Consumption of Milk-Protein Combined with Green Tea Modulates Diet-Induced Thermogenesis
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Subjects
2.2. Experimental Design
| GT | PL | |
|---|---|---|
| Total polyphenols | 207.1 | |
| Total catechins | 169.0 | |
| Epigallocatechin gallate | 84.5 | |
| Caffeine | 2.1 | |
| Soy oil | 316.9 | 528.2 |
| Total filling weight (mg/capsule) | 528.2 | 528.2 |
| Total weight (mg/capsule) | 757.0 | 757.0 |
| Total weight (mg/testday) | 1584.6 | 1584.6 |
2.3. Statistical Analysis
3. Results
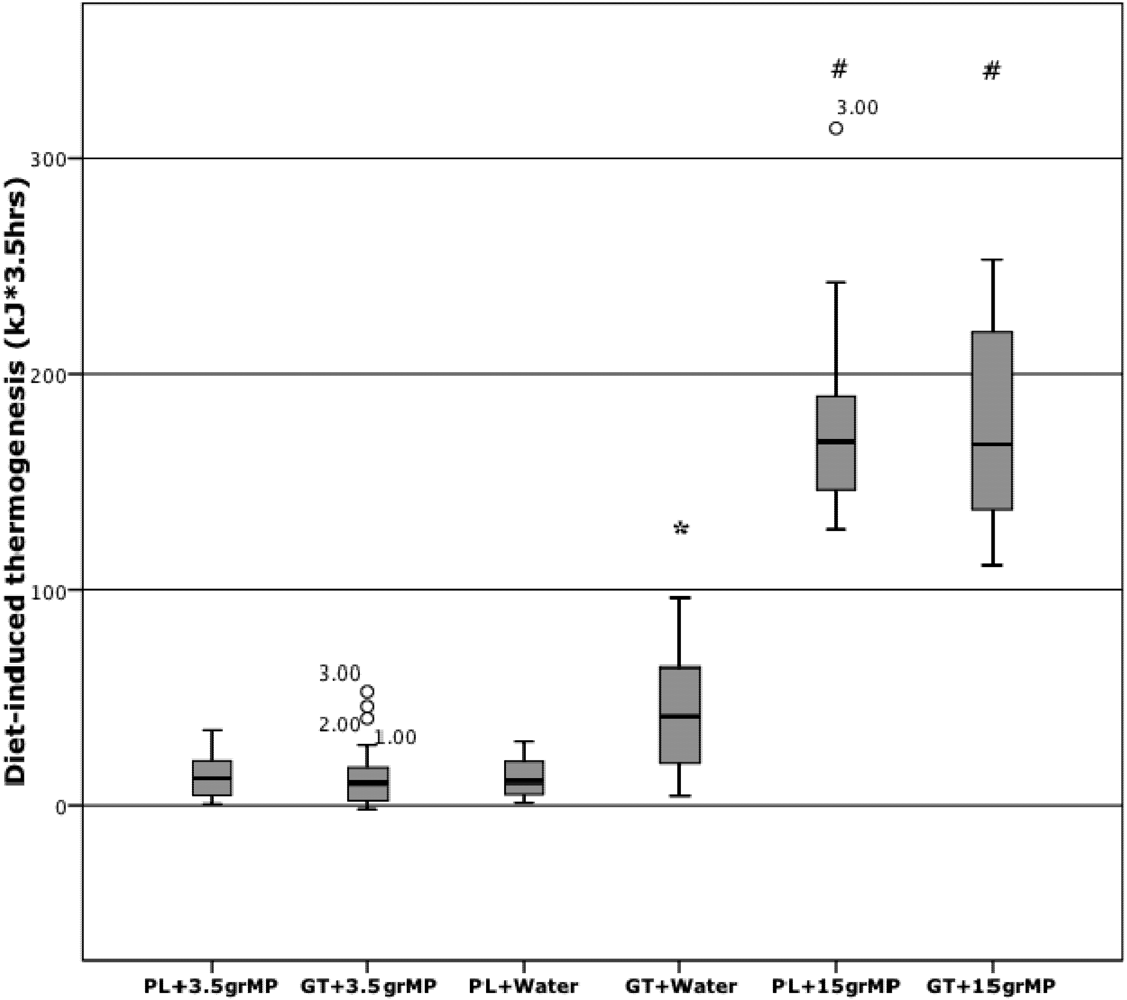
4. Discussion
5. Conclusions
Acknowledgements
Conflict of Interest
References
- Seidell, J.C. Obesity in Europe. Obes. Res. 1995, 3, 89s–93s. [Google Scholar]
- Dulloo, A.G.; Duret, C.; Rohrer, D.; Girardier, L.; Mensi, N.; Fathi, M.; Chantre, P.; Vandermander, J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr. 1999, 70, 1040–1045. [Google Scholar]
- Berube-Parent, S.; Pelletier, C.; Dore, J.; Tremblay, A. Effects of encapsulated green tea and Guarana extracts containing a mixture of epigallocatechin-3-gallate and caffeine on 24 h energy expenditure and fat oxidation in men. Br. J. Nutr. 2005, 94, 432–436. [Google Scholar]
- Westerterp-Plantenga, M.S.; Lejeune, M.P.; Kovacs, E.M. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes. Res. 2005, 13, 1195–1204. [Google Scholar]
- Auvichayapat, P.; Prapochanung, M.; Tunkamnerdthai, O.; Sripanidkulchai, B.O.; Auvichayapat, N.; Thinkhamrop, B.; Kunhasura, S.; Wongpratoom, S.; Sinawat, S.; Hongprapas, P. Effectiveness of green tea on weight reduction in obese Thais: A randomized, controlled trial. Physiol. Behav. 2008, 93, 486–491. [Google Scholar]
- Hursel, R.; Westerterp-Plantenga, M.S. Green tea catechin plus caffeine supplementation to a high-protein diet has no additional effect on body weight maintenance after weight loss. Am. J. Clin. Nutr. 2009, 89, 822–830. [Google Scholar]
- Brown, P.J.; Wright, W.B. An investigation of the interactions between milk proteins and tea polyphenols. J. Chromatogr. A 1963, 11, 504–514. [Google Scholar]
- Haslam, E. Polyphenol-protein interactions. Biochem. J. 1974, 139, 285–288. [Google Scholar]
