Body Mass Index (BMI) Trajectories from Birth to 11.5 Years: Relation to Early Life Food Intake
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants
2.2. Measures
2.2.1. Outcome Variable: Trajectories of BMI
2.2.2. Predictor variables
2.2.2.1. Infant Feeding
2.2.2.2. Dietary Intake around 18 Months
2.2.2.3. Parental Characteristics
2.3. Statistical Analysis
3. Results
| Subjects included in this analysis (N = 370) | Subjects lost to follow up at 11.5 years (N = 246) | Difference between included and lost to follow-up | ||
|---|---|---|---|---|
| Variable | n (%) | n (%) | P-value 1 | |
| Child’s gender | ||||
| Male | 187 (51) | 125 (51) | 0.95 | |
| Female | 183 (49) | 121 (49) | ||
| Maternal smoking in pregnancy | ||||
| No | 292 (79) | 174 (71) | 0.02 | |
| Yes | 78 (21) | 72 (29) | ||
| Age of parents at child’s birth (years, mean ± SD) | ||||
| Mother | 29.0 ± 5.1 | 27.6 ± 5.6 | 0.002 | |
| Father | 31.2 ± 5.9 | 30.2 ± 6.3 | 0.047 | |
| Australian born | ||||
| Mother | 272 (74) | 185 (75) | 0.64 | |
| Father | 255 (69) | 166 (68) | 0.71 | |
| Tertiary educated | ||||
| Mother | 191 (52) | 85 (35) | <0.001 | |
| Father | 175 (47) | 90 (37) | 0.009 | |
| Full-time employment | ||||
| Mother | 183 (49) | 95 (39) | 0.008 | |
| Father | 323 (87) | 195 (79) | 0.008 | |
| Primigravida | 131 (35) | 68 (28) | 0.04 | |
| Sex | Number of classes | Parameters | AIC 1 | BIC 2 | aBIC 3 | Entropy | LMR 4P-Value |
|---|---|---|---|---|---|---|---|
| Males | 1 | 20 | 10566 | 10631 | 10567 | - | - |
| 2 | 37 | 9620 | 9740 | 9622 | 0.92 | 0.057 | |
| 3 | 54 | 9318 | 9492 | 9321 | 0.83 | 0.137 | |
| 4 | 71 | 9164 | 9394 | 9169 | 0.87 | 0.126 | |
| 5 | 88 | 9021 | 9305 | 9026 | 0.85 | 0.707 | |
| Females | 1 | 20 | 9793 | 9857 | 9794 | - | - |
| 2 | 37 | 9112 | 9231 | 9114 | 0.86 | <0.001 | |
| 3 | 54 | 8976 | 9149 | 8978 | 0.85 | 0.442 | |
| 4 | 71 | 8892 | 9120 | 8895 | 0.81 | 0.353 | |
| 5 | 88 | 8830 | 9112 | 8834 | 0.79 | 0.731 |
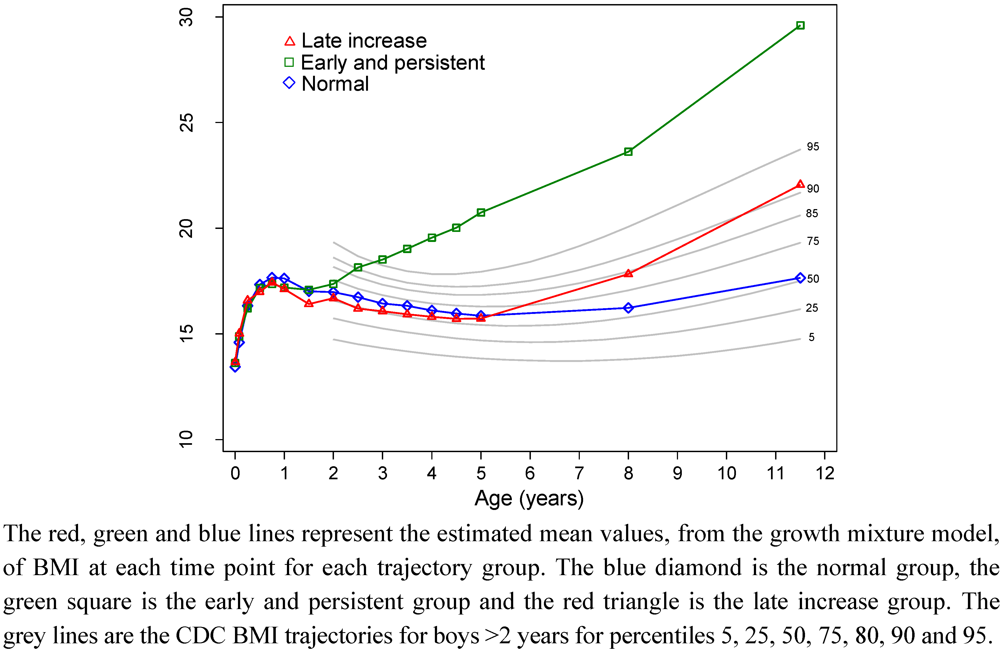

| Boys BMI trajectory group | Girls BMI trajectory group | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Normal | Early and persistent | Late BMI increase | P 1 | Overall | Normal | Early and persistent | Late BMI increase | P 1 | ||||||||||
| N | n | (%) | n | (%) | n | (%) | N | n | (%) | n | (%) | n | (%) | ||||||
| Overall Sample | 187 | 114 | (61) | 22 | (12) | 51 | (27) | 183 | 113 | (62) | 22 | (12) | 48 | (26) | |||||
| Dietary predictors | |||||||||||||||||||
| Breastfeeding duration | |||||||||||||||||||
| 0 to <3 months | 73 | 45 | (62) | 8 | (11) | 20 | (27) | 0.27 | 75 | 49 | (65) | 8 | (11) | 18 | (24) | 0.25 | |||
| 3 to <6 months | 29 | 19 | (66) | 6 | (21) | 4 | (14) | 33 | 23 | (70) | 1 | (3) | 9 | (27) | |||||
