The Effect of Diabetes-Specific Enteral Nutrition Formula on Cardiometabolic Parameters in Patients with Type 2 Diabetes: A Systematic Review and Meta–Analysis of Randomised Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. Types of Studies and Participants
2.2. Type of Intervention
2.3. Types of Outcome Measures
- Blood glucose parameters—Fasting blood glucose and glycated haemoglobin.
- Lipid profile: Total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol and triglycerides.
2.4. Search Strategy
2.5. Data Extraction
2.6. Assessment of Risk of Bias and Evaluation of Quality
2.7. Statistical Analysis
2.8. Effect Size
3. Results
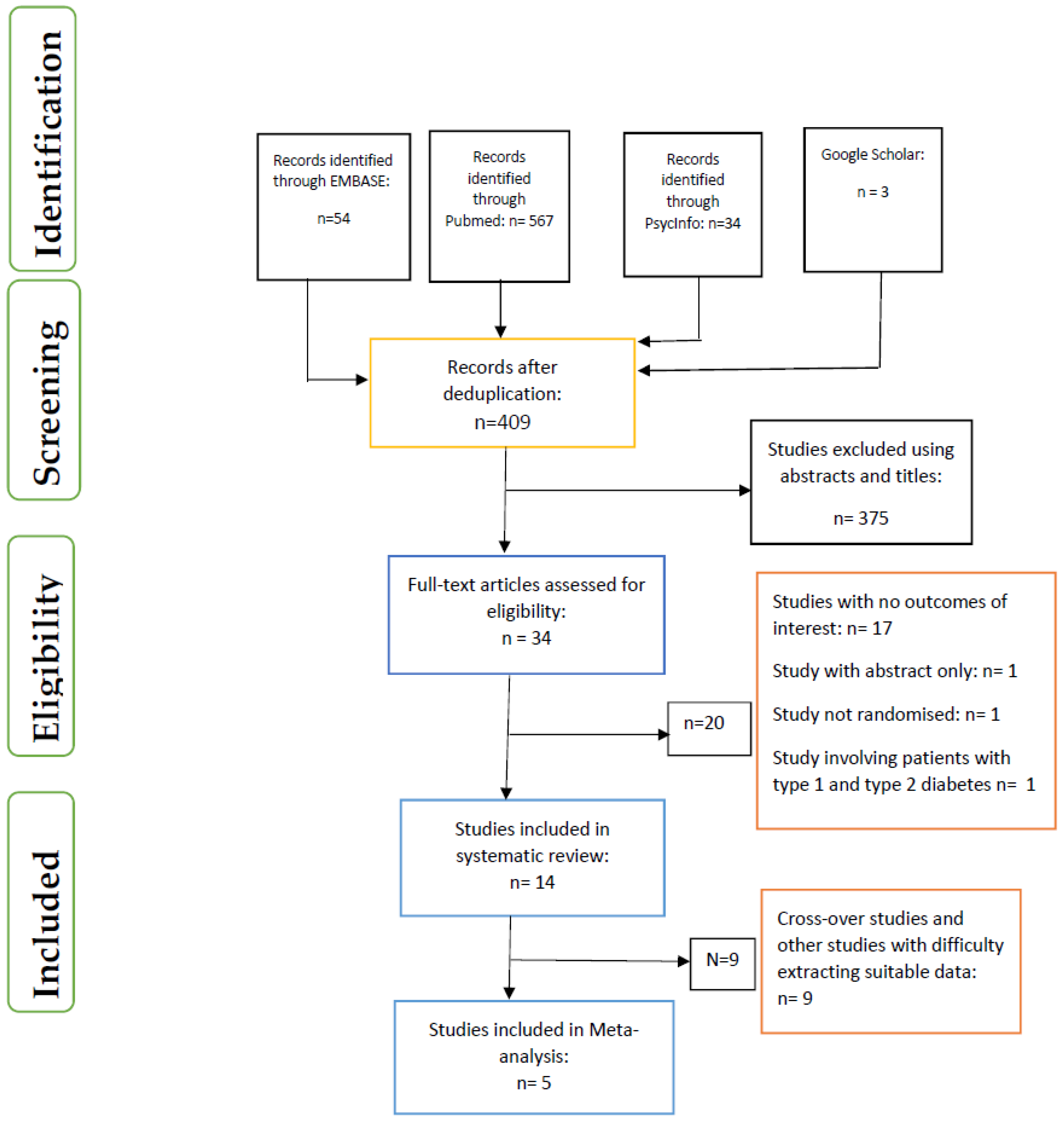
3.1. Data Inclusion Decisions
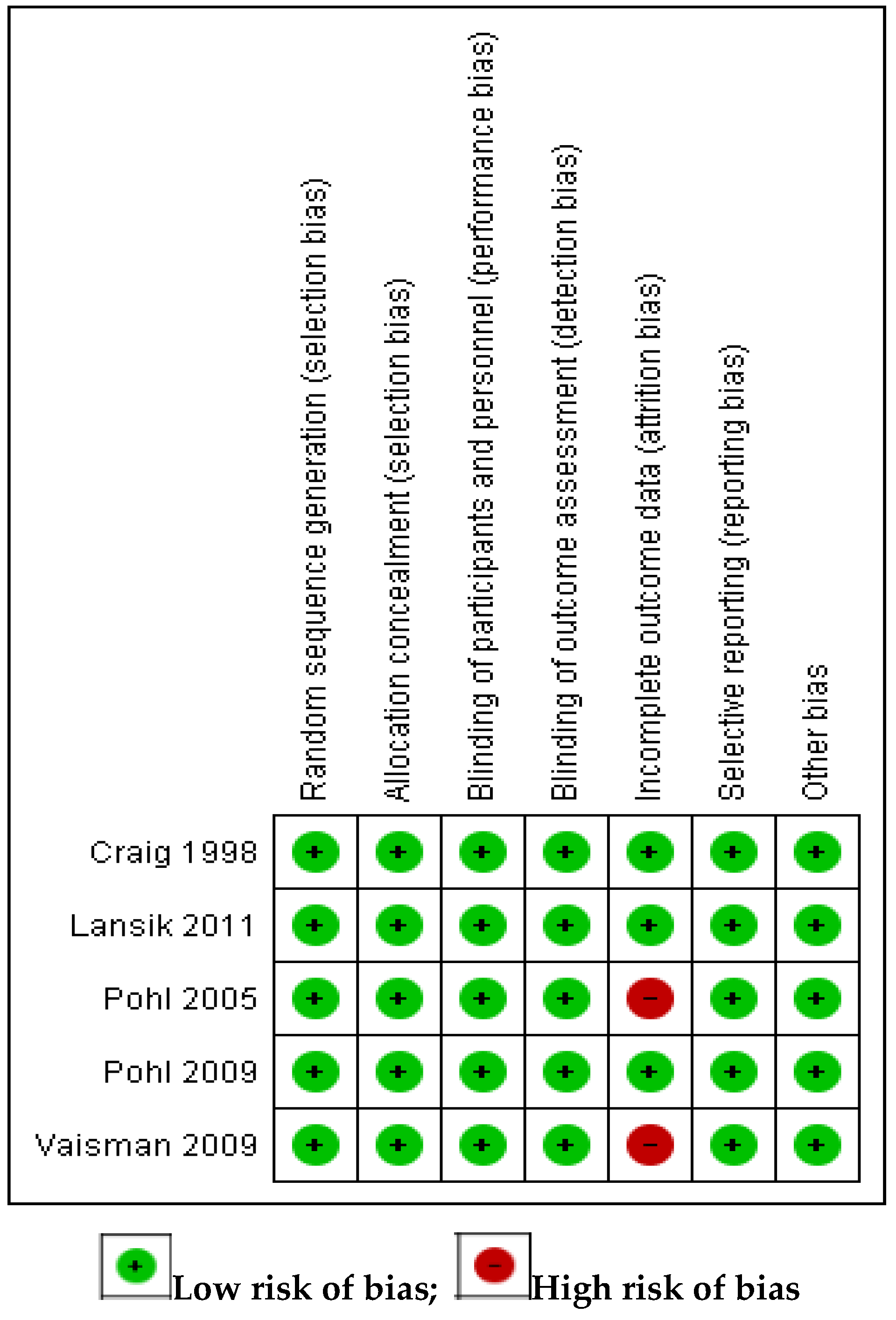
3.2. Assessment of Risk of Bias in Included Studies
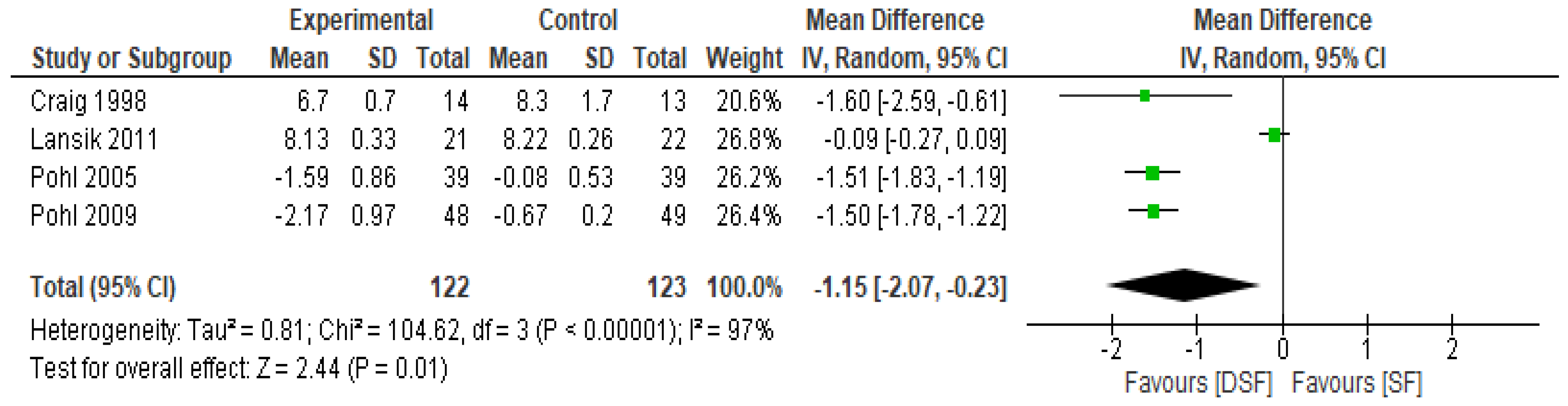
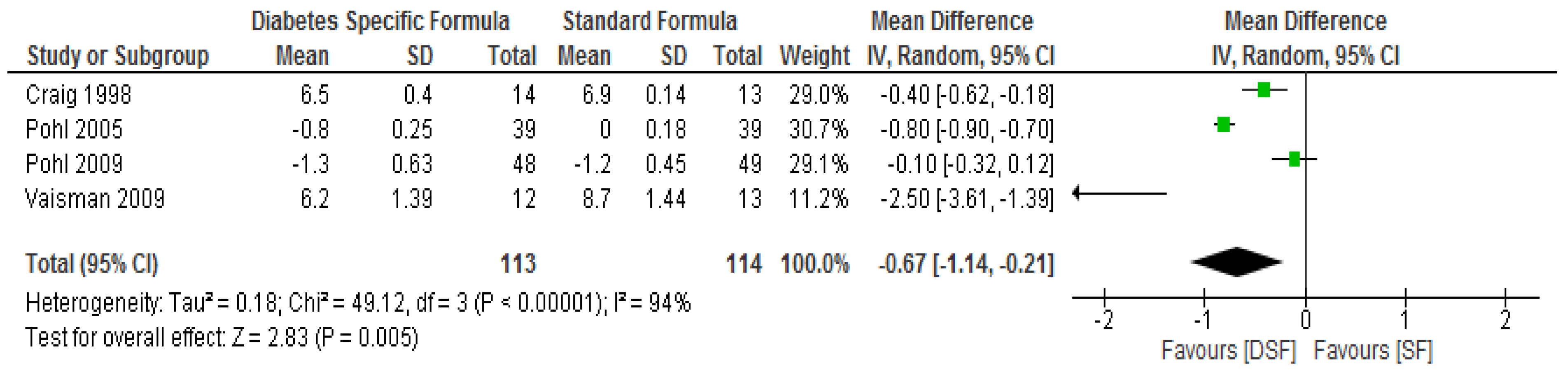
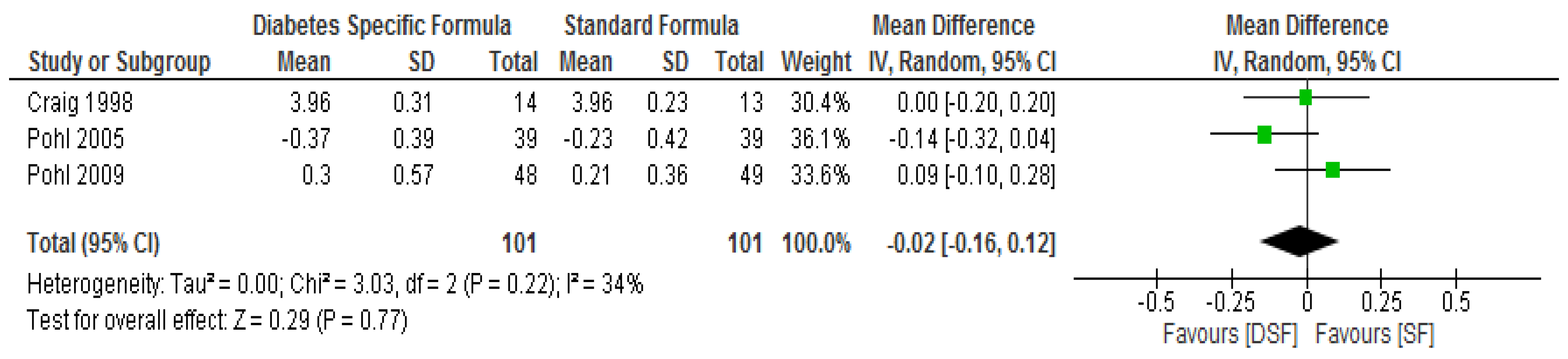
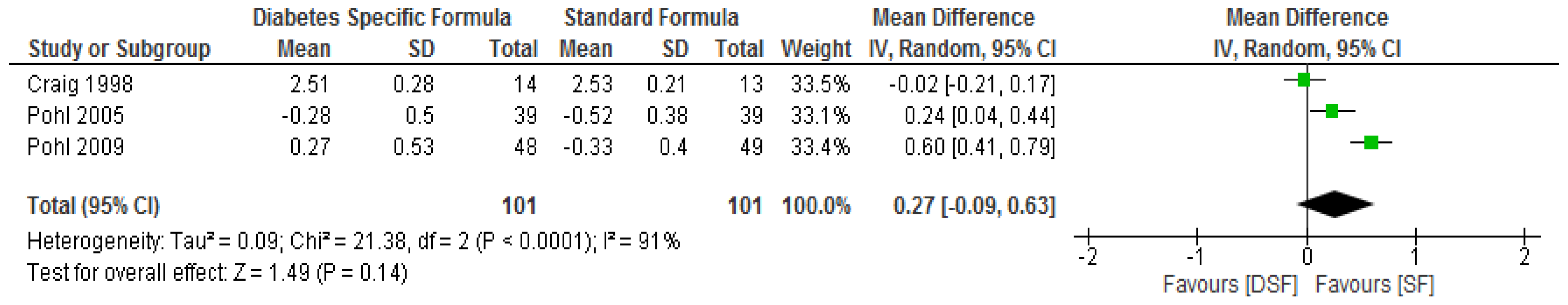
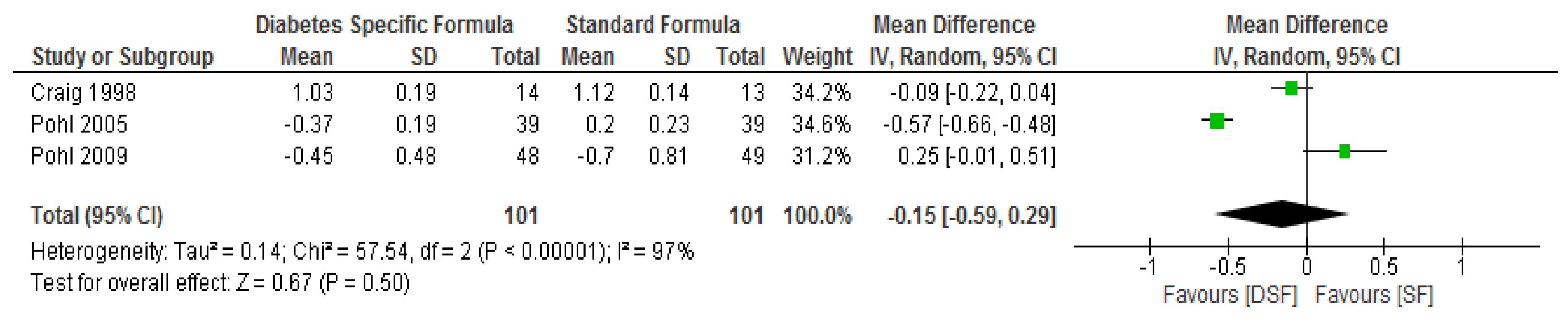
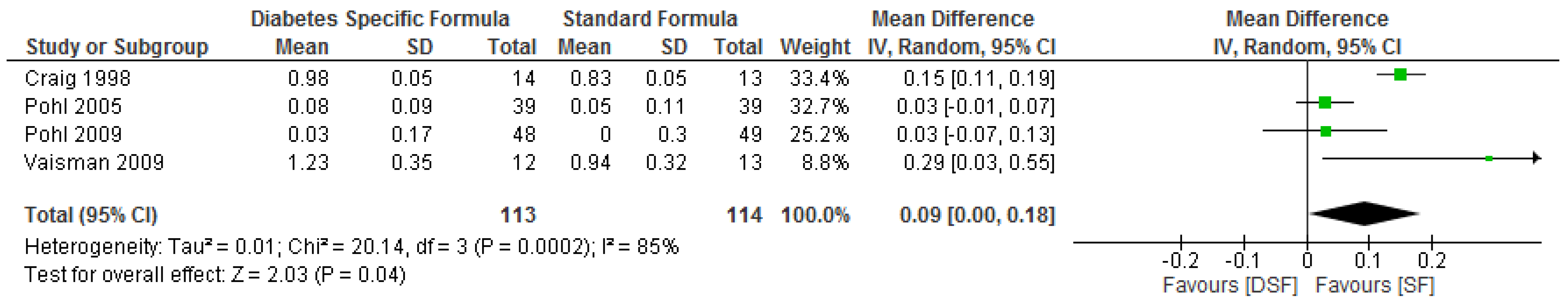
3.3. The Effect of DSF on Lipid Profile
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- DeFronzo, R.A.; Ratner, R.E.; Han, J.; Kim, D.D.; Fineman, M.S.; Baron, A.D. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005, 28, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Jansink, R.; Braspenning, J.; Laurant, M.; Keizer, E.; Elwyn, G.; Weijden, T.D.; Grol, R. Minimal improvement of nurses’ motivational interviewing skills in routine diabetes care one year after training: A cluster randomized trial. BMC Fam. Pract. 2013, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Chronic Conditions (NCCCC). Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update); Royal College of Physicians: London, UK, 2008. [Google Scholar]
