Folate Status of Reproductive Age Women and Neural Tube Defect Risk: The Effect of Long-Term Folic Acid Supplementation at Doses of 140 µg and 400 µg per Day
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Participants
2.2. Study Protocol
2.3. Supplements
2.4. Dietary Folate Assessment
2.5. Blood Sampling and Laboratory Analysis
2.6. Statistical Analysis
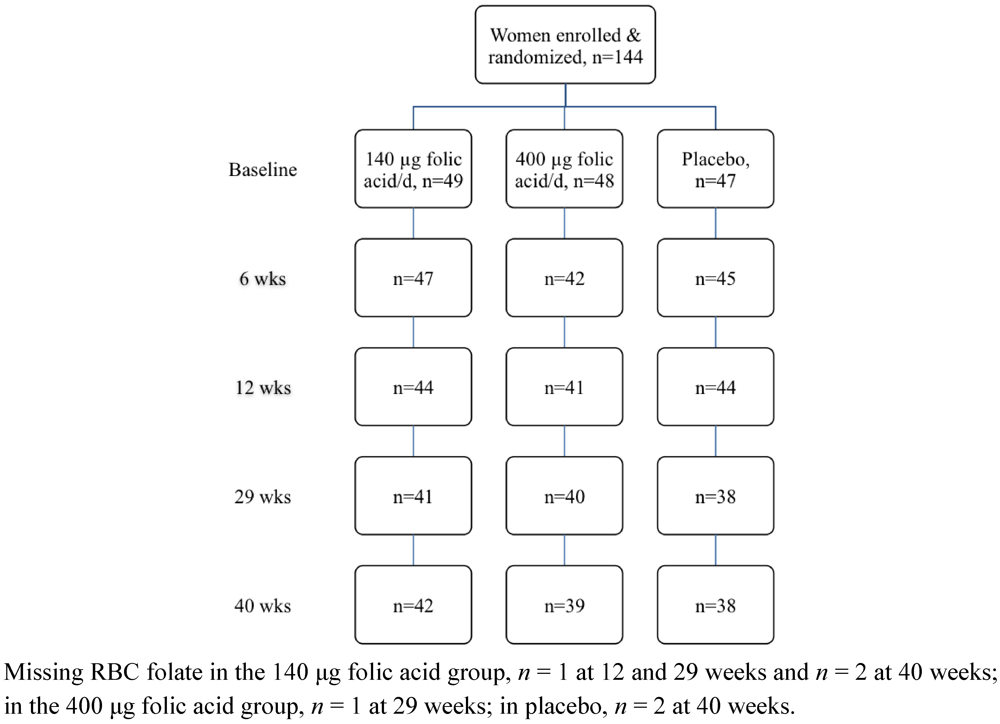
3. Results
| All (n = 144) | Placebo (n = 47) | 140 µg folic acid/day (n = 49) | 400 µg folic acid/day (n = 48) | |
|---|---|---|---|---|
| Age (y) 2 | 24.5 ± 5.9 | 24.5 ± 5.6 | 24.2 ± 6.2 | 24.9 ± 5.8 |
| Weight (kg) 2 | 65.5 ± 12.3 | 65.5 ± 12.2 | 66.5 ± 12.1 | 64.5 ± 12.7 |
| BMI (kg/m²) 2 | 23.7 ± 3.9 | 23.6 ± 3.8 | 24.2 ± 4.0 | 23.4 ± 4.0 |
| BMI Category, n (%) | ||||
| <18.5 | 7 (5) | 2 (4) | 2 (4) | 3 (6) |
| 18.5–24.9 | 96 (67) | 32 (68) | 31 (63) | 33 (69) |
| 25.0–29.9 | 32 (22) | 10 (21) | 12 (25) | 10 (21) |
| ≥30.0 | 9 (6) | 3 (6) | 4 (8) | 2 (4) |
| Ethnicity, n (%) 3 | ||||
| New Zealand European | 109 (76) | 35 (75) | 35 (71) | 39 (81) |
| Maori and Pacific Peoples | 7 (5) | 4 (9) | 2 (4) | 1 (2) |
| Asian | 17 (12) | 6 (13) | 8 (16) | 3 (6) |
| Other ethnicities | 11 (8) | 2 (4) | 4 (8) | 5 (10) |
| Education, n (%) 3 | ||||
| Secondary school or less | 17 (12) | 3 (6) | 9 (18) | 5 (10) |
| Post-secondary education | 105 (73) | 39 (83) | 31 (63) | 35 (73) |
| Advanced degree | 22 (15) | 5 (11) | 9 (18) | 8 (17) |
| Oral contraceptive use, n (%) | 63 (44) | 22 (47) | 20 (41) | 21 (44) |
| Dietary Folate (µg/day) 4,5 | 255.0 ± 1.5 | 246.7 ± 1.5 | 248.0 ± 1.6 | 271.5 ± 1.5 |
| Plasma folate (nmol/L) 4 | 19.9 ± 1.8 | 21.4 ± 1.6 | 19.2 ± 1.8 | 19.3 ± 1.9 |
| <6.8 nmol/L, n (%) | 2 (1) | 0 (0) | 2 (1) | 0 (0) |
| Red blood cell folate (nmol/L) 4 | 753.2 ± 1.6 | 808.5 ± 1.5 | 700.4 ± 1.6 | 756.8 ± 1.6 |
| <317 nmol/L, n (%) | 4 (3) | 1 (2) | 1 (2) | 2 (4) |
| <906 nmol/L, n (%) | 95 (66) | 30 (64) | 33 (67) | 32 (67) |
| Measurement and treatment group | Baseline | Week 6 | Week 12 | Week 29 | Week 40 | Difference in change from baseline to 40 weeks (%) 2 |