| ≥6 months | 85 | 50 | (59) | 8 | (9) | 27 | (32) | 75 | 41 | (55) | 13 | (17) | 21 | (28) | |||||
| Introduced solids by 3 months | |||||||||||||||||||
| No | 125 | 72 | (58) | 17 | (14) | 36 | (29) | 0.18 | 125 | 75 | (60) | 16 | (13) | 34 | (27) | 0.30 | |||
| Yes | 42 | 30 | (71) | 2 | (5) | 10 | (24) | 35 | 26 | (74) | 3 | (9) | 6 | (17) | |||||
| Non-dietary predictors | |||||||||||||||||||
| CAPS diet intervention group | |||||||||||||||||||
| Control | 88 | 52 | (59) | 10 | (11) | 26 | (30) | 0.81 | 95 | 60 | (63) | 9 | (10) | 26 | (27) | 0.54 | |||
| Active | 99 | 62 | (63) | 12 | (12) | 25 | (25) | 88 | 53 | (60) | 13 | (15) | 22 | (25) | |||||
| Parental weight status 2 | |||||||||||||||||||
| Both parents overweight or obese | 63 | 36 | (57) | 6 | (10) | 21 | (33) | 0.19 | 73 | 45 | (62) | 10 | (14) | 18 | (25) | 0.68 | |||
| Only mother overweight or obese | 29 | 13 | (45) | 4 | (14) | 12 | (41) | 20 | 11 | (55) | 4 | (20) | 5 | (25) | |||||
| Only father overweight or obese | 45 | 27 | (60) | 6 | (13) | 12 | (27) | 54 | 33 | (61) | 4 | (7) | 17 | (31) | |||||
| Both normal | 21 | 16 | (76) | 2 | (10) | 3 | (14) | 11 | 9 | (82) | 0 | (0) | 2 | (18) | |||||
| Undefined | 29 | 22 | (76) | 4 | (14) | 3 | (10) | 25 | 15 | (60) | 4 | (16) | 6 | (24) | |||||
| Fathers BMI | |||||||||||||||||||
| Normal | 45 | 28 | (62) | 5 | (11) | 12 | (27) | 0.19 | 24 | 16 | (67) | 3 | (13) | 5 | (21) | 0.95 | |||
| Overweight | 64 | 41 | (64) | 3 | (5) | 20 | (31) | 77 | 46 | (60) | 10 | (13) | 21 | (27) | |||||
| Obese | 44 | 22 | (50) | 9 | (20) | 13 | (30) | 50 | 32 | (64) | 4 | (8) | 14 | (28) | |||||
| Missing/refused | 34 | 23 | (68) | 5 | (15) | 6 | (18) | 32 | 19 | (59) | 5 | (16) | 8 | (25) | |||||
| Mothers BMI | |||||||||||||||||||
| Normal | 67 | 46 | (69) | 7 | (10) | 14 | (21) | 0.045 | 67 | 42 | (63) | 5 | (7) | 20 | (30) | 0.48 | |||
| Overweight | 45 | 29 | (64) | 4 | (9) | 12 | (27) | 51 | 34 | (67) | 8 | (16) | 9 | (18) | |||||
| Obese | 47 | 20 | (43) | 6 | (13) | 21 | (45) | 42 | 22 | (52) | 6 | (14) | 14 | (33) | |||||
| Missing/refused | 28 | 19 | (68) | 5 | (18) | 4 | (14) | 23 | 15 | (65) | 3 | (13) | 5 | (22) | |||||
| Child's grandparents ethnicity/country of birth 3 | |||||||||||||||||||
| Caucasian | 100 | 62 | (62) | 8 | (8) | 30 | (30) | 0.36 | 98 | 60 | (61) | 12 | (12) | 26 | (27) | 0.86 | |||
| European | 17 | 10 | (59) | 4 | (24) | 3 | (18) | 18 | 11 | (61) | 1 | (6) | 6 | (33) | |||||
| Middle Eastern/ Indian/Asian | 19 | 9 | (47) | 4 | (21) | 6 | (32) | 24 | 17 | (71) | 2 | (8) | 5 | (21) | |||||
| Undefined | 51 | 33 | (65) | 6 | (12) | 12 | (24) | 43 | 25 | (58) | 7 | (16) | 11 | (26) | |||||
| Cigarettes during pregnancy | |||||||||||||||||||
| None | 146 | 90 | (62) | 15 | (10) | 41 | (28) | 0.53 | 146 | 96 | (66) | 15 | (10) | 35 | (24) | 0.04 | |||