- Luck, G.; Liao, H.; Murray, N.J.; Grimmer, H.R.; Warminski, E.E.; Williamson, M.P.; Lilley, T.H.; Haslam, E. Polyphenols, astringency and proline-rich proteins. Phytochemistry 1994, 37, 357–371. [Google Scholar]
- Siebert, K.J.; Troukhanova, N.V.; Lynn, P.Y. Nature of polyphenol-protein interactions. J. Argric. Food Chem. 1996, 44, 80–85. [Google Scholar]
- Arts, M.J.; Haenen, G.R.; Voss, H.P.; Bast, A. Masking of antioxidant capacity by the interaction of flavonoids with protein. Food Chem. Toxicol. 2001, 39, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Jobstl, E.; Howse, J.R.; Fairclough, J.P.; Williamson, M.P. Noncovalent cross-linking of casein by epigallocatechin gallate characterized by single molecule force microscopy. J. Agric. Food Chem. 2006, 54, 4077–4081. [Google Scholar]
- Serafini, M.; Ghiselli, A.; Ferro-Luzzi, A. In vivo antioxidant effect of green and black tea in man. Eur. J. Clin. Nutr. 1996, 50, 28–32. [Google Scholar] [PubMed]
- Langley-Evans, S.C. Antioxidant potential of green and black tea determined using the ferric reducing power (FRAP) assay. Int. J. Food Sci. Nutr. 2000, 51, 181–188. [Google Scholar]
- Krul, C.; Luiten-Schuiten, A.; Tenfelde, A.; van Ommen, B.; Verhagen, H.; Havenaar, R. Antimutagenic activity of green tea and black tea extracts studied in a dynamicin vitro gastrointestinal model. Mutat. Res. 2001, 474, 71–85. [Google Scholar]
- Arts, M.J.; Haenen, G.R.; Wilms, L.C.; Beetstra, S.A.; Heijnen, C.G.; Voss, H.P.; Bast, A. Interactions between flavonoids and proteins: Effect on the total antioxidant capacity. J. Agric. Food Chem. 2002, 50, 1184–1187. [Google Scholar]
- Lorenz, M.; Jochmann, N.; von Krosigk, A.; Martus, P.; Baumann, G.; Stangl, K.; Stangl, V. Addition of milk prevents vascular protective effects of tea. Eur. Heart J. 2007, 28, 219–223. [Google Scholar]
- van het Hof, K.H.; Kivits, G.A.; Weststrate, J.A.; Tijburg, L.B. Bioavailability of catechins from tea: The effect of milk. Eur. J. Clin. Nutr. 1998, 52, 356–359. [Google Scholar]
- Leenen, R.; Roodenburg, A.J.; Tijburg, L.B.; Wiseman, S.A. A single dose of tea with or without milk increases plasma antioxidant activity in humans. Eur. J. Clin. Nutr. 2000, 54, 87–92. [Google Scholar]