- Public Health England. 3.8 Million People in England Now Have Diabetes. 2016. Available online: https://www.gov.uk/government/news/38-million-people-in-england-now-have-diabetes (accessed on 1 February 2019).
- National Health Service (NHS) Digital and Healthcare Quality Improvement Partnership. National diabetes audit, 2015–2016 Report 1: Care Processes and Treatment Targets. 2017. Available online: http://www.content.digital.nhs.uk/catalogue/PUB23241/nati-diab-rep1-audi-2015-16.pdf (accessed on 1 February 2019).
- Diabetes UK. State of the Nation Report. 2015. Available online: https://www.diabetes.org.uk/Documents/About%20Us/What%20we%20say/State%20of%20the%20nation%202014.pdf (accessed on 1 February 2019).
- Holman, N.; Young, B.; Gadsby, R. What is the current prevalence of diagnosed and yet to be diagnosed diabetes in the UK. Diabetes Med. 2014, 31, 510–511. [Google Scholar] [CrossRef] [PubMed]
- Diabetes UK. Diabetes in the UK 2012: Key Statistics on Diabetes. 2012. Available online: http://tinyurl.com/owcyr7b (accessed on 1 February 2019).
- Holmes, C.; Dyer, P. Diabetes training for nurses: The effectiveness of an inpatient diabetes half-day workshop. J. Diabetes Nurs. 2013, 17, 86–94. [Google Scholar]
- Pereira, D.A.; da Silva Campos Costa, N.M.; Lima Sousa, A.L.; Veiga Jardim, P.B.; de Oliveira Zanini, C.R. The effect of educational intervention on the disease knowledge of diabetes mellitus patients. Rev. Lat. Am. De Enferm. 