. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 941–955. [Google Scholar] [CrossRef]
- Zambell, K.L.; Keim, N.L.; Van Loan, M.D.; Gale, B.; Benito, P.; Kelley, D.S.; Nelson, G.J. Conjugated linoleic acid supplementation in humans: Effects on body composition and energy expenditure. Lipids 2000, 35, 777–782. [Google Scholar] [CrossRef]
- Oostenbrug, G.S.; Mensink, R.P.; Hardeman, M.R.; De Vries, T.; Brouns, F.; Hornstra, G. Exercise performance, red blood cell deformability, and lipid peroxidation: Effects of fish oil and vitamin E. J. Appl. Physiol. 1997, 83, 746–752. [Google Scholar]
- Buckley, J.D.; Burgess, S.; Murphy, K.J.; Howe, P.R. DHA-rich fish oil lowers heart rate during submaximal exercise in elite Australian Rules footballers. J. Sci. Med. Sport 2009, 12, 503–507. [Google Scholar] [CrossRef]
- Raastad, T.; Hostmark, A.T.; Stromme, S.B. Omega-3 fatty acid supplementation does not improve maximal aerobic power, anaerobic threshold and running performance in well-trained soccer players. Scand. J. Med. Sci. Sports 1997, 7, 25–31. [Google Scholar]
- Peoples, G.E.; McLennan, P.L.; Howe, P.R.; Groeller, H. Fish oil reduces heart rate and oxygen consumption during exercise. J. Cardiovasc. Pharmacol. 2008, 52, 540–547. [Google Scholar]
- Brilla, L.R.; Landerholm, T.E. Effect of fish oil supplementation and exercise on serum lipids and aerobic fitness. J. Sports Med. Phys. Fitness 1990, 30, 173–180. [Google Scholar]
- Guezennec, C.Y.; Nadaud, J.F.; Satabin, P.; Leger, F.; Lafargue, P. Influence of polyunsaturated fatty acid diet on the hemorrheological response to physical exercise in hypoxia. Int. J. Sports Med. 1989, 10, 286–291. [Google Scholar]
- Ernst, E.; Saradeth, T.; Achhammer, G. n-3 fatty acids and acute-phase proteins. Eur. J. Clin. Invest. 1991, 21, 77–82. [Google Scholar] [CrossRef]
- Toft, A.D.; Thorn, M.; Ostrowski, K.; Asp, S.; Moller, K.; Iversen, S.; Hermann, C.; Sondergaard, S.R.; Pedersen, B.K. N-3 polyunsaturated fatty acids do not affect cytokine response to strenuous exercise. J. Appl. Physiol. 2000, 89, 2401–2406. [Google Scholar]
- Lenn, J.; Uhl, T.; Mattacola, C.; Boissonneault, G.; Yates, J.; Ibrahim, W.; Bruckner, G. The effects of fish oil and isoflavones on delayed onset muscle soreness. Med. Sci. Sports Exerc. 2002, 34, 1605–1613. [Google Scholar] [CrossRef]
- Kreider, R.B.; Ferreira, M.P.; Greenwood, M.; Wilson, M.; Almada, A.L. Effects of conjugated linoleic acid supplementation during resistance training on body composition, bone density, strength, and selected hematological markers. J. Strength Cond. Res. 2002, 16, 325–334. [Google Scholar]
- Lambert, E.V.; Goedecke, J.H.; Bluett, K.; Heggie, K.; Claassen, A.; Rae, D.E.; West, S.; Dugas, J.; Dugas, L.; Meltzeri, S.; Charlton, K.; Mohede, I. Conjugated linoleic acid versus high-oleic acid sunflower oil: Effects on energy metabolism, glucose tolerance, blood lipids, appetite and body composition in regularly exercising individuals. Br. J. Nutr. 2007, 97, 1001–1011. [Google Scholar] [CrossRef]
- Macaluso, F.M.; Catanese, P.; Ardizzone, N.M.; Marino Gammazza, A.; Bonsignore, G.; Lo Giudice, G.; Stampone, T.; Barone, R.; Farina, F.; Di Felice, V. Effect of conjugated linoleic acid on testosterone levels in vitro and in vivo. J. Strength Cond. Res. 2012, 26, 1667–1674. [Google Scholar] [CrossRef]
- Thom, E.; Wadstein, J.; Gudmundsen, O. Conjugated linoleic acid reduces body fat in healthy exercising humans. J. Int. Med. Res. 2001, 29, 392–396. [Google Scholar]
- Colakoglu, S.; Colakoglu, M.; Taneli, F.; Cetinoz, F.; Turkmen, M. Cumulative effects of conjugated linoleic acid and exercise on endurance development, body composition, serum leptin and insulin levels. J. Sports Med. Phys. Fitness 2006, 46, 570–577. [Google Scholar]
