Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search
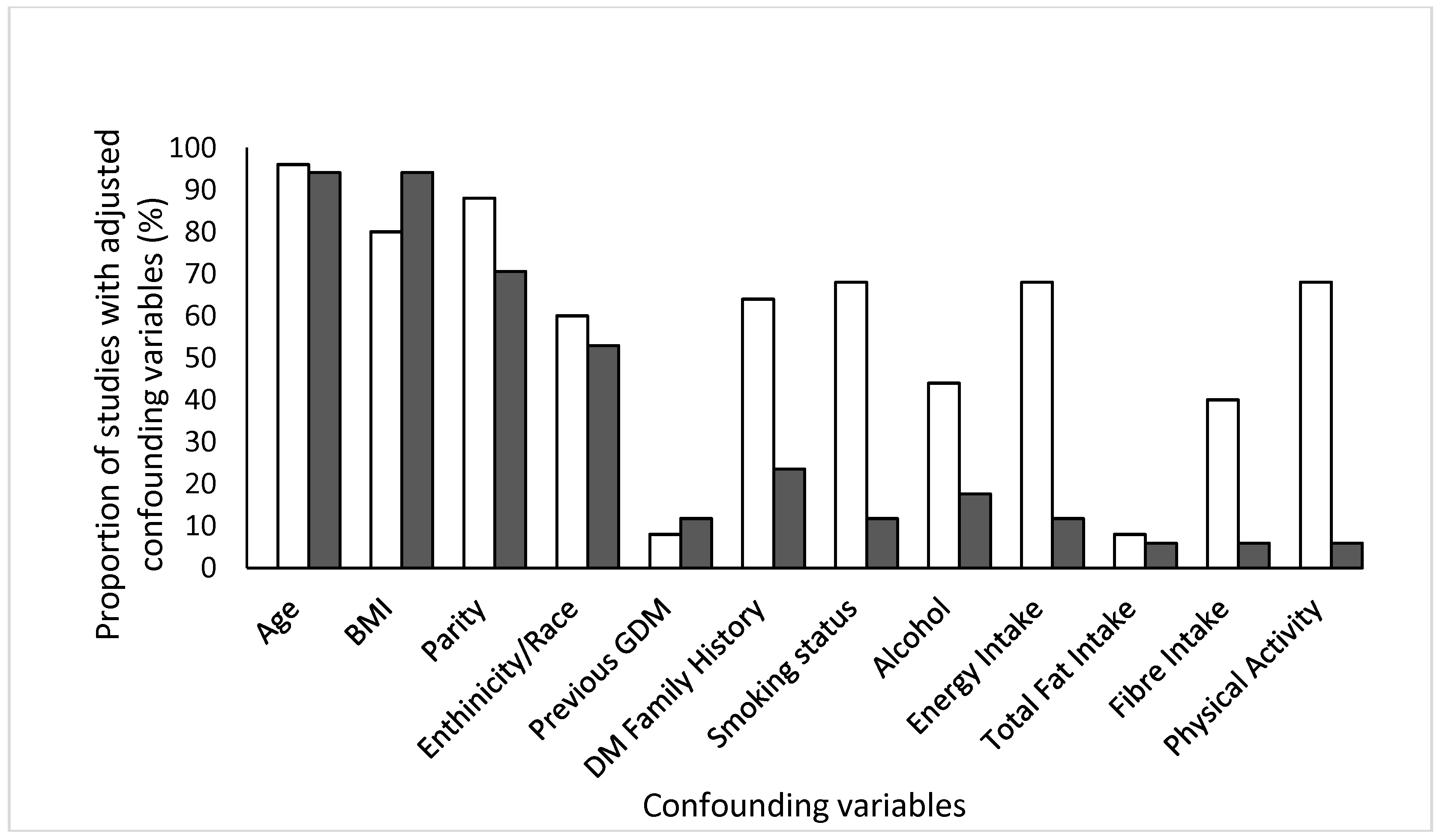
2.3. Quality Assessment and Data Extraction
2.4. Statistical Analysis
3. Results
3.1. Studies Identified
3.2. General Characteristics of Studies
3.3. Diet Related Studies
3.3.1. Carbohydrates (Fruit, Fiber, Beverages, Potato)
3.3.2. Fat Intake (i.e., Total, Monounsaturated Fatty Acids, Dietary Cholesterol, Egg Intake)
3.4.3. Protein Intake (i.e., Meat, Iron, Heme)
3.3.4. Caffeine
3.3.5. Fast Food Intake
3.3.6. Calcium/Dairy Intake
3.3.7. Recognised Dietary Patterns
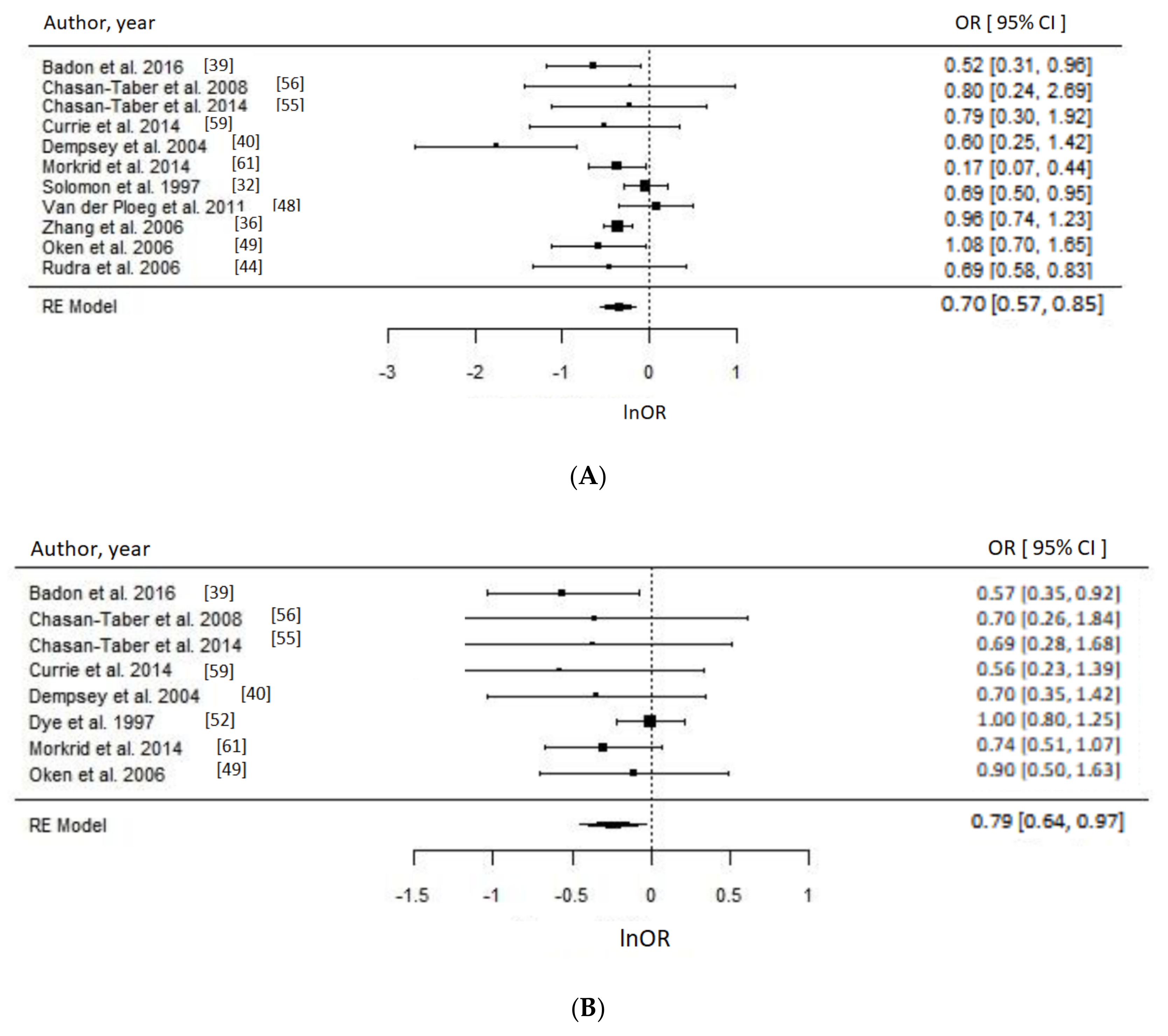
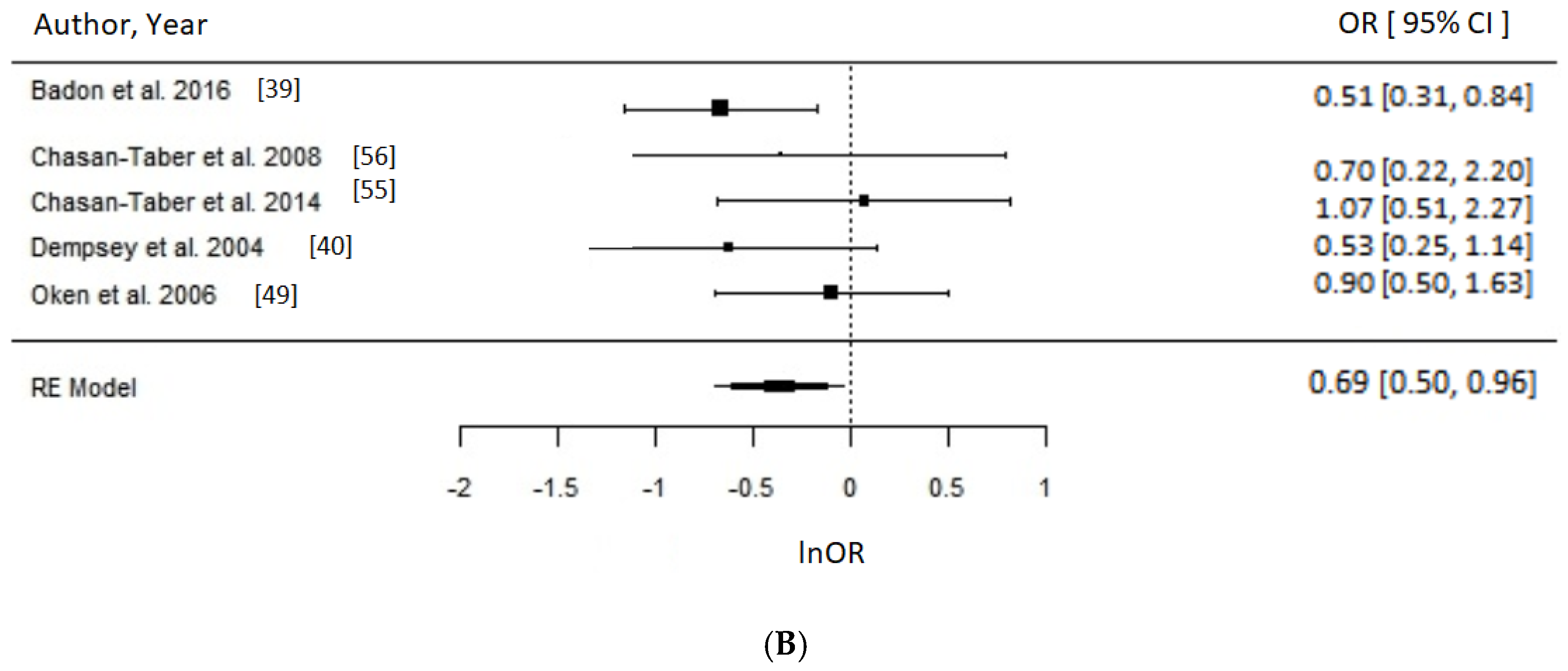
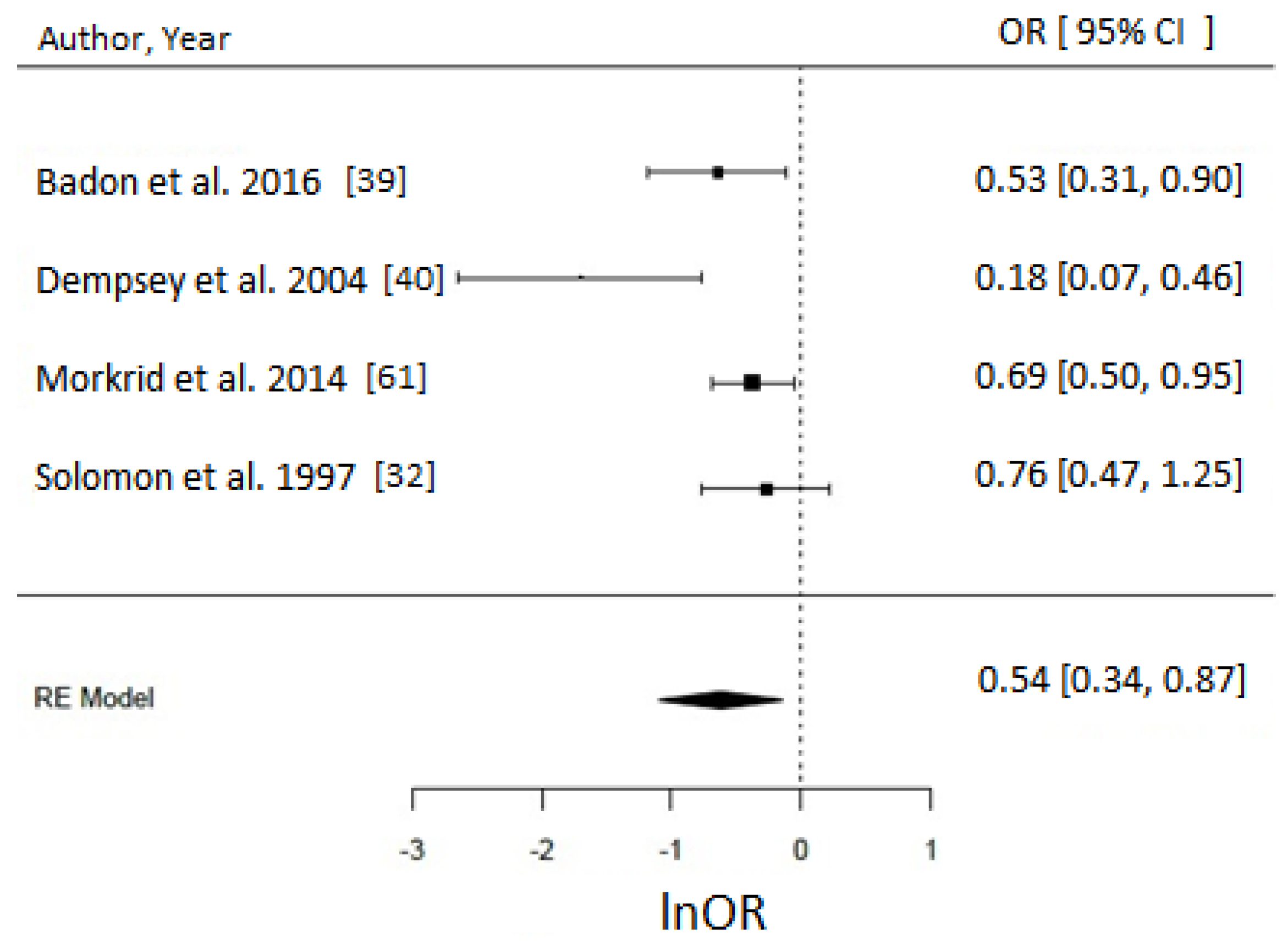
3.4. Physical Activity
Meta-Analysis and Assessment of Bias
4. Discussion
4.1. Diet and GDM Risk
4.2. Physical Activity and GDM