|---|---|---|---|---|---|---|
| Plasma folate (nmol/L) | ||||||
| Placebo | 21.1 (17.8, 24.4) | 20.3 (17.1, 23.5) | 19.7 (16.6, 22.9) | 20.6 (17.2, 24.1) | 21.0 (17.4, 24.5) | – |
| 140 μg folic acid/day | 19.5 (16.5, 22.5) | 26.6 (22.5, 30.8) | 28.0 (23.6, 32.5) | 31.0 (25.9, 36.0) | 29.0 (24.3, 33.7) | 49.8 (18.1, 90.1) 3 |
| 400 μg folic acid/day | 19.2 (16.3, 22.2) | 37.3 (31.2, 43.3) | 38.3 (32.1, 44.6) | 37.3 (31.1, 43.4) | 39.5 (33.0, 46.1) | 107.0 (62.7, 163.8) 4,5 |
| RBC folate (nmol/L) | ||||||
| Placebo | 794.4 (696.7, 892.1) | 763.8 (668.8, 858.8) | 707.8 (619.2, 796.5) | 729.0 (634.3, 823.7) | 819.0 (711.1, 926.8) | – |
| 140 μg folic acid/day | 711.8 (626.1, 797.5) | 834.5 (732.8, 936.1) | 849.0 (743.4, 954.7) | 987.0 (861.8, 1112.2) | 1111.3 (970.3, 1252.3) | 51.4 (29.1, 77.6) 4 |
| 400 μg folic acid/day | 755.5 (664.2, 846.8) | 910.8 (796.2, 1025.3) | 940.1 (821.2, 1059.1) | 1121.7 (977.9, 1265.4) | 1273.4 (1110.2, 1436.6) | 63.4 (39.2, 92.0) 4 |
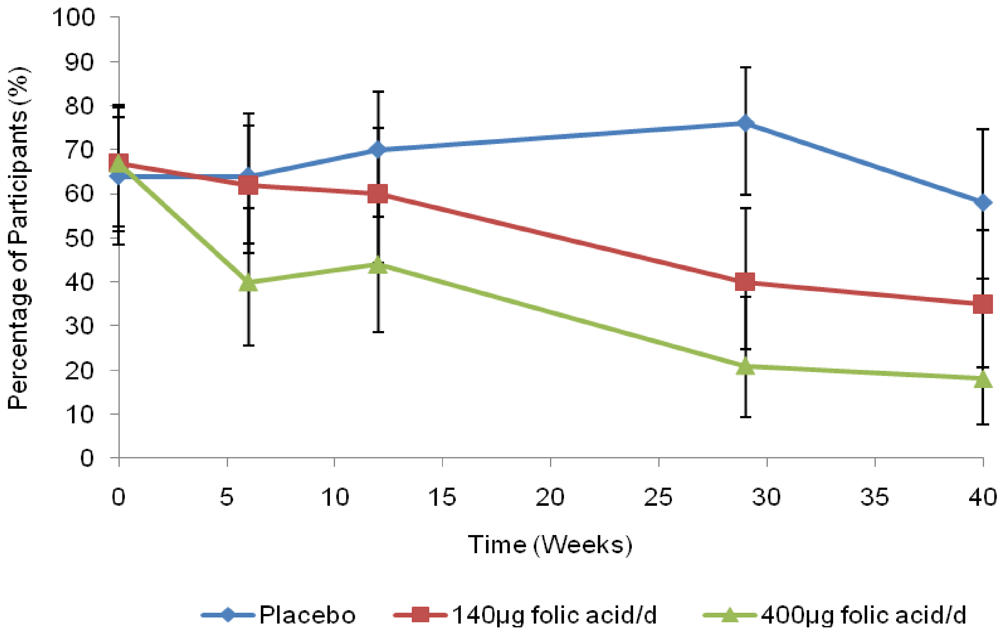
4. Discussion and Conclusions
Acknowledgements
References
- Department of Health Scottish Office Home and Health Department, Welsh Office, Department of Health and Social Services, Northern Ireland, Report from an Expert Advisory Group on Folic Acid and the Prevention of Neural Tube Defects; Department of Health: London, UK, 1992.
- Public Health Service, Centers for Disease Control and Prevention. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Morb. Mortal. Wkly. Rep. 1992, 41, 1–7.
- National Health and Medical Research Council, Revised Statement on the Relationship between Dietary Folic Acid and Neural Tube Defects Such As Spina Bifida; NHMRC: Melbourne, Australia, 1993.
- Commission of the European Communities, Nutrient and Energy Intakes for the European Community; Reports of the Scientific Committee for Food Thirty-First Series; Office for Official Publications of the European Communities: Luxembourg, 1993.
- Mulinare, J.; Cordero, J.F.; Erickson, J.D.; Berry, R.J. Periconceptional use of multivitamins and the occurrence of neural tube defects. JAMA 1988, 260, 3141–3145. [Google Scholar]