| 1–10/day | 28 | 16 | (57) | 6 | (21) | 6 | (21) | 22 | 13 | (59) | 4 | (18) | 5 | (23) | |||||
| 11–40/day | 13 | 8 | (62) | 1 | (8) | 4 | (31) | 15 | 4 | (27) | 3 | (20) | 8 | (53) | |||||
| Father’s Education | |||||||||||||||||||
| ≤10 years of school | 63 | 33 | (52) | 9 | (14) | 21 | (33) | 0.20 | 57 | 32 | (56) | 7 | (12) | 18 | (32) | 0.13 | |||
| 11–12 years of school | 37 | 27 | (73) | 5 | (14) | 5 | (14) | 34 | 18 | (53) | 8 | (24) | 8 | (24) | |||||
| Tertiary Education | 85 | 54 | (64) | 8 | (9) | 23 | (27) | 90 | 61 | (68) | 7 | (8) | 22 | (24) | |||||
| Mother’s Education | |||||||||||||||||||
| ≤10 years of school | 53 | 30 | (57) | 6 | (11) | 17 | (32) | 0.72 | 57 | 38 | (67) | 3 | (5) | 16 | (28) | 0.047 | |||
| 11–12 years of school | 34 | 24 | (71) | 3 | (9) | 7 | (21) | 35 | 15 | (43) | 8 | (23) | 12 | (34) | |||||
| Tertiary Education | 100 | 60 | (60) | 13 | (13) | 27 | (27) | 91 | 60 | (66) | 11 | (12) | 20 | (22) | |||||
| Father worked full time before baby born | |||||||||||||||||||
| No | 22 | 12 | (55) | 4 | (18) | 6 | (27) | 0.59 | 25 | 17 | (68) | 2 | (8) | 6 | (24) | 0.73 | |||
| Yes | 165 | 102 | (62) | 18 | (11) | 45 | (27) | 158 | 96 | (61) | 20 | (13) | 42 | (27) | |||||
| Mother worked full time before baby born | |||||||||||||||||||
| No | 90 | 56 | (62) | 7 | (8) | 27 | (30) | 0.24 | 97 | 57 | (59) | 7 | (7) | 33 | (34) | 0.01 | |||
| Yes | 97 | 58 | (60) | 15 | (15) | 24 | (25) | 86 | 56 | (65) | 15 | (17) | 15 | (17) | |||||
| Boys BMI trajectory group | Girls BMI trajectory group | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal( n = 98) | Early and persistent( n = 16) | Late BMI increase( n = 41) | P-value | Normal( n = 92) | Early and persistent ( n = 18) | Late BMI increase( n = 33) | P-value | |||||||||||
| Variable | Unit | Mean | SD | Mean | SD | Mean | SD | Unadjusted 1 | Adjusted 2 | Mean | SD | Mean | SD | Mean | SD | Unadjusted 1 | Adjusted 2 | |
| Total energy | MJ | 4.35 | 0.83 | 4.32 | 1.15 | 4.68 | 1.14 | 0.16 | 4.25 | 0.93 | 4.41 | 0.96 | 4.13 | 1.32 | 0.66 | |||
| Protein | 100 g of protein | 0.40 | 0.11 | 0.42 | 0.11 | 0.43 | 0.12 | 0.27 | 0.27 | 0.39 | 0.11 | 0.40 | 0.09 | 0.38 | 0.13 | 0.82 | 0.87 | |
| Percentage of total energy from protein | % | 15.46 | 2.65 | 16.78 | 2.99 | 15.52 | 2.51 | 0.18 | 15.50 | 3.02 | 15.57 | 2.44 | 15.84 | 2.51 | 0.84 | |||
| Fat | 100 g of fat | 0.43 | 0.12 | 0.44 | 0.13 | 0.45 | 0.15 | 0.77 | 0.15 | 0.41 | 0.11 | 0.43 | 0.10 | 0.42 | 0.14 | 0.91 | 0.58 | |
| Percentage of total energy from fat | % | 36.47 | 5.76 | 37.32 | 4.58 | 34.91 | 5.07 | 0.21 | 35.88 | 5.82 | 35.86 | 5.64 | 37.48 | 5.45 | 0.37 | |||