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- James, R.G., III; Alberti, K.G.M.M.; Davidson, M.B.; DeFronzo, R.A.; The Expert Committee on the, D.; Classification of Diabetes, M. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997, 20, 1183–1197. [Google Scholar]
- Standards of Medical Care in Diabetes—2014. Diabetes Care 2013, 37, S14.
- Guariguata, L.; Linnenkamp, U.; Beagley, J.; Whiting, D.R.; Cho, N.H. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res. Clin. Pract. 2014, 103, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Lin, A.; Russell, H. Adopting the new World Health Organization diagnostic criteria for gestational diabetes: How the prevalence changes in a high-risk region in Australia. Diabetes Res. Clin. Pract. 2017, 129, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Zhao, B.; Wang, E.J.; Nimbal, V.; Osmundson, S.; Kunz, L.; Popat, R.A.; Chung, S.; Palaniappan, L.P. Racial/Ethnic Differences in Gestational Diabetes Prevalence and Contribution of Common Risk Factors: Racial/ethnic differences in GDM risk factors. Paediatr. Perinat. Epidemiol. 2015, 29, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Wilmot, E.G.; Mansell, P. Diabetes and pregnancy. Clin. Med. 2014, 14, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Lehnen, H.; Zechner, U.; Haaf, T. Epigenetics of gestational diabetes mellitus and offspring health: The time for action is in early stages of life. Mol. Hum. Reprod. 2013, 19, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Alwan, N.A.; West, J.; Brown, S.; McKinlay, C.J.D.; Farrar, D.; Crowther, C.A. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Castilla, C.; Mauricio, D.; Hernandez, M. Role of Medical Nutrition Therapy in the Management of Gestational Diabetes Mellitus. Curr. Diabetes Rep. 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, S.B.; Rönö, K.; Klemetti, M.M.; Roine, R.P.; Lindström, J.; Erkkola, M.; Kaaja, R.J.; Pöyhönen-Alho, M.; Tiitinen, A.; Huvinen, E.; et al. Gestational Diabetes Mellitus Can Be Prevented by Lifestyle Intervention: The Finnish Gestational Diabetes Prevention Study (RADIEL): A Randomized Controlled Trial. Diabetes Care 2016, 39, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. Br. Med. J. 2014, 349, g4490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Goodyear, L.J. Exercise and type 2 diabetes: Molecular mechanisms regulating glucose uptake in skeletal muscle. Adv. Physiol. Educ. 2014, 38, 308–314. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans; U.S. Department of Health and Human Services: Washington, DC, USA, 2008.
- Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report; U.S. Department of Health and Human Services: Washington, DC, USA, 2018.