- Milunsky, A.; Jick, H.; Jick, S.S.; Bruell, C.L.; MacLaughlin, D.S.; Rothman, K.J.; Willett, W. Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects. JAMA 1989, 262, 2847–2852. [Google Scholar]
- Werler, M.M.; Shapiro, S.; Mitchell, A.A. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA 1993, 269, 1257–1261. [Google Scholar]
- Shaw, G.M.; Schaffer, D.; Velie, E.M.; Morland, K.; Harris, J.A. Periconceptional vitamin use, dietary folate, and the occurrence of neural tube defects. Epidemiology 1995, 6, 219–226. [Google Scholar]
- Khoury, M.J.; Shaw, G.M.; Moore, C.A.; Lammer, E.J.; Mulinare, J. Does periconceptional multivitamin use reduce the risk of neural tube defects associated with other birth defects? Data from two population-based case-control studies. Am. J. Med. Genet. 1996, 61, 30–36. [Google Scholar]
- Berry, R.J.; Li, Z.; Erickson, J.D.; Li, S.; Moore, C.A.; Wang, H.; Mulinare, J.; Zhao, P.; Wong, L.Y.; Gindler, J.; Hong, S.X.; Correa, A. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N. Engl. J. Med. 1999, 341, 1485–1490. [Google Scholar] [PubMed]
- Daly, L.E.; Kirke, P.N.; Molloy, A.; Weir, D.G.; Scott, J.M. Folate levels and neural tube defects. Implications for prevention. JAMA 1995, 274, 1698–1702. [Google Scholar] [PubMed]
- Pfeiffer, C.M.; Johnson, C.L.; Jain, R.B.; Yetley, E.A.; Picciano, M.F.; Rader, J.I.; Fisher, K.D.; Mulinare, J.; Osterloh, J.D. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988–2004. Am. J. Clin. Nutr. 2007, 86, 718–727. [Google Scholar]
- Honein, M.A.; Paulozzi, L.J.; Mathews, T.J.; Erickson, J.D.; Wong, L.Y.C. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 2001, 285, 2981–2986. [Google Scholar]
- Williams, L.J.; Mai, C.T.; Edmonds, L.D.; Shaw, G.M.; Kirby, R.S.; Hobbs, C.A.; Sever, L.E.; Miller, L.A.; Meaney, F.J.; Levitt, M. Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology 2002, 66, 33–39. [Google Scholar]
- De Wals, P.; Tairou, F.; Van Allen, M.I.; Uh, S.H.; Lowry, R.B.; Sibbald, B.; Evans, J.A.; Van den Hof, M.C.; Zimmer, P.; Crowley, M. Reduction in Neural-Tube Defects after Folic Acid Fortification in Canada. N. Engl. J. Med. 2007, 357, 135–142. [Google Scholar]