2012, 20, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Wyness, L. Understanding the role of diet in type 2 diabetes prevention. Br. J. Commun. Nurs. 2009, 14, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O. The role of nutrition and hydration in disease prevention and patient safety. Br. J. Nurs. 2017, 26, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Arinzon, Z.; Shabat, S.; Shuval, I.; Peisakh, A.; Berner, Y. Prevalence of diabetes mellitus in elderly patients received enteral nutrition long-term care service. Arch. Gerontol. Geriatr. 2008, 47, 383–393. [Google Scholar] [CrossRef]
- Wong, V.W.; Manoharan, M.; Mak, M. Managing hyperglycaemia in patients with diabetes on enteral nutrition: The role of a specialized diabetes team. Eur. J. Clin. Nutr. 2014, 68, 1305–1308. [Google Scholar] [CrossRef]
- Oyibo, S.; Sagi, S.; Home, C. Glycaemic control during enteral tube feeding in patients with diabetes who have had a stroke: A twice-daily insulin regimen. Pract. Diabetes 2012, 29, 135–139. [Google Scholar] [CrossRef]
- Elia, M.; Ceriello, A.; Laube, H.; Sinclair, A.J.; Engfer, M.; Stratton, R.J. Enteral nutritional support and use of diabetes specific formulas for patients with diabetes. Diabetes Care 2005, 28, 2267–2279. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Paris, A.; Álvarez Hernández, J.; Ballesteros-Pomar, M.D.; Botella-Romero, F.; León-Sanz, M.; Martín-Palmero, Á.; Olveira, G. Evidence-based recommendations and expert consensus on enteral nutrition in the adult patient with diabetes mellitus or hyperglycemia. Nutrition 2017, 41, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Lansink, M.; Rouws, C.H.F.C.; van Laere, K.M.J.; Frost, G.S. Administration of a new diabetes specific enteral formula results in an improved 24 h glucose profile in type 2 diabetic patients. Diabetes Res. Clin. Pract. 