| Carbohydrates | 100 g of carbohydrates | 1.26 | 0.27 | 1.22 | 0.37 | 1.40 | 0.33 | 0.04 | 0.19 | 1.26 | 0.35 | 1.30 | 0.37 | 1.19 | 0.44 | 0.51 | 0.69 | |
| Percentage of total energy from Carbohydrates | % | 49.72 | 7.51 | 47.65 | 6.30 | 51.26 | 6.55 | 0.21 | 50.30 | 7.77 | 50.22 | 7.33 | 48.49 | 6.81 | 0.49 | |||
| Boys BMI trajectory group | Girls BMI trajectory group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal( n = 98) | Early and persistent( n = 16) | Late BMI increase( n = 41) | Normal( n = 92) | Early and persistent( n = 18) | Late BMI increase( n = 33) | |||||
| Food group | Units | Median | Median | Median | P-value 1 | Median | Median | Median | P-value 1 | |
| Core Foods | ||||||||||
| Dairy foods 2 | g | 554.3 | 433.1 | 527.2 | 0.24 | 473.1 | 576.5 | 480.9 | 0.45 | |
| % of total energy | 38.1 | 33.4 | 35.9 | 0.56 | 37.0 | 40.6 | 32.7 | 0.28 | ||
| Milk 3 | g | 478.8 | 401.1 | 407.0 | 0.46 | 398.7 | 510.0 | 380.8 | 0.74 | |
| % of total energy | 29.4 | 24.8 | 23.5 | 0.53 | 28.0 | 26.7 | 25.3 | 0.85 | ||
| Fruit | g | 57.3 | 78.0 | 79.7 | 0.20 | 60.7 | 61.3 | 57.7 | 0.75 | |
| % of total energy | 3.8 | 4.0 | 5.0 | 0.52 | 3.6 | 5.4 | 4.2 | 0.73 | ||
| Vegetables | g | 43.2 | 57.3 | 36.3 | 0.49 | 35.3 | 43.3 | 43.0 | 0.48 | |
| % of total energy | 3.6 | 3.4 | 2.8 | 0.64 | 3.8 | 3.6 | 3.8 | 0.97 | ||
| Cereals 4 | g | 60.7 | 52.1 | 63.4 | 0.79 | 54.2 | 59.9 | 58.3 | 0.87 | |
| % of total energy | 13.3 | 14.4 | 14.1 | 0.94 | 14.0 | 13.4 | 14.1 | 0.86 | ||
| Meats | g | 18.6 | 38.8 | 24.1 | 0.01 | 26.4 | 25.4 | 35.0 | 0.57 | |
| % of total energy | 4.4 | 9.2 | 4.1 | 0.01 | 5.6 | 4.9 | 7.3 | 0.38 | ||
| Extra Foods | ||||||||||
| Total extra foods 5 | g | 118.3 | 131.3 | 124.3 | 0.81 | 113.7 | 112.4 | 82.5 | 0.60 | |
| % of total energy | 24.0 | 26.2 | 24.5 | 1.00 | 24.8 | 25.1 | 24.8 | 0.99 | ||
| Non-milk beverages 6 | g | 128.3 | 148.3 | 150.9 | 0.54 | 133.3 | 139.2 | 100.0 | 0.50 | |
| % of total energy | 6.2 | 8.5 | 6.4 | 0.70 | 7.2 | 5.9 | 5.6 | 0.27 | ||
| Sweetened drinks 7 | g | 15.0 | 31.7 | 17.8 | 0.93 | 21.7 | 7.5 | 1.0 | 0.23 | |
| % of total energy | 2.1 | 4.0 | 2.1 | 0.41 | 3.4 | 1.2 | 2.1 | 0.26 | ||
| Fried potatoes | g | 3.3 | 0.0 | 3.3 | 0.73 | 5.2 | 1.7 | 1.3 | 0.67 | |
| % of total energy | 0.9 | 0.0 | 0.7 | 0.76 | 1.4 | 0.5 | 0.3 | 0.73 | ||
| Salty snacks 8 | g | 0.0 | 0.0 | 0.0 | 0.98 | 0.0 | 0.0 | 0.0 | 0.76 | |
| % of total energy | 0.0 | 0.0 | 0.0 | 0.98 | 0.0 | 0.0 | 0.0 | 0.79 | ||
| Confectionery | g | 1.3 | 3.3 | 4.0 | 0.41 | 4.0 | 3.8 | 3.3 | 0.99 | |
| % of total energy | 0.4 | 1.6 | 1.0 | 0.47 | 1.7 | 1.9 | 1.9 | 0.99 | ||
| Cereal based foods 9 | g | 18.0 | 11.0 | 26.7 | 0.15 | 22.5 | 28.5 | 18.5 | 0.56 | |
| % of total energy | 7.1 | 4.6 | 8.6 | 0.19 | 9.1 | 8.4 | 7.0 | 0.59 | ||