- De Wals, P.; Rusen, I.D.; Lee, N.S.; Morin, P.; Niyonsenga, T. Trend in prevalence of neural tube defects in Quebec. Clin. Mol. Teratol. 2003, 67, 919–923. [Google Scholar]
- Ray, J.G.; Meier, C.; Vermeulen, M.J.; Boss, S.; Wyatt, P.R.; Cole, D.E. Association of neural tube defects and folic acid food fortification in Canada. Lancet 2002, 360, 2047–2048. [Google Scholar]
- Persad, V.L.; van den Hof, M.C.; Dube, J.M.; Zimmer, P. Incidence of open neural tube defects in Nova Scotia after folic acid fortification. CMAJ 2002, 167, 241–245. [Google Scholar]
- Liu, S.; West, R.; Randell, E.; Longerich, L.; O’Connor, K.S.; Scott, H.; Crowley, M.; Lam, A.; Prabhakaran, V.; McCourt, C. A comprehensive evaluation of food fortification with folic acid for the primary prevention of neural tube defects. BMC Pregnancy Childbirth 2004, 4, 20. [Google Scholar] [Green Version]
- Canfield, M.A.; Collins, J.S.; Botto, L.D.; Williams, L.J.; Mai, C.T.; Kirby, R.S.; Pearson, K.; Devine, O.; Mulinare, J. Changes in the birth prevalence of selected birth defects after grain fortification with folic acid in the United States: findings from a multi-state population-based study. Clin. Mol. Teratol. 2005, 73, 679–689. [Google Scholar]
- Proposed Amendment to the New Zealand Folic Acid Standard; NZFSA Public Discussion Paper No. 10/09; New Zealand Food Safety Authority: Wellington, New Zealand, July 22 2009.
- Australian New Zealand Clinical Trials Registry. Effect of mandatory folic acid fortification policy on folate status of New Zealand women of childbearing age. ACTRN12609000215224. Available online: http://www.anzctr.org.au/trial_view.aspx?ID=83422 (accessed on 4 December 2008).
- Lohman, T.J.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Publications: Champaign, IL, USA, 1988. [Google Scholar]
- New Zealand Food Composition Database (FOODfiles); New Zealand Institute for Crop & Food Research Limited and Ministry of Health: Christchurch, New Zealand, 2006.