- Pinkoski, C.; Chilibeck, P.D.; Candow, D.G.; Esliger, D.; Ewaschuk, J.B.; Facci, M.; Farthing, J.P.; Zello, G.A. The effects of conjugated linoleic acid supplementation during resistance training. Med. Sci. Sports Exerc. 2006, 38, 339–348. [Google Scholar]
- Cartwright, I.J.; Pockley, A.G.; Galloway, J.H.; Greaves, M.; Preston, F.E. The effects of dietary omega-3 polyunsaturated fatty acids on erythrocyte membrane phospholipids, erythrocyte deformability and blood viscosity in healthy volunteers. Atherosclerosis 1985, 55, 267–281. [Google Scholar] [CrossRef]
- Solomon, S.A.; Cartwright, I.; Pockley, G.; Greaves, M.; Preston, F.E.; Ramsay, L.E.; Waller, P.C. A placebo-controlled, double-blind study of eicosapentaenoic acid-rich fish oil in patients with stable angina pectoris. Curr. Med. Res. Opin. 1990, 12, 1–11. [Google Scholar]
- Bruckner, G.; Webb, P.; Greenwell, L.; Chow, C.; Richardson, D. Fish oil increases peripheral capillary blood cell velocity in humans. Atherosclerosis 1987, 66, 237–245. [Google Scholar] [CrossRef]
- Helge, J.W.; Wu, B.J.; Willer, M.; Daugaard, J.R.; Storlien, L.H.; Kiens, B. Training affects muscle phospholipid fatty acid composition in humans. J. Appl. Physiol. 2001, 90, 670–677. [Google Scholar]
- Fetterman, J.W., Jr.; Zdanowicz, M.M. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am. J. Health Syst. Pharm. 2009, 66, 1169–1179. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; McAnulty, S.R.; Jin, F.; Maxwell, K.R. n-3 polyunsaturated fatty acids do not alter immune and inflammation measures in endurance athletes. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 536–546. [Google Scholar]
- Ye, L.; Su, Z.J.; Ge, R.S. Inhibitors of testosterone biosynthetic and metabolic activation enzymes. Molecules 2011, 16, 9983–10001. [Google Scholar] [CrossRef]
- Staples, C.R.; Burke, J.M.; Thatcher, W.W. Influence of supplemental fats on reproductive tissues and performance of lactating cows. J. Dairy Sci. 1998, 81, 856–871. [Google Scholar] [CrossRef]
- Sebokova, E.; Garg, M.L.; Clandinin, M.T. Modulation of receptor-mediated gonadotropin action in rat testes by dietary fat. Am. J. Physiol. 1988, 254, E708–E712. [Google Scholar]
- Sebokova, E.; Garg, M.L.; Wierzbicki, A.; Thomson, A.B.; Clandinin, M.T. Alteration of the lipid composition of rat testicular plasma membranes by dietary (n-3) fatty acids changes the responsiveness of Leydig cells and testosterone synthesis. J. Nutr. 1990, 120, 610–618. [Google Scholar]
- Castellano, C.A.; Audet, I.; Laforest, J.P.; Matte, J.J.; Suh, M. Fish oil diets alter the phospholipid balance, fatty acid composition, and steroid hormone concentrations in testes of adult pigs. Theriogenology 2011, 76, 1134–1145. [Google Scholar] [CrossRef]
- Montano, M.; Zimmer, K.E.; Dahl, E.; Berg, V.; Olsaker, I.; Skaare, J.U.; Murk, A.J.; Ropstad, E.; Verhaegen, S. Effects of mixtures of persistent organic pollutants (POPs) derived from cod liver oil on H295R steroidogenesis. Food. Chem. Toxicol. 2011, 49, 2328–2335. [Google Scholar]
- May, K.C.; Bobe, G.; Mueller, C.J.; Cannon, M.J. Conjugated linoleic acid decreases prostaglandin synthesis in bovine luteal cells in vitro. Mol. Reprod. Dev. 2011, 78, 328–336. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Tipton, K.D.; Doyle, D.; Phillips, S.M.; Cortiella, J.; Wolfe, R.R. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am. J. Physiol. 1998, 275, E864–E871. [Google Scholar]
- Kadi, F.; Bonnerud, P.; Eriksson, A.; Thornell, L.E. The expression of androgen receptors in human neck and limb muscles: Effects of training and self-administration of androgenic-anabolic steroids. Histochem. Cell Biol. 2000, 113, 25–29. [Google Scholar] [CrossRef]