- Hollman, P.C.; van het Hof, K.H.; Tijburg, L.B.; Katan, M.B. Addition of milk does not affect the absorption of flavonols from tea in man. Free Radic. Res. 2001, 34, 297–300. [Google Scholar]
- Reddy, V.C.; Vidya Sagar, G.V.; Sreeramulu, D.; Venu, L.; Raghunath, M. Addition of milk does not alter the antioxidant activity of black tea. Ann. Nutr. Metab. 2005, 49, 189–195. [Google Scholar]
- McCarty, M.F. Promotion of hepatic lipid oxidation and gluconeogenesis as a strategy for appetite control. Med. Hypotheses 1994, 42, 215–225. [Google Scholar]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar]
- Palmatier, M.A.; Kang, A.M.; Kidd, K.K. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biol. Psychiatry 1999, 46, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R.; Donkers, J.H.; Fredrix, E.W.; Boekhoudt, P. Energy intake, physical activity and body weight: A simulation model. Br. J. Nutr. 1995, 73, 337–347. [Google Scholar]
- Schoffelen, P.F.; Westerterp, K.R.; Saris, W.H.; Ten Hoor, F. A dual-respiration chamber system with automated calibration. J. Appl. Physiol. 1997, 83, 2064–2072. [Google Scholar] [PubMed]
- Hursel, R.; van der Zee, L.; Westerterp-Plantenga, M.S. Effects of a breakfast yoghurt, with additional total whey protein or caseinomacropeptide-depleted alpha-lactalbumin-enriched whey protein, on diet-induced thermogenesis and appetite suppression. Br. J. Nutr. 2010, 103, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrere, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hursel, R.; Westerterp-Plantenga, M.S. Consumption of Milk-Protein Combined with Green Tea Modulates Diet-Induced Thermogenesis. Nutrients 2011, 3, 725-733. https://doi.org/10.3390/nu3080725
Hursel R, Westerterp-Plantenga MS. Consumption of Milk-Protein Combined with Green Tea Modulates Diet-Induced Thermogenesis. Nutrients. 2011; 3(8):725-733. https://doi.org/10.3390/nu3080725
Chicago/Turabian StyleHursel, Rick, and Margriet S. Westerterp-Plantenga. 2011. "Consumption of Milk-Protein Combined with Green Tea Modulates Diet-Induced Thermogenesis" Nutrients 3, no. 8: 725-733. https://doi.org/10.3390/nu3080725