2009, 84, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Hise, M.E.; Fuhrman, M.P. The Effect of Diabetes Specific Enteral Formulae on Clinical and Glycaemic Indicators; Parrish, C.R., Ed.; Nutrition Issues in Gastroenterology Series 74; Shugar Publishing: New York, NY, USA, 2009; pp. 20–36. [Google Scholar]
- Buranapin, S.; Siangruangsang, S.; Chantapanich, V.; Hengjeerajarus, N. The comparative study of diabetic specific formula and standard formula on postprandial plasma glucose control in type 2 DM patients. J. Med. Assoc. Thail. 2014, 97, 582–588. [Google Scholar]
- McMahon, M.M.; Nystrom, E.; Braunschweig, C.; Miles, J.; Compher, C. The American Society of Parenteral and Enteral Nutrition (ASPEN) Clinical Guidelines: Nutrition support of adult patients with hyperglycaemia. J. Parenter. Enter. Nutr. 2013, 37, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Ojo O and Brooke J Evaluation of the role of enteral nutrition in managing patients with Diabetes: A systematic review. Nutrients 2014, 6, 5142–5152. [CrossRef]
- Jones, S.; Honnor, M.; Castro, E.; Alsmadi, A. Management of people with diabetes receiving artificial nutrition: A review. J. Diabetes Nurs. 2017, 21, 179–183. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma, G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme (CASP) Case Control Study Checklist. Available online: http://docs.wixstatic.com/ugd/dded87_afbfc99848f64537a53826e1f5b30b5c.pdf (accessed on 29 January 2019).