- Molloy, A.M.; Scott, J.M. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997, 281, 43–53. [Google Scholar]
- Gibson, R.S. Principles of Nutritional Assessment, 2nd ed; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Venn, B.J.; Green, T.J.; Moser, R.; Mann, J.I. Comparison of the effect of low-dose supplementation with l-5-methyltetrahydrofolate or folic acid on plasma homocysteine: a randomized placebo-controlled study. Am. J. Clin. Nutr. 2003, 77, 658–662. [Google Scholar]
- Hao, L.; Yang, Q.H.; Li, Z.; Bailey, L.B.; Zhu, J.H.; Hu, D.J.; Zhang, B.L.; Erickson, J.D.; Zhang, L.; Gindler, J.; Li, S.; Berry, R.J. Folate status and homocysteine response to folic acid doses and withdrawal among young Chinese women in a large-scale randomized double-blind trial. Am. J. Clin. Nutr. 2008, 88, 448–457. [Google Scholar]
- Lamers, Y.; Prinz-Langenohl, R.; Moser, R.; Pietrzik, K. Supplementation with [6S]-5-methyltetrahydrofolate or folic acid equally reduces plasma total homocysteine concentrations in healthy women. Am. J. Clin. Nutr. 2004, 79, 473–478. [Google Scholar]
- Molloy, A.M.; Brody, L.C.; Mills, J.L.; Scott, J.M.; Kirke, P.N. The search for genetic polymorphisms in the homocysteine/folate pathway that contribute to the etiology of human neural tube defects. Clin. Mol. Teratol. 2009, 85, 285–294. [Google Scholar]
- Tighe, P.; Ward, M.; McNulty, H.; Finnegan, O.; Dunne, A.; Strain, J.; Molloy, A.M.; Duffy, M.; Pentieva, K.; Scott, J.M. A dose-finding trial of the effect of long-term folic acid intervention: implications for food fortification policy. Am. J. Clin. Nutr. 2011, 93, 11–18. [Google Scholar]
- Bailey, R.L.; Dodd, K.W.; Gahche, J.J.; Dwyer, J.T.; McDowell, M.A.; Yetley, E.A.; Sempos, C.A.; Burt, V.L.; Radimer, K.L.; Picciano, M.F. Total folate and folic acid intakes from foods and dietary supplements in the United States: 2003–2006. Am. J. Clin. Nutr. 2010, 91, 231–237. [Google Scholar]
- Shakur, Y.A.; Garriguet, D.; Corey, P.; O’Connor, D.L. Folic acid fortification above mandated levels results in a low prevalence of folate inadequacy among Canadians. Am. J. Clin. Nutr. 2010, 92, 818–825. [Google Scholar]
- Bar-Oz, B.; Koren, G.; Nguyen, P.; Kapur, B.M. Folate fortification and supplementation—are we there yet? Reprod. Toxicol. 2008, 25, 408–412. [Google Scholar]
- Nguyen, P.; Tam, C.; O’Connor, D.L.; Kapur, B.; Koren, G. Steady state folate concentrations achieved with 5 compared with 1.1 mg folic acid supplementation among women of childbearing age. Am. J. Clin. Nutr. 2009, 89, 844–852. [Google Scholar] [PubMed]
- Heseker, H.B.; Mason, J.B.; Selhub, J.; Rosenberg, I.H.; Jacques, P.F. Not all cases of neural-tube defect can be prevented by increasing the intake of folic acid. Br. J. Nutr. 2009, 102, 173–180. [Google Scholar]
- Mosley, B.S.; Cleves, M.A.; Siega-Riz, A.M.; Shaw, G.M.; Canfield, M.A.; Waller, D.K.; Werler, M.M.; Hobbs, C.A. Neural tube defects and maternal folate intake among pregnancies conceived after folic acid fortification in the United States. Am. J. Epidemiol. 2009, 169, 9–17. [Google Scholar]
- Mills, J.L.; Carter, T.C. Invited commentary: Preventing neural tube defects and more via food fortification? Am. J. Epidemiol. 2009, 169, 18–21. [Google Scholar] [PubMed]
- New Zealand Food Safety Authority. Folic Acid—Key Issues—NZFSA Response Science. Available online: http://www.nzfsa.govt.nz/consultation/folic-acid/nzfsa-response/folic-acid-key-issues-nzfsa-response.pdf (accessed on 22 October 2010).