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Ventura, A.K.; Loken, E.; Birch, L.L. Developmental trajectories of girls’ BMI across childhood and adolescence. Obesity 2009, 17, 2067–2074. [Google Scholar] [CrossRef]
- Li, C.; Goran, M.I.; Kaur, H.; Nollen, N.; Ahluwalia, J.S. Developmental trajectories of overweight during childhood: Role of early life factors. Obesity 2007, 15, 760–771. [Google Scholar] [CrossRef]
- Mustillo, S.; Worthman, C.; Erkanli, A.; Keeler, G.; Angold, A.; Costello, E.J. Obesity and psychiatric disorder: Developmental trajectories. Pediatrics 2003, 111, 851–859. [Google Scholar] [CrossRef]
- Huang, R.-C.; de Klerk, N.H.; Smith, A.; Kendall, G.E.; Landau, L.I.; Mori, T.A.; Newnham, J.P.; Stanley, F.J.; Oddy, W.H.; Hands, B.; et al. Lifecourse childhood adiposity trajectories associated with adolescent insulin resistance. Diabetes Care 2011, 34, 1019–1025. [Google Scholar] [CrossRef]
- Pryor, L.E.; Tremblay, R.E.; Boivin, M.; Touchette, E.; Dubois, L.; Genolini, C.; Liu, X.; Falissard, B.; Cote, S.M. Developmental trajectories of body mass index in early childhood and their risk factors: An 8-year longitudinal study. Arch. Pediatr. Adolesc. Med. 2011, 165, 906–912. [Google Scholar] [CrossRef]
- Balistreri, K.S.; van Hook, J. Trajectories of overweight among us school children: A focus on social and economic characteristics. Matern. Child Health J. 2011, 15, 610–619. [Google Scholar] [CrossRef]
- Garden, F.L.; Marks, G.B.; Almqvist, C.; Simpson, J.M.; Webb, K.L. Infant and early childhood dietary predictors of overweight at age 8 years in the caps population. Eur. J. Clin. Nutr. 2011, 65, 454–462. [Google Scholar] [CrossRef]
- Marks, G.B.; Mihrshahi, S.; Kemp, A.S.; Tovey, E.R.; Webb, K.; Almqvist, C.; Ampon, R.D.; Crisafulli, D.; Belousova, E.G.; Mellis, C.M.; et al. Prevention of asthma during the first 5 years of life: A randomized controlled trial. J. Allergy Clin. Immunol. 2006, 118, 53–61. [Google Scholar] [CrossRef]
- Toelle, B.G.; Ng, K.K.W.; Crisafulli, D.; Belousova, E.G.; Almqvist, C.; Webb, K.; Tovey, E.R.; Kemp, A.S.; Mellis, C.M.; Leeder, S.R.; et al. Eight-year outcomes of the childhood asthma prevention study. J. Allergy Clin. Immunol. 2010, 126, 388–389. [Google Scholar] [CrossRef]
- Mihrshahi, S.; Peat, J.K.; Webb, K.; Tovey, E.R.; Marks, G.B.; Mellis, C.M.; Leeder, S.R. The childhood asthma prevention study (caps): Design and research protocol of a randomized trial for the primary prevention of asthma. Control. Clin. Trials 2001, 22, 333–354. [Google Scholar] [CrossRef]
- Mihrshahi, S.; Vukasin, N.; Forbes, S.; Wainwright, C.; Krause, W.; Ampon, R.; Mellis, C.; Marks, G.; Peat, J. Are you busy for the next 5 years? Recruitment in the childhood asthma prevention study (caps). Respirology 2002, 7, 147–151. [Google Scholar] [CrossRef]