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- The Nordic Cochrane Centre. Review Manager (RevMan). In Computer Program; Version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2014. [Google Scholar]
- Pohl, M.; Mayr, P.; Mertl-Roetzer, M.; Lauster, F.; Lerch, M.; Eriksen, J.; Haslbeck, M.; Rahlfs, V.W. Glycaemic control in type II diabetic tube-fed patients with a new enteral formula low in carbohydrates and high in monounsaturated fatty acids: A randomised controlled trial. Eur. J. Clin. Nutr. 2005, 59, 1221–1232. [Google Scholar] [CrossRef]
- Pohl, M.; Mayr, P.; Mertl-Roetzer, M.; Lauster, F.; Haslbeck, M.; Hipper, B.; Steube, D.; Tietjen, M.; Eriksen, J.; Rahlfs, V.W. Glycemic control in patients with type 2 diabetes mellitus with a disease-specific enteral formula: Stage II of a randomized, controlled multicenter trial. J. Parenter. Enter. Nutr. 2009, 33, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.D.; Nicholson, S.; Silverstone, F.A.; Kennedy, R.D.; Coble Voss, A.; Allison, S. Use of a reduced-carbohydrate, modified-fat enteral formula for improving metabolic control and clinical outcomes in long-term care residents with type 2 diabetes: Results of a pilot trial. Nutrition 1998, 14, 529–534. [Google Scholar] [CrossRef]
- Lansink, M.; van Laere, K.M.; Vendrig, L.; Rutten, G.E. Lower postprandial glucose responses at baseline and after 4 weeks use of a diabetes-specific formula in diabetes type 2 patients. Diabetes Res. Clin. Pract. 2011, 93, 421–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaisman, N.; Lansink, M.; Rouws, C.H.; van Laere, K.M.; Segal, R.; Niv, E.; Bowling, T.E.; Waitzberg, D.L.; Morleyf, J.E. Tube feeding with a diabetes-specific feed for 12 weeks improves glycaemic control in type 2 diabetes patients. Clin. Nutr. 2009, 28, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Alish, C.J.; Garvey, W.T.; Maki, K.C.; Sacks, G.S.; Hustead, D.S.; Hegazi, R.A.; Mustad, V.A. A diabetes-specific enteral formula improves glycemic variability in patients with type 2 diabetes. Diabetes Technol. Ther. 2010, 12, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Misra, A.; Nanda, K.; Pandey, R.M.; Garg, V.; Ganguly, S.; Cheung, L. Efficacy and tolerance of a diabetes specific formula in patients with type 2 diabetes mellitus: An open label, randomized, crossover study. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Hofman, Z.; Lansink, M.; Rouws, C.; van Drunen, J.D.E.; Kuipers, H. Diabetes specific tube feed results in improved glycaemic and triglyceridaemic control during 6 h continuous feeding in diabetes patients. e-SPEN 2007, 2, 44–50. [Google Scholar] [CrossRef]
- Lansink, M.; Hofman, Z.; Genovese, S.; Rouws, C.H.F.C.; Ceriello, A. Improved Glucose Profile in Patients with Type 2 Diabetes with a New, High-Protein, Diabetes-Specific Tube Feed During 4 Hours of Continuous Feeding. JPEN J. Parenter. Enter. Nutr. 2017, 41, 968–975. [Google Scholar] [CrossRef]
- Mesejo, A.; Montejo-Gonzalez, J.C.; Vaquerizo-Alonso, C.; Lobo-Tamer, G.; Zabarte-Martinez, M.; Herrero-Meseguer, J.I.; Escirbano, J.A.; Malpica, A.B.; Lozano, F.M. Diabetes-specific enteral nutrition formula in hyperglycemic, mechanically ventilated, critically ill patients: A prospective, open-label, blind-randomized, multicenter study. Crit. Care 2015, 19, 390. [Google Scholar] [CrossRef]
- Voss, A.C.; Maki, K.C.; Garvey, W.T.; Hustead, D.S.; Alish, C.; Fix, B.; Mustad, V.A. Effect of two carbohydrate-modified tube-feeding formulas on metabolic responses in patients with type 2 diabetes. Nutrition 2008, 24, 990–997. [Google Scholar] [CrossRef]
- Vanschoonbeek, K.; Lansink, M.; van Laere, K.M.; Senden, J.M.; Verdijk, L.B.; van Loon, L.J. Slowly digestible carbohydrate sources can be used to attenuate the postprandial glycemic response to the ingestion of diabetes-specific enteral formulas. Diabetes Educ. 2009, 35, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Vahabzadeh, D.; Valizadeh Hasanloei, M.A.; Vahdat Shariatpanahi, Z. Effect of high-fat, low-carbohydrate enteral formula versus standard enteral formula in hyperglycemic critically ill patients: A randomized clinical trial. Int. J. Diabetes Dev. Ctries 2019, 39, 173–180. [Google Scholar] [CrossRef]
- Hofman, Z.; van Drunen, J.D.E.; de Later, C.; Kuipers, H. The Glycaemic index of standard and diabetes specific enteral formulas. Asia Pac. J. Clin. Nutr. 2006, 15, 412–417. [Google Scholar] [PubMed]