- New Zealand Ministry of Health. Nutrition—Folate/Folic Acid (Last updated 4 October 2010). 2010. Available online: http://www.moh.govt.nz/moh.nsf/indexmh/nutrition-folate#ntd2 (accessed on 22 October 2010).
- Bower, C.; Klerk, N.; Hickling, S.; Ambrosini, G.; Flicker, L.; Geelhoed, E.; Milne, E. Assessment of the potential effect of incremental increases in folic acid intake on neural tube defects in Australia and New Zealand. Aust. N. Z. J. Public Health 2006, 30, 369–374. [Google Scholar]
- Wald, N.J.; Law, M.R.; Morris, J.K.; Wald, D.S. Quantifying the effect of folic acid. Lancet 2001, 358, 2069–2073. [Google Scholar]
- Ward, M.; McNulty, H.; McPartlin, J.; Strain, J.J.; Weir, D.G.; Scott, J.M. Plasma homocysteine, a risk factor for cardiovascular disease, is lowered by physiological doses of folic acid. QJM 1997, 90, 519–524. [Google Scholar]
- Quinlivan, E.P.; Gregory, J.F., III. Effect of food fortification on folic acid intake in the United States. Am. J. Clin. Nutr. 2003, 77, 221–225. [Google Scholar]
- Berry, R.J.; Bailey, L.; Mulinare, J.; Bower, C. Fortification of flour with folic acid. Food Nutr. Bull. 2010, 31, S22–S35. [Google Scholar]
- Ministry of Health, Improving the Folate Intake in New Zealand: Policy Implications; Public Health Intelligence Occasional Bulletin Number 18; Ministry of Health: Wellington, New Zealand, 2003.
- Schader, I.; Corwin, P. How many pregnant women in Christchurch are using folic acid supplements in early pregnancy? N. Z. Med. J. 1999, 112, 463–465. [Google Scholar] [PubMed]
- Ferguson, E.L.; Skeaff, C.M.; Bourn, D.M. Folate Status of Representative Populations in Dunedin: Issues for Folate Fortification; A report prepared for the Ministry of Health and the Australian NZ Food Authority; University of Otago: Dunedin, New Zealand, 2000. [Google Scholar]
- Dobson, I.; Devenish, C.; Skeaff, C.M.; Green, T.J. Periconceptional folic acid use among women giving birth at Queen Mary Maternity Hospital in Dunedin. Aust. N. Z. J. Obstet. Gynaecol. 2006, 46, 534–537. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hursthouse, N.A.; Gray, A.R.; Miller, J.C.; Rose, M.C.; Houghton, L.A. Folate Status of Reproductive Age Women and Neural Tube Defect Risk: The Effect of Long-Term Folic Acid Supplementation at Doses of 140 µg and 400 µg per Day. Nutrients 2011, 3, 49-62. https://doi.org/10.3390/nu3010049
Hursthouse NA, Gray AR, Miller JC, Rose MC, Houghton LA. Folate Status of Reproductive Age Women and Neural Tube Defect Risk: The Effect of Long-Term Folic Acid Supplementation at Doses of 140 µg and 400 µg per Day. Nutrients. 2011; 3(1):49-62. https://doi.org/10.3390/nu3010049
Chicago/Turabian StyleHursthouse, Nicola A., Andrew R. Gray, Jody C. Miller, Meredith C. Rose, and Lisa A. Houghton. 2011. "Folate Status of Reproductive Age Women and Neural Tube Defect Risk: The Effect of Long-Term Folic Acid Supplementation at Doses of 140 µg and 400 µg per Day" Nutrients 3, no. 1: 49-62. https://doi.org/10.3390/nu3010049