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar]
- Macaluso, F.; Brooks, N.E.; Van de Vyver, M.; Van Tubbergh, K.; Niesler, C.U.; Myburgh, K.H. Satellite cell count, VO2max, and p38 MAPK in inactive to moderately active young men. Scand. J. Med. Sci. Sports 2012, 22, e38–e44. [Google Scholar] [CrossRef]
- Macaluso, F.; Brooks, N.E.; Niesler, C.U.; Myburgh, K.H. Satellite cell pool expansion is affected by skeletal muscle characteristics. Muscle Nerve 2012. [Google Scholar] [CrossRef]
- Kadi, F.; Charifi, N.; Denis, C.; Lexell, J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve 2004, 29, 120–127. [Google Scholar] [CrossRef]
- Macaluso, F.; Myburgh, K.H. Current evidence that exercise can increase the number of adult stem cells. J. Muscle Res. Cell. Motil. 2012, 33, 187–198. [Google Scholar] [CrossRef]
- Sinha-Hikim, I.; Taylor, W.E.; Gonzalez-Cadavid, N.F.; Zheng, W.; Bhasin, S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: Up-regulation by androgen treatment. J. Clin. Endocrinol. Metab. 2004, 89, 5245–5255. [Google Scholar]
- Powers, M.L.; Florini, J.R. A direct effect of testosterone on muscle cells in tissue culture. Endocrinology 1975, 97, 1043–1047. [Google Scholar] [CrossRef]
- Sinha-Hikim, I.; Cornford, M.; Gaytan, H.; Lee, M.L.; Bhasin, S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J. Clin. Endocrinol. Metab. 2006, 91, 3024–3033. [Google Scholar]
- Ryan, A.J. Anabolic steroids are fool’s gold. Fed. Proc. 1981, 40, 2682–2688. [Google Scholar]
- Hartgens, F.; Kuipers, H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004, 34, 513–554. [Google Scholar] [CrossRef]
- Storer, T.W.; Magliano, L.; Woodhouse, L.; Lee, M.L.; Dzekov, C.; Dzekov, J.; Casaburi, R.; Bhasin, S. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J. Clin. Endocrinol. Metab. 2003, 88, 1478–1485. [Google Scholar] [CrossRef]
- Rogerson, S.; Weatherby, R.P.; Deakin, G.B.; Meir, R.A.; Coutts, R.A.; Zhou, S.; Marshall-Gradisnik, S.M. The effect of short-term use of testosterone enanthate on muscular strength and power in healthy young men. J. Strength Cond. Res. 2007, 21, 354–361. [Google Scholar]
- Husak, J.F.; Irschick, D.J.; Meyers, J.J.; Lailvaux, S.P.; Moore, I.T. Hormones, sexual signals, and performance of green anole lizards (Anolis carolinensis). Horm. Behav. 2007, 52, 360–367. [Google Scholar] [CrossRef]
- Leslie, M.; Forger, N.G.; Breedlove, S.M. Sexual dimorphism and androgen effects on spinal motoneurons innervating the rat flexor digitorum brevis. Brain Res. 1991, 561, 269–273. [Google Scholar] [CrossRef]
- Blanco, C.E.; Popper, P.; Micevych, P. Anabolic-androgenic steroid induced alterations in choline acetyltransferase messenger RNA levels of spinal cord motoneurons in the male rat. Neuroscience 1997, 78, 873–882. [Google Scholar]
- Tamaki, T.; Uchiyama, S.; Uchiyama, Y.; Akatsuka, A.; Roy, R.R.; Edgerton, V.R. Anabolic steroids increase exercise tolerance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E973–E981. [Google Scholar]
- Husak, J.F.; Irschick, D.J. Steroid use and human performance: Lessons for integrative biologists. Integr. Comp. Biol. 2009, 49, 354–364. [Google Scholar] [CrossRef]
- Pearlman, W.H.; Crepy, O. Steroid-protein interaction with particular reference to testosterone binding by human serum. J. Biol. Chem. 1967, 242, 182–189. [Google Scholar]
- Weitzel, L.R.; Sandoval, P.A.; Mayles, W.J.; Wischmeyer, P.E. Performance-enhancing sports supplements: Role in critical care. Crit. Care Med. 2009, 37, S400–S409. [Google Scholar] [CrossRef]
- Fry, A.C.; Kraemer, W.J.; Stone, M.H.; Warren, B.J.; Fleck, S.J.; Kearney, J.T.; Gordon, S.E. Endocrine responses to overreaching before and after 1 year of weightlifting. Can. J. Appl. Physiol. 1994, 19, 400–410. [Google Scholar] [CrossRef]