- Löf, M. Physical activity pattern and activity energy expenditure in healthy pregnant and non-pregnant Swedish women. Eur. J. Clin. Nutr. 2011, 65, 1295–1301. [Google Scholar]
- Evenson, K.R.; Wen, F. Prevalence and correlates of objectively measured physical activity and sedentary behavior among US pregnant women. Prev. Med. 2011, 53, 39–43. [Google Scholar] [CrossRef] [PubMed]
- American Dietetic Association. Evidence Analysis Manual: Steps in the Academy: Evidence Analysis Process; Research and Strategic Business Development: Chicago, IL, USA, 2012. [Google Scholar]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Multiple Outcomes or Time-Points within a Study; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 225–238. [Google Scholar]
- Deeks, J.J.; Altman, D.G. Effect Measures for Meta-Analysis of Trials with Binary Outcomes. Syst. Rev. Health Care Meta-Anal. Context 2008. [Google Scholar] [CrossRef]
- Nakagawa, S.; Noble, D.W.A.; Senior, A.M.; Lagisz, M. Meta-evaluation of meta-analysis: Ten appraisal questions for biologists. BMC Biol. 2017, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Bowers, K.; Tobias, D.K.; Hu, F.B.; Zhang, C. Prepregnancy Dietary Protein Intake, Major Dietary Protein Sources, and the Risk of Gestational Diabetes Mellitus: A prospective cohort study. Diabetes Care 2013, 36, 2001–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, W.; Bowers, K.; Tobias, D.K.; Olsen, S.F.; Chavarro, J.; Vaag, A.; Kiely, M.; Zhang, C. Prepregnancy low-carbohydrate dietary pattern and risk of gestational diabetes mellitus: A prospective cohort study. Am. J. Clin. Nutr. 2014, 99, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Tobias, D.K.; Olsen, S.F.; Zhang, C. Pre-pregnancy fried food consumption and the risk of gestational diabetes mellitus: A prospective cohort study. Diabetologia 2014, 57, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Tobias, D.K.; Hu, F.B.; Chavarro, J.E.; Zhang, C. Pre-pregnancy potato consumption and risk of gestational diabetes mellitus: Prospective cohort study. BMJ 2016, 352, h6898. [Google Scholar] [CrossRef] [PubMed]
- Bowers, K.; Yeung, E.; Williams, M.A.; Qi, L.; Tobias, D.K.; Hu, F.B.; Zhang, C. A prospective study of prepregnancy dietary iron intake and risk for gestational diabetes mellitus. Diabetes Care 2011, 34, 1557–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowers, K.; Tobias, D.K.; Yeung, E.; Hu, F.B.; Zhang, C. A prospective study of prepregnancy dietary fat intake and risk of gestational diabetes. Am. J. Clin. Nutr. 2012, 95, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, F.B.; Yeung, E.; Willett, W.; Zhang, C. Prospective study of pre-gravid sugar-sweetened beverage consumption and the risk of gestational diabetes mellitus. Diabetes Care 2009, 32, 2236–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Hu, F.B.; Yeung, E.; Tobias, D.K.; Willett, W.C.; Zhang, C. Prepregnancy consumption of fruits and fruit juices and the risk of gestational diabetes mellitus: A prospective cohort study. Diabetes Care 2012, 35, 1079–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, C.G.; Willett, W.C.; Carey, V.J.; Rich-Edwards, J.; Hunter, D.J.; Colditz, G.A.; Stampfer, M.J.; Speizer, F.E.; Spiegelman, D.; Manson, J.E. A prospective study of pregravid determinants of gestational diabetes mellitus. J. Am. Med. Assoc. 1997, 278, 1078–1083. [Google Scholar] [CrossRef]
- Tobias, D.K.; Zhang, C.; Chavarro, J.; Bowers, K.; Rich-Edwards, J.; Rosner, B.; Mozaffarian, D.; Hu, F.B. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am. J. Clin. Nutr. 2012, 96, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, S.; Solomon, C.G.; Hu, F.B. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care 2006, 29, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Schulze, M.B.; Solomon, C.G.; Hu, F.B. A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetologia 2006, 49, 2604–2613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Solomon, C.G.; Manson, J.E.; Hu, F.B. A prospective study of pregravid physical activity and sedentary behaviors in relation to the risk for gestational diabetes mellitus. Arch. Intern. Med. 2006, 166, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tobias, D.K.; Chavarro, J.E.; Bao, W.; Wang, D.; Ley, S.H.; Hu, F.B. Adherence to healthy lifestyle and risk of gestational diabetes mellitus: Prospective cohort study. BMJ 2014, 349, g5450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeney, K.L.; Williams, M.A.; Schiff, M.A.; Qiu, C.; Sorensen, T.K. Coffee consumption and the risk of gestational diabetes mellitus. Acta Obstet. Gynecol. Scand. 2007, 86, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Badon, S.E.; Wartko, P.D.; Qiu, C.; Sorensen, T.K.; Williams, M.A.; Enquobahrie, D.A. Leisure Time Physical Activity and Gestational Diabetes Mellitus in the Omega Study. Med. Sci. Sports Exerc. 2016, 48, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.C.; Sorensen, T.K.; Williams, M.A.; Lee, I.; Miller, R.S.; Dashow, E.E.; Luthy, D.A. Prospective study of gestational diabetes mellitus risk in relation to maternal recreational physical activity before and during pregnancy. Am. J. Epidemiol. 