- Ojo, O.; Ojo, O.O.; Adebowale, F.; Wang, X.H. The Effect of Dietary Glycaemic Index on Glycaemia in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2018, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.T.; Lampe, J.W.; Schwarz, Y.; Breymeyer, K.L.; Noar, K.A.; Song, X.; Neuhouser, M.L. Low Glycemic Load Experimental Diet More Satiating Than High Glycemic Load Diet. Nutr. Cancer 2012, 64, 666–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Population | Patients with type 2 diabetes and on enteral nutrition irrespective of type of feeding tube. | Patients with type 1 diabetes. |
| Pregnant women with gestational diabetes. | ||
| Healthy individuals without diabetes on enteral nutrition. | ||
| Patients with diabetes on parenteral nutrition and parenteral plus enteral nutrition. | ||
| Studies involving animals | ||
| Intervention | Diabetes specific formulas | Parenteral nutrition, parenteral plus enteral nutrition. |
| (Oral nutrition supplement or enteral tube feeding) | ||
| Comparator | Standard formulas (Oral nutrition supplement or enteral tube feeding) | Parenteral nutrition and parenteral plus enteral nutrition. |
| Outcomes | Cardiometabolic parameters | Qualitative outcomes such as patient feelings. |
| Study Design: | Randomised Controlled Trials | Letters, comments, reviews, qualitative studies |
| Patient/Population | Intervention | Comparator | Study Designs | Combining Search Terms | |
|---|---|---|---|---|---|
| Patients with type 2 diabetes | Diabetes specific formulas | Standard formulas | Randomised Controlled Trial | ||
| Type 2 diabetes OR type 2 diabetes mellitus OR Diabetes complications OR diabetes mellitus, type 2 | Diabetes specific formula OR Diabetes specific form* OR Enteral nutrition OR Enteral* OR Enteral feed OR Enteral feed* OR Enteral form* OR Diabetes formula OR tube feeding OR enteral feeding | Randomised Controlled Trial OR Randomized Controlled Trial OR Randomized Controlled study OR RCT OR Randomized* OR controlled clinical trial OR placebo OR randomly OR trial OR groups | Column 1 AND Column 2 AND Column 3 |
| Citation | Country | Length of Study | Study Type/Design | Sample Size/Description | Age (Years) | Type of Enteral Formula/Feeding Method | Duration of Diabetes (Years) | Study Results/Conclusion |
|---|---|---|---|---|---|---|---|---|
| Ceriello et al. [18] | Netherlands | 24 h | Randomized, controlled, double-blind, cross-over | n = 11 | Mean ± SEM | The DSF had 1 kcal/mL and low GI and/or slowly digestible CHO. The SF was isocaloric fibre containing formula. | Mean ± SEM | Administration of DSF lowered glucose profiles. |
| 67.2 ± 1.3 | Bolus Feeding | 6.6 ± 1.4 years | Using DSF resulted in significantly lower 24 h and postprandial glucose profiles than fibre-containing SF after bolus administration. | |||||
| Buranapin et al. [20] | Thailand | 180 min | Single centre, prospective, randomized, double blind, cross-over study. | n = 30 | Mean ± SD | 55% CHO, 15% protein, 30% fat for DSF and SF. However, DSF substituted sucrose for combination of fructose, polydextrose and FOS. Bolus Feeding | More than 6 months. | DSF resulted in significantly lower postprandial blood glucose concentration than SF. |
| Administration of oral DSF and SF | 60.93 ± 11.71 | |||||||
| Pohl et al. [29] | Germany | 12 weeks | Randomized, double-blind, controlled, multi-centre trial. | n = 78 | Median (Range) | DSF contained 37% energy as CHO, 45% as fat, 18% as protein, SF contained 52% energy as CHO, 30% of energy as total fat and 18% as protein. | No data | DSF formula resulted in a more effective glycaemia control than SF, and was comparable in safety. |
| Test group (DSF): 71 (42–86) | Continuous Feeding. | DSF significantly decreased triglycerides compared with SF, but differences were not significant in relation to total cholesterol, HDL and LDL cholesterols. | ||||||
| Control group (SF): 72 (51–87) | ||||||||
| Pohl et al. [30] | Germany | 84 days | Parallel design. | n = 97 | Median (Range) | DSF contained 37% energy as CHO, 45% as fat, 18% as protein, SF contained 52% energy as CHO, 30% of energy as total fat and 18% as protein. | No data | Compared to SF, DSF significantly lowered FBG and improved glycaemic control. |
| Stage two of a randomized, prospective, double-blind, controlled, multicentre, parallel group study | DSF: 74 (44–91) | Continuous Feeding. | There were no significant differences between the two groups with respect to TG, TC, HDL and LDL cholesterols. | |||||
| SF: 69 (53–86) | ||||||||
| Craig et al. [31] | USA–New York State | 3 months | Randomized, double-blind, controlled, parallel group 3 months pilot trial. | n = 34 | DSF: 82 ± 3 (range 52–94) | Per 1000 mL, DSF contained 1000 kcal, 41.8 g protein, 93. 7 g CHO, 55.7 g fat. SF contained 1060 kcal, 44.4 g protein, 151.7 CHO, 35.9 g fat. Continuous or intermittent feeding. | No data | DSF resulted in lower fasting serum glucose and HbA1c than SF. |