- Kraemer, W.J. Endocrine responses to resistance exercise. Med. Sci. Sports Exerc. 1988, 20, S152–S157. [Google Scholar]
- Fahrner, C.L.; Hackney, A.C. Effects of endurance exercise on free testosterone concentration and the binding affinity of sex hormone binding globulin (SHBG). Int. J. Sports Med. 1998, 19, 12–15. [Google Scholar] [CrossRef]
- Grandys, M.; Majerczak, J.; Duda, K.; Zapart-Bukowska, J.; Kulpa, J.; Zoladz, J.A. Endurance training of moderate intensity increases testosterone concentration in young, healthy men. Int. J. Sports Med. 2009, 30, 489–495. [Google Scholar] [CrossRef]
- Volek, J.S.; Kraemer, W.J.; Bush, J.A.; Incledon, T.; Boetes, M. Testosterone and cortisol in relationship to dietary nutrients and resistance exercise. J. Appl. Physiol. 1997, 82, 49–54. [Google Scholar]
- Sallinen, J.; Pakarinen, A.; Ahtiainen, J.; Kraemer, W.J.; Volek, J.S.; Hakkinen, K. Relationship between diet and serum anabolic hormone responses to heavy-resistance exercise in men. Int. J. Sports Med. 2004, 25, 627–633. [Google Scholar] [CrossRef]
- Herrmann, J.L.; Abarbanell, A.M.; Weil, B.R.; Manukyan, M.C.; Poynter, J.A.; Wang, Y.; Coffey, A.C.; Meldrum, D.R. Gender dimorphisms in progenitor and stem cell function in cardiovascular disease. J. Cardiovasc. Transl. Res. 2010, 3, 103–113. [Google Scholar]
- Kim, S.W.; Hwang, J.H.; Cheon, J.M.; Park, N.S.; Park, S.E.; Park, S.J.; Yun, H.J.; Kim, S.; Jo, D.Y. Direct and indirect effects of androgens on survival of hematopoietic progenitor cells in vitro. J. Korean Med. Sci. 2005, 20, 409–416. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Abarbanell, A.; Tan, J.; Weil, B.; Herrmann, J.; Meldrum, D.R. Both endogenous and exogenous testosterone decrease myocardial STAT3 activation and SOCS3 expression after acute ischemia and reperfusion. Surgery 2009, 146, 138–144. [Google Scholar] [CrossRef]
- Serradifalco, C.; Catanese, P.; Rizzuto, L.; Cappello, F.; Barresi, V.; Nunnari, C.M.; Zummo, G.; Di Felice, V. Embryonic and foetal Islet-1 positive cells in human hearts are also positive to c-Kit. Eur. J. Histochem. 2011, 55, e41. [Google Scholar]
- Di Felice, V.; Zummo, G. Tetralogy of fallot as a model to study cardiac progenitor cell migration and differentiation during heart development. Trends Cardiovasc. Med. 2009, 19, 130–135. [Google Scholar] [CrossRef]
- Di Felice, V.; De Luca, A.; Colorito, M.L.; Montalbano, A.; Ardizzone, N.M.; Macaluso, F.; Gammazza, A.M.; Cappello, F.; Zummo, G. Cardiac stem cell research: An elephant in the room? Anat. Rec. (Hoboken) 2009, 292, 449–454. [Google Scholar] [CrossRef]
- Thiene, G.; Corrado, D.; Rigato, I.; Basso, C. Why and how to support screening strategies to prevent sudden death in athletes. Cell Tissue Res. 2012, 348, 315–318. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Macaluso, F.; Barone, R.; Catanese, P.; Carini, F.; Rizzuto, L.; Farina, F.; Di Felice, V. Do Fat Supplements Increase Physical Performance? Nutrients 2013, 5, 509-524. https://doi.org/10.3390/nu5020509
Macaluso F, Barone R, Catanese P, Carini F, Rizzuto L, Farina F, Di Felice V. Do Fat Supplements Increase Physical Performance? Nutrients. 2013; 5(2):509-524. https://doi.org/10.3390/nu5020509
Chicago/Turabian StyleMacaluso, Filippo, Rosario Barone, Patrizia Catanese, Francesco Carini, Luigi Rizzuto, Felicia Farina, and Valentina Di Felice. 2013. "Do Fat Supplements Increase Physical Performance?" Nutrients 5, no. 2: 509-524. https://doi.org/10.3390/nu5020509
APA StyleMacaluso, F., Barone, R., Catanese, P., Carini, F., Rizzuto, L., Farina, F., & Di Felice, V. (2013). Do Fat Supplements Increase Physical Performance? Nutrients, 5(2), 509-524. https://doi.org/10.3390/nu5020509