2004, 159, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Yanez, C.; Qiu, C.F.; Gelaye, B.; Enquobahrie, D.A.; Williams, M.A. Risk of gestational diabetes mellitus in relation to maternal dietary calcium intake. Public Health Nutr. 2017, 20, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Frederick, I.O.; Zhang, C.; Sorensen, T.K.; Enquobahrie, D.A.; Williams, M.A. Risk of Gestational Diabetes Mellitus in Relation to Maternal Egg and Cholesterol Intake. Am. J. Epidemiol. 2011, 173, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zhang, C.; Gelaye, B.; Enquobahrie, D.A.; Frederick, I.O.; Williams, M.A. Gestational diabetes mellitus in relation to maternal dietary heme iron and nonheme iron intake. Diabetes Care 2011, 34, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Rudra, C.B.; Williams, M.A.; Lee, I.M.; Miller, R.S.; Sorensen, T.K. Perceived exertion in physical activity and risk of gestational diabetes mellitus. Epidemiology 2006, 17, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Gresham, E.; Collins, C.E.; Mishra, G.D.; Byles, J.E.; Hure, A.J. Diet quality before or during pregnancy and the relationship with pregnancy and birth outcomes: The Australian Longitudinal Study on Women’s Health. Public Health Nutr. 2016, 19, 2975–2983. [Google Scholar] [CrossRef] [PubMed]
- Schoenaker, D.A.J.M.; Soedamah-Muthu, S.S.; Mishra, G.D. Quantifying the mediating effect of body mass index on the relation between a Mediterranean diet and development of maternal pregnancy complications: The Australian Longitudinal Study on Women’s Health. Am. J. Clin. Nutr. 2016, 104, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Schoenaker, D.A.; Soedamah-Muthu, S.S.; Callaway, L.K.; Mishra, G.D. Pre-pregnancy dietary patterns and risk of gestational diabetes mellitus: Results from an Australian population-based prospective cohort study. Diabetologia 2015, 58, 2726–2735. [Google Scholar] [CrossRef] [PubMed]
- Van der Ploeg, H.P.; Van Poppel, M.N.M.; Chey, T.; Bauman, A.E.; Brown, W.J. The role of pre-pregnancy physical activity and sedentary behaviour in the development of gestational diabetes mellitus. J. Sci. Med. Sport 2011, 14, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Ning, Y.; Rifas-Shiman, S.L.; Radesky, J.S.; Rich-Edwards, J.W.; Gillman, M.W. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstet. Gynecol. 2006, 108, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Radesky, J.S.; Oken, E.; Rifas-Shiman, S.L.; Kleinman, K.P.; Rich-Edwards, J.W.; Gillman, M.W. Diet during early pregnancy and development of gestational diabetes. Paediatr. Perinat. Epidemiol. 2008, 22, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Baptiste-Roberts, K.; Ghosh, P.; Nicholson, W.K. Pregravid Physical Activity, Dietary Intake, and Glucose Intolerance During Pregnancy. J. Women’s Health 2011, 20, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Dye, T.D.; Knox, K.L.; Artal, R.; Aubry, R.H.; Wojtowycz, M.A. Physical activity, obesity, and diabetes in pregnancy. Am. J. Epidemiol. 1997, 146, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Putnam, K.F.; Mueller, L.A.; Magann, E.F.; Thagard, A.; Johnson, A.M.; Ounpraseuth, S.T.; Morrison, J.C. Evaluating effects of self-reported domestic physical activity on pregnancy and neonatal outcomes in “stay at home” military wives. Military Med. 2013, 178, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.L.; Lombard, C.B.; Teede, H.J. Understanding health behaviours in a cohort of pregnant women at risk of gestational diabetes mellitus: An observational study. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Chasan-Taber, L.; Silveira, M.; Lynch, K.E.; Pekow, P.; Braun, B.; Manson, J.E.; Solomon, C.G.; Markenson, G. Physical activity before and during pregnancy and risk of abnormal glucose tolerance among Hispanic women. Diabetes Metab. 2014, 40, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Chasan-Taber, L.; Schmidt, M.D.; Pekow, P.; Sternfeld, B.; Manson, J.E.; Solomon, C.G.; Braun, B.; Markenson, G. Physical activity and gestational diabetes mellitus among Hispanic women. J. Women’s Health 2008, 17, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Behboudi-Gandevani, S.; Safary, K.; Moghaddam-Banaem, L.; Lamyian, M.; Goshtasbi, A.; Alian-Moghaddam, N. The Relationship Between Maternal Serum Iron and Zinc Levels and Their Nutritional Intakes in Early Pregnancy with Gestational Diabetes. Biol. Trace Element Res. 2013, 154, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, S.N.; Laughon, S.K.; Catov, J.M.; Olsen, J.; Bech, B.H. First trimester coffee and tea intake and risk of gestational diabetes mellitus: A study within a national birth cohort. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Currie, L.; Woolcott, C.; Fell, D.; Armson, B.; Dodds, L. The Association Between Physical Activity and Maternal and Neonatal Outcomes: A. Prospective Cohort. Mater. Child Health J. 2014, 18, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, R.; Rafique, G.; Badruddin, S.; Qureshi, R.; Cue, R.; Gray-Donald, K. Increased body fat percentage and physical inactivity are independent predictors of gestational diabetes mellitus in South Asian women. Eur. J. Clin. Nutr. 2007, 61, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Morkrid, K.; Jenum, A.K.; Berntsen, S.; Sletner, L.; Richardsen, K.R.; Vangen, S.; Holme, I.; Birkeland, K.I. Objectively recorded physical activity and the association with gestational diabetes. Scand. J. Med. Sci. Sports 2014, 24, e389–e397. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Martinez-Gonzalez, M.A.; Basterra-Gortari, F.J.; Gea, A.; Barbagallo, M.; Bes-Rastrollo, M. Fast food consumption and gestational diabetes incidence in the SUN project. PLoS ONE 2014, 9, e106627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamanos, B.; Thanopoulou, A.; Anastasiou, E.; Assaad-Khalil, S.; Albache, N.; Bachaoui, M.; Slama, C.B.; El Ghomari, H.; Jotic, A.; Lalic, N.; et al. Relation of the Mediterranean diet with the incidence of gestational diabetes. Eur. J. Clin. Nutr. 2014, 68, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Shanshan, L.; Chavarro, J.E.; Tobias, D.K.; Yeyi, Z.; Hu, F.B.; Cuilin, Z.; Bao, W.; Li, S.; Zhu, Y.; et al. Low Carbohydrate-Diet Scores and Long-term Risk of Type 2 Diabetes Among Women With a History of Gestational Diabetes Mellitus: A Prospective Cohort Study. Diabetes Care 2016, 39, 43–49. [Google Scholar]
- Radd-Vagenas, S.; Kouris-Blazos, A.; Singh, M.F.; Flood, V.M. Evolution of Mediterranean diets and cuisine: Concepts and definitions. Asia Pacific J. Clin. Nutr. 2017, 26, 749–763. [Google Scholar]
- Schoenaker, D.A.J.M.; Mishra, G.D.; Callaway, L.K.; Soedamah-Muthu, S.S. The Role of Energy, Nutrients, Foods, and Dietary Patterns in the Development of Gestational Diabetes Mellitus: A Systematic Review of Observational Studies. Diabetes Care 2016, 39, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Donazar-Ezcurra, M.; Lopez-del Burgo, C.; Bes-Rastrollo, M. Primary prevention of gestational diabetes mellitus through nutritional factors: A systematic review. BMC Pregnancy Childbirth 2017, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.J.; Cho, E.; Willett, W.C. Energy adjustment of nutrient intakes is preferable to adjustment using body weight and physical activity in epidemiological analyses. Public Health Nutr. 2014, 17, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Lundeen, E.A.; Stein, A.D. The sugar-sweetened beverage wars: Public health and the role of the beverage industry. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.C.; Barclay, A.W. Declining consumption of added sugars and sugar-sweetened beverages in Australia: A challenge for obesity prevention. Am. J. Clin. Nutr. 2017, 105, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Colchero, M.A.; Rivera-Dommarco, J.; Popkin, B.M.; Ng, S.W. In Mexico, Evidence Of Sustained Consumer Response Two Years After Implementing A Sugar-Sweetened Beverage Tax. Health Aff. 2017, 36, 564. [Google Scholar] [CrossRef] [PubMed]
- Statistics, A.B.o. Australian Health Survey: Nutrition First Results—Foods and Nutrients, 2011-12. Available online: http://www.abs.gov.au/ausstats/abs@.nsf/lookup/4364.0.55.007main+features7102011-12 (accessed on 27 April 2018).
- Collison, K.S.; Zaidi, M.Z.; Subhani, S.N.; Al-Rubeaan, K.; Shoukri, M.; Al-Mohanna, F.A. Sugar-sweetened carbonated beverage consumption correlates with BMI, waist circumference, and poor dietary choices in school children. BMC Public Health 2010, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Panagiotakos, D.; Giugliano, D. A journey into a Mediterranean diet and type 2 diabetes: A systematic review with meta-analyses. BMJ Open 2015, 5, e008222. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Feskens, E.J.M.; Sluik, D.; van Woudenbergh, G.J. Meat consumption, diabetes and its complications. Curr. Diabetes Rep. 2013, 13, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Jansson, N.; Nilsfelt, A.; Gellerstedt, M.; Wennergren, M.; Rossander-Hulthén, L.; Powell, T.L.; Jansson, T.; Avd för Datavetenskap och, I.; Institutionen för Ekonomi och, I.; Högskolan, V. Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am. J. Clin. Nutr. 2008, 87, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Arem, H.; Moore, S.C.; Patel, A.; Hartge, P.; Berrington De Gonzalez, A.; Visvanathan, K.; Campbell, P.T.; Freedman, M.; Weiderpass, E.; Adami, H.O.; et al. Leisure time physical activity and mortality: A detailed pooled analysis of the dose-response relationship. JAMA Intern. Med. 2015, 175, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Australia, S.M. Position Statement: Exercise in Pregnancy and the Postpartum Period. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwi6ld64lajbAhWGspQKHSGjAFsQFgglMAA&url=https%3A%2F%2Fsma.org.au%2Fsma-site-content%2Fuploads%2F2017%2F08%2FSMA-Position-Statement-Exercise-Pregnancy.pdf&usg=AOvVaw38jppDGEL15r9CaWakIFQU (accessed on 9 March 2018).