| 80 ± 2 (range-SF: 52–100) | No significant differences between the DSF and SF groups with respect to LDL cholesterol and TG 3 months post intervention, but the DSF group had significantly higher level of HDL cholesterol than the SF group. | |||||||
| Lansink et al. [32] | Netherlands | 4 weeks | Randomized, controlled, double-blind, parallel-group study. | n = 44 | Mean ± SD | DSF contained 1 kcal/mL, 47 Energy% CHO, 19 Energy% protein, 34 Energy% fat and 2 g fibres/100 mL. The SF contained 50 Energy% CHO, 16 Energy% protein, 34 Energy% fat and 1.5 g fibres/100 mL. | Mean (Range) DSF: 84 (18–216) months | DSF significantly lowered postprandial glucose compared with SF. |
| DSF: 65.2 ± 7.4 SF: 64.2 ± 5.9 | Bolus Feeding | SF: 66 (10–504) months | Levels of TG, TC, HDL and LDL cholesterols were not significantly different between the two groups at baseline and 4 weeks post intervention. | |||||
| Vaisman et al. [33] | No data | 12 weeks | Randomized, controlled, double-blind, parallel group study. | n = 25 | Total: 76.2 ± 12.8 years | DSF contained 100 kcal, 45 Energy% CHO, 38 Energy% fat, 17 Energy% protein and 1.5 g/100 kcal fibre. SF contained 100 kcal, 55 Energy% CHO, 30 Energy% fat, 15 Energy% protein, 2 g/100 kcal fibre. | Mean ± SD | The DSF significantly reduced HbA1c compared to SF. No significant effect was found with respect to fasting blood glucose. |
| DSF: 73.0 ± 14.7 | Bolus, Continuous or intermittent feeding. | Total: 8.6 ± 7.6 years DSF: 5.0 ± 4.9 | DSF significantly increased HDL cholesterol, but differences were not significant in relation to TG, TC and LDL cholesterol compared with SF. | |||||
| SF: 79.2 ± 10.4 | ||||||||
| SF: 12.6 ± 8.4 | ||||||||
| Alish et al. [34] | USA | 10 days | Randomized, double blind, two treatment, crossover design. | n = 12 | Mean ± SEM | DSF had 1.2 kcal/mL, 114.5 g CHO, 17 g/L fibre, 60 g/L protein, 60 g/L fat. SF had 1.2 kcal/mL, 169.4 g CHO, 18 g/L fibre, 55.5 g/L protein, 39.3 g/L fat. | NS | Use of DSF produced lower postprandial glycaemic and insulinemic responses, reduced glycaemic variability, and resulted in less hyperglycaemia, reduced short acting insulin requirements. |
| DSF (Postprandial response protocol) vs. SF (Continuous glucose monitoring). | Postprandial: 63.1 ± 1.9 | Continuous Feeding. | ||||||
| Continuous feed: 74.1 ± 4.0 | ||||||||
| Gulati et al. [35] | India | 8 months | Open-label, randomized, crossover, pilot single centre study. | n = 40 | 35–60 years | DSF administered was 55 g in 210 mL of water to make 250 mL at standard reconstitution (1 kcal/mL) which can be used as tube feed or oral nutrition supplement. The SF was isocaloric Meal. | No data | DSF demonstrated lower blood glucose and insulin post meal levels than SF. |
| Bolus Feeding | The level of HDL cholesterol was significantly higher in the DSF group compared with the SF group after intervention, but differences were not significant in relation to TG, TC and LDL cholesterol. | |||||||
| Hofman et al. [36] | Netherlands | 360 min | Randomized, double blind, cross over study involving SF (A), DSF with moderate amount of carbohydrate and MUFA (B) and Test feed with low amount of carbohydrate and high amount of fat (C) | n = 12 | 63 ± 9.4 years | DSF (45 Energy% CHO, 26 Energy% MUFA), SF (49 Energy% CHO, 21 Energy% MUFA). Continuous Feeding. | No data | DSF showed significantly lower glucose levels compared with SF. |
| With respect to TG level, the DSF B with a lower amount of fat showed significantly lower levels than test feed C. | ||||||||
| Lansink et al. [37] | Netherlands | 8 h | Randomized, controlled, double-blind cross-over study | n = 24 | Mean ± SD | The DSF had 1.5 kcal/mL, high protein, a mixture of 6 different dietary fibre and low GI CHO. SF was isocaloric fibre containing formula. | Median (Minimum and Maximum) | Administration of a new, high-protein DSF during 4 h of continuous feeding resulted in lower glucose and insulin levels compared with a fiber-containing SF. DSF may contribute to lower glucose levels in these patients. |
| 64.6 ± 10.7 | Continuous Feeding. | 76.5 months (13, 303) | ||||||
| Mesejo et al. [38] | Spain | 2 years | Prospective, open-label, randomized study | n = 157 | Median (Q1–Q3) | Per 100 mL, DSF had 100 kcal, 5.7 g protein, 8.2 g CHO, 4.4 g fat. SF had 100 kcal, 5/7 g protein, 10.93/15.3 g CHO, 3.79/5.3 g fat. | No data | DSFs lowered insulin requirements, improved glycaemic control and reduced the risk of acquired infections relative to SF. |