- Mihrshahi, S.; Ampon, R.; Webb, K.; Almqvist, C.; Kemp, A.; Hector, D.; Marks, G.B. The association between infant feeding practices and subsequent atopy among children with a family history of asthma. Clin. Exp. Allergy 2007, 37, 671–679. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics, National Nutrition Survey: Users’ Guide, 1995; Australian Bureau of Statistics: Canberra, Australia, 1998.
- Webb, K.L.; Lahti-Koski, M.; Rutishauser, I.; Hector, D.J.; Knezevic, N.; Gill, T.; Peat, J.K.; Leeder, S.R. CAPS Team. Consumption of “extra” foods (energy-dense, nutrient-poor) among children aged 16–24 months from western Sydney, Australia. Public Health Nutr. 2006, 9, 1035–1044. [Google Scholar]
- Muthen, L.K.; Muthen, B.O. Mplus User's Guide, 6th ed; Muthen & Muthen: Los Angeles, CA, USA, 2010. [Google Scholar]
- McArdle, J.J.; Epstein, D. Latent growth curves within developmental structural equation models. Child Dev. 1987, 58, 110–133. [Google Scholar] [CrossRef]
- Grimm, K.J.; Ram, N.; Hamagami, F. Nonlinear growth curves in developmental research. Child Dev. 2011, 82, 1357–1371. [Google Scholar] [CrossRef]
- Nylund, K.L.; Asparouhov, T.; Muthen, B.O. Deciding on the number of classes in latent class analysis and growth mixture modeling: A monte carlo simulation study. Struct. Equ. Model. Multidiscip. J. 2007, 14, 535–569. [Google Scholar] [CrossRef]
- Kuczmarski, R.J.; Ogden, C.L.; Guo, S.S.; Grummer-Strawn, L.M.; Flegal, K.M.; Mei, Z.; Wei, R.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. 2000 CDC Growth Charts for the United States: Methods and Development; Vital and Health Statistics Series 11; National Center for Health Statistics: Hyattsville, MD, USA, 2002. [Google Scholar]
- Bradlee, M.L.; Singer, M.R.; Qureshi, M.M.; Moore, L.L. Food group intake and central obesity among children and adolescents in the Third National Health and Nutrition Examination Survey (NHANES III). Public Health Nutr. 2010, 13, 797–805. [Google Scholar] [CrossRef]
- Gillis, L.J.; Bar-Or, O. Food away from home, sugar-sweetened drink consumption and juvenile obesity. J. Am. Coll. Nutr. 2003, 22, 539–545. [Google Scholar]
- Wang, Y.; Beydoun, M.A. Meat consumption is associated with obesity and central obesity among US adults. Int. J. Obes. 2009, 33, 621–628. [Google Scholar] [CrossRef]
- Webb, K.; Rutishauser, I.; Katz, T.; Knezevic, N.; Lahti-Koski, M.; Peat, J.; Mihrshahi, S. Meat consumption among 18-month-old children participating in the childhood asthma prevention study. Nutr. Diet. 2005, 62, 12–20. [Google Scholar] [CrossRef]
- Obesity: Dietary and Developmental Influences; Woodward-Lopez, G.; Ritchie, L.D.; Gerstein, D.E.; Crawford, P.B. (Eds.) Taylor & Francis: Boca Raton, FL, USA, 2006.