- Evenson, K.R.; Barakat, R.; Brown, W.J.; Dargent-Molina, P.; Haruna, M.; Mikkelsen, E.M.; Mottola, M.F.; Owe, K.M.; Rousham, E.K.; Yeo, S. Guidelines for Physical Activity During Pregnancy: Comparisons From Around the World; SAGE Publications: Los Angeles, CA, USA, 2014; Volume 8, pp. 102–121. [Google Scholar]
- Bennie, J.A.; Pedisic, Z.; Van Uffelen, J.G.Z.; Gale, J.; Banting, L.K.; Vergeer, I.; Stamatakis, E.; Bauman, A.E.; Biddle, S.J.H. The descriptive epidemiology of total physical activity, muscle-strengthening exercises and sedentary behaviour among Australian adults—Results from the National Nutrition and Physical Activity Survey. BMC Public Health 2016, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, G.; Lee, I.M.; Hamer, M.; Stamatakis, E. Association of “Weekend Warrior” and Other Leisure Time Physical Activity Patterns With Risks for All-Cause, Cardiovascular Disease, and Cancer Mortality. JAMA Intern. Med. 2017, 177, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Chastin, S.F.M.; De Craemer, M.; De Cocker, K.; Powell, L.; Van Cauwenberg, J.; Dall, P.; Hamer, M.; Stamatakis, E. How does light-intensity physical activity associate with adult cardiometabolic health and mortality? Systematic review with meta-analysis of experimental and observational studies. Br. J. Sports Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cordero, Y.; Mottola, M.F.; Vargas, J.; Blanco, M.; Barakat, R. Exercise Is Associated with a Reduction in Gestational Diabetes Mellitus. Med. Sci. Sports Exerc. 2015, 47, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Bennett, W.L.; Liu, S.H.; Yeh, H.C.; Nicholson, W.K.; Gunderson, E.P.; Lewis, C.E.; Clark, J.M. Changes in weight and health behaviors after pregnancies complicated by gestational diabetes mellitus: The CARDIA study. Obesity 2013, 21, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Deierlein, A.L.; Siega-Riz, A.M.; Evenson, K.R. Physical Activity During Pregnancy and Risk of Hyperglycemia. J. Women’s Health 2012, 21, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Saldana, T.M.; Siega-Riz, A.M.; Adair, L.S. Effect of macronutrient intake on the development of glucose intolerance during pregnancy. Am. J. Clin. Nutr. 2004, 79, 479–486. [Google Scholar] [CrossRef] [PubMed]
- He, J.R.; Yuan, M.Y.; Chen, N.N.; Lu, J.H.; Hu, C.Y.; Mai, W.B.; Zhang, R.F.; Pan, Y.H.; Qiu, L.; Wu, Y.F.; et al. Maternal dietary patterns and gestational diabetes mellitus: A large prospective cohort study in China. Br. J. Nutr. 2015, 113, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Higgins JPT, G.S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available online: http://handbook-5-1.cochrane.org/ (accessed on 17 April 2018).




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mijatovic-Vukas, J.; Capling, L.; Cheng, S.; Stamatakis, E.; Louie, J.; Cheung, N.W.; Markovic, T.; Ross, G.; Senior, A.; Brand-Miller, J.C.; et al. Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 698. https://doi.org/10.3390/nu10060698
Mijatovic-Vukas J, Capling L, Cheng S, Stamatakis E, Louie J, Cheung NW, Markovic T, Ross G, Senior A, Brand-Miller JC, et al. Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients. 2018; 10(6):698. https://doi.org/10.3390/nu10060698
Chicago/Turabian StyleMijatovic-Vukas, Jovana, Louise Capling, Sonia Cheng, Emmanuel Stamatakis, Jimmy Louie, N. Wah Cheung, Tania Markovic, Glynis Ross, Alistair Senior, Jennie C. Brand-Miller, and et al. 2018. "Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis" Nutrients 10, no. 6: 698. https://doi.org/10.3390/nu10060698
APA StyleMijatovic-Vukas, J., Capling, L., Cheng, S., Stamatakis, E., Louie, J., Cheung, N. W., Markovic, T., Ross, G., Senior, A., Brand-Miller, J. C., & Flood, V. M. (2018). Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients, 10(6), 698. https://doi.org/10.3390/nu10060698