| New generation DSF: 57 (43–70) | Continuous Feeding. | Plasma levels of cholesterol and TG were similar across the three treatment groups. | ||||||
| SF: 60 (45–71) | ||||||||
| Control DSF: 58 (46–68) | ||||||||
| Voss et al. [39] | USA | 240 min | Randomized cross over-study | n = 48 | Mean ± SEM | DSF had 1 kcal/mL, 47.8 g CHO, 7.2 g fibre, 20.9 g protein, 27.2 g fat. SF had 1.06 kcal/mL, 73 g CHO, 7.2 g fibre, 20.9 g protein, 16.4 g fat. | DSF resulted in lower postprandial blood glucose response compared with SF. | |
| Double-blinded with three-treatments | 56 ± 1.4 years | Bolus Feeding | ||||||
| Vanschoonbeek et al. [40] | Netherlands | 10 days | Randomized, double-blind, cross over study. | n = 15 | Mean ± SEM | Per 100 mL, DSF had 98 kcal, 1.44 g fibre and 5.44/50 (g/energy%) of fat. SF had 100 kcal, 1.4 g of fibre, 3.4/30 (g/energy%) of fat.), | Mean ± SEM | DSF rich in lowly digestible carbohydrate sources can be equally effective in lowering the postprandial blood glucose response as low-carbohydrate, high-fat enteral formulas without elevating the plasma triglyceride response. |
| 63 ± 1 years | Bolus Feeding | 9 ± 2 years | ||||||
| Study Reference | Interventions | Pre-and Post Intervention | Fasting Blood Glucose mmol/L Mean ± SD/Median (Quartiles) | Glycated Haemoglobin % Mean ± SD/Median (Quartiles) | Total Cholesterol mmol/L Mean ± SD/Median (Quartiles) | LDL Cholesterol mmol/L Mean ± SD/Median (Quartiles) | HDL Cholesterol mmol/L Mean ± SD/Median (Quartiles) | Triglycerides mmol/L Mean ± SD/Median (Quartiles) |
|---|---|---|---|---|---|---|---|---|
| Pohl. et al. [29] | DSF, n = 39 | Change from baseline | ** ∆−1.59 (−3.38 to −0.06) | ** ∆−0.8 (−1.5 to −0.5) | ** ∆−0.37 (−1.00 to 0.56) | ** ∆−0.28 (−1.46 to 0.53) | ** ∆0.08 (−0.06 to 0.28) | ** ∆−0.37 (−0.36 to 0.38) |
| SF, n = 39 | Change from baseline | ** ∆−0.08 (−1.34 to 0.79) | ** ∆0.0 (−0.4 to 0.3) | ** ∆−0.23 (−1.22 to 0.46) | ** ∆−0.52 (−1.48 to 0.04) | ** ∆0.05 (−0.10 to 0.32) | ** ∆0.203 (−0.07 to 0.84) | |
| Pohl et al. [30] | DSF, n = 48 | Change from baseline | ** ∆−2.17 (−2.55/−1.33) | ** ∆−1.30 (−2.60/−0.10) | ** ∆0.30 (−1.22/1.06) | ** ∆0.27 (−0.71/1.40 | ** ∆0.03 (−0.26/0.4) | ** ∆−0.45 (−1.65/0.27) |
| SF, n = 49 | Change from baseline | ** ∆−0.67 (−0.90/−0.10) | ** ∆−1.20 (−2.35/−0.55) | ** ∆0.21 (−1.02/0.48) | ** ∆−0.33 (−1.03/0.56) | ** ∆0.00 (−0.22/0.28) | ** ∆−0.70 (−1.50/1.73) | |
| Craig et al. [31] | DSF, n = 14 | Baseline | * 7.3 ± 0.4 | * 6.9 ± 0.3 | * 4.16 ± 0.31 | * 2.66 ± 0.23 | * 1.01 ± 0.05 | * 0.97 ± 0.13 |
| Final | 6.7 ± 0.7 | 6.5 ± 0.4 | 3.96 ± 0.31 | 2.51 ± 0.28 | 0.98 ± 0.05 | 0.91 ± 0.17g/L | ||
| SF, n = 13 | Baseline | * 6.9 ± 0.6 | * 6.9 ± 0.5 | * 4.21 ± 0.18 | * 2.69 ± 0.15 | * 0.98 ± 0.05 | * 0.9 ± 0.07 | |
| Final | 8.3 ± 1.7 | 6.9 ± 0.14 | 3.96 ± 0.23 | 2.53 ± 0.21 | 0.83 ± 0.05 | 1.06 ± 0.12 g/L | ||
| Lansink et al. [32] | DSF, n = 21 | Baseline | * 8.32 ± 0.33 | No data | No data | No data | No data | No data |
| Final | 8.13 ± 0.33 | |||||||
| SF, n = 22 | Baseline | * 7.73 ± 0.22 | No data | No data | No data | No data | No data | |
| Final | 8.22 ± 0.26 | |||||||
| Vaisman et al. [33] | DSF, n = 12 | Baseline | No data | *** 6.9 ± 0.3 | No data | No data | *** 1.04 ± 0.08 | No data |
| Final | 6.2 ± 0.4 | 1.23 ± 0.10 | ||||||
| SF, n = 13 | Baseline | No data | *** 7.9 ± 0.3 | No data | No data | *** 1.06 ± 0.08 | No data | |
| Final | 8.7 ± 0.4 | 0.94 ± 0.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojo, O.; Weldon, S.M.; Thompson, T.; Crockett, R.; Wang, X.-H. The Effect of Diabetes-Specific Enteral Nutrition Formula on Cardiometabolic Parameters in Patients with Type 2 Diabetes: A Systematic Review and Meta–Analysis of Randomised Controlled Trials. Nutrients 2019, 11, 1905. https://doi.org/10.3390/nu11081905
Ojo O, Weldon SM, Thompson T, Crockett R, Wang X-H. The Effect of Diabetes-Specific Enteral Nutrition Formula on Cardiometabolic Parameters in Patients with Type 2 Diabetes: A Systematic Review and Meta–Analysis of Randomised Controlled Trials. Nutrients. 2019; 11(8):1905. https://doi.org/10.3390/nu11081905
Chicago/Turabian StyleOjo, Omorogieva, Sharon Marie Weldon, Trevor Thompson, Rachel Crockett, and Xiao-Hua Wang. 2019. "The Effect of Diabetes-Specific Enteral Nutrition Formula on Cardiometabolic Parameters in Patients with Type 2 Diabetes: A Systematic Review and Meta–Analysis of Randomised Controlled Trials" Nutrients 11, no. 8: 1905. https://doi.org/10.3390/nu11081905