- Woodward-Lopez, G.; Kao, J.; Ritchie, L. To what extent have sweetened beverages contributed to the obesity epidemic? Public Health Nutr. 2011, 14, 499–509. [Google Scholar] [CrossRef]
- Webb, K.; Rutishauser, I.; Knezevic, N. Foods, nutrients and portions consumed by a sample of Australian children aged 16–24 months. Nutr. Diet. 2008, 65, 56–65. [Google Scholar] [CrossRef]
- Arenz, S.; Ruckerl, R.; Koletzko, B.; von Kries, R. Breast-feeding and childhood obesity—A systematic review. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1247–1256. [Google Scholar] [CrossRef]
- Crume, T.L.; Ogden, L.G.; Mayer-Davis, E.J.; Hamman, R.F.; Norris, J.M.; Bischoff, K.J.; McDuffie, R.; Dabelea, D. The impact of neonatal breast-feeding on growth trajectories of youth exposed and unexposed to diabetes in utero: The EPOCH study. Int. J. Obes. (Lond.) 2012, 36, 529–534. [Google Scholar] [CrossRef]
- Buyken, A.E.; Karaolis-Danckert, N.; Remer, T.; Bolzenius, K.; Landsberg, B.; Kroke, A. Effects of breastfeeding on trajectories of body fat and BMI throughout childhood. Obesity 2008, 16, 389–395. [Google Scholar] [CrossRef]
- Najman, J.M.; Lanyon, A.; Andersen, M.; Williams, G.; Bor, W.; O’Callaghan, M. Socioeconomic status and maternal cigarette smoking before, during and after a pregnancy. Aust. N. Z. J. Public Health 1998, 22, 60–66. [Google Scholar] [CrossRef]
- Wang, Y.C.; Gortmaker, S.L.; Sobol, A.M.; Kuntz, K.M. Estimating the energy gap among us children: A counterfactual approach. Pediatrics 2006, 118, e1721–e1733. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Garden, F.L.; Marks, G.B.; Simpson, J.M.; Webb, K.L. Body Mass Index (BMI) Trajectories from Birth to 11.5 Years: Relation to Early Life Food Intake. Nutrients 2012, 4, 1382-1398. https://doi.org/10.3390/nu4101382
Garden FL, Marks GB, Simpson JM, Webb KL. Body Mass Index (BMI) Trajectories from Birth to 11.5 Years: Relation to Early Life Food Intake. Nutrients. 2012; 4(10):1382-1398. https://doi.org/10.3390/nu4101382
Chicago/Turabian StyleGarden, Frances L., Guy B. Marks, Judy M. Simpson, and Karen L. Webb. 2012. "Body Mass Index (BMI) Trajectories from Birth to 11.5 Years: Relation to Early Life Food Intake" Nutrients 4, no. 10: 1382-1398. https://doi.org/10.3390/nu4101382
APA StyleGarden, F. L., Marks, G. B., Simpson, J. M., & Webb, K. L. (2012). Body Mass Index (BMI) Trajectories from Birth to 11.5 Years: Relation to Early Life Food Intake. Nutrients, 4(10), 1382-1398. https://doi.org/10.3390/nu4101382




