Dietary Administration of Olive Mill Wastewater Extract Reduces Campylobacter spp. Prevalence in Broiler Chickens
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Design and Animal Management
2.2. Campylobacter spp. Isolation and Identification
2.3. Statistical Analyses
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Messineo, A.; Volpe, R.; Asdrubali, F. Evaluation of net energy obtainable from combustion of stabilized olive mill by-products. Energies 2012, 5, 1384–1397. [Google Scholar] [CrossRef]
- Goula, A.M.; Lazarides, H.N. Integrated processes can turn industrial food waste into valuable food by-products and/or ingredients: The cases of olive mill and pomegranate wastes. J. Food Eng. 2015, 167, 45–50. [Google Scholar] [CrossRef]
- Carraro, L.; Fasolato, L.; Montemurro, F.; Martino, M.E.; Balzan, S.; Servili, M.; Novelli, E.; Cardazzo, B. Polyphenols from olive mill waste affect biofilm formation and motility in Escherichia coli K-12. Microb. Biotechnol. 2014, 7, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Zbakh, H.; El Abbassi, A. Potential use of olive mill wastewater in the preparation of functional beverages: A review. J. Funct. Foods 2012, 4, 53–65. [Google Scholar] [CrossRef]
- Molina Alcaide, E.; Nefzaoui, A. Recycling of olive oil by-products: Possibilities of utilization in animal nutrition. Int. Biodeterior. Biodegrad. 1996, 38, 227–235. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Taticchi, A.; Urbani, S.; Di Maio, I.; Veneziani, G.; Selvaggini, R. New approaches to virgin olive oil quality, technology, and by-products valorization. Eur. J. Lipid Sci. Technol. 2015, 117, 1882–1892. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Castellini, C.; Cardinali, R.; Mourvaki, E.; Moscati, L.; Battistacci, L.; Servili, M.; Taticchi, A. Olive cake dietary supplementation in rabbit: Immune and oxidative status. Ital. J. Anim. Sci. 2007, 6 (Suppl. S1), 761–763. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Mourvaki, E.; Cardinali, R.; Servili, M.; Ruggeri, S.; Mattioli, S.; Taticchi, A.; Esposto, S.; Castellini, C. Effect of dietary supplementation with olive pomaces on the performance and meat quality of growing rabbits. Meat Sci. 2012, 92, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Branciari, R.; Ranucci, D.; Miraglia, D.; Urbani, S.; Esposto, S.; Servili, M. Effect of dietary treatment with olive oil by-product (olive cake) on physico-chemical, sensory and microbial characteristics of beef during storage. Ital. J. Food Saf. 2015, 4, 225–229. [Google Scholar] [CrossRef]
- European Food Safety Authority. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 2015, 13, 3991–4156. [Google Scholar]
- Servili, M.; Esposto, S.; Veneziani, G.; Urbani, S.; Taticchi, A.; Di Maio, I.; Selvaggini, R.; Sordini, B.; Montedoro, G.F. Improvement of bioactive content in virgin olive oil with an olive-vegetation water concentrate produced by membrane treatment. Food Chem. 2011, 124, 1308–1315. [Google Scholar] [CrossRef]
- Tang, S.Z.; Kerry, J.P.; Sheehan, D.; Buckley, D.J.; Morrissey, P.A. Dietary tea catechins and iron-induced lipid oxidation in chicken meat, liver and heart. Meat Sci. 2000, 56, 285–290. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Goñí, I.; Centeno, C.; Sayago-Ayerdy, S.G.; Arija, I.; Saura-Calixto, F. Effect of grape pomace concentrate and vitamin E on digestibility of polyphenols and antioxidant activity in chickens. Poult. Sci. 2008, 87, 307–316. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Poultry, 9th Revised EditionNational Academic Press: Washington, DC, USA, 1994; pp. 26–32.
- Galarini, R.; Giusepponi, D.; Moretti, S.; Saluti, G.; Ortenzi, R.; Valiani, A. Residues in poultry fed with high content of polyphenols. In Proceedings of the EuroResidue VIII Conference, Egmond aan Zee, The Netherlands, 23–25 May 2016.
- Wang, G.; Clark, C.G.; Taylor, T.M.; Pucknell, C.; Barton, C.; Price, L.; Woodward, D.L.; Rodgers, F.G. Colony Multiplex PCR Assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J. Clin. Microbiol. 2002, 40, 4744–4747. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, D.; Ranucci, D.; Branciari, R.; Cioffi, A.; Mammoli, R.; Cenci Goga, B.T.; Avellini, P. Prevalence of Campylobacter jejuni and Campylobacter coli in chicken hybrids with different growth rates, reared according to conventional and “Free-Range” production methods. Vet. Res. Commun. 2007, 31 (Suppl. S1), 381–384. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Kassem, I.I.; Shen, Z.; Rajashekara, G.; Zhang, Q. Campylobacter in poultry: Ecology and potential interventions. Avian Dis. 2015, 59, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Brenes, M.; Garcia, A.; Romero, C.; de Castro, A. Bactericidal activity of Glutaraldehyde-like compounds from olive products. J. Food Prot. 2009, 12, 2611–2614. [Google Scholar]
- Larif, M.; Ouhssine, M.; Soulaymani, A.; Elmidaoui, A. Potential effluent oil mills and antibacterial activity polyphenols against some pathogenic strains. Res. Chem. Intermed. 2015, 41, 1213–1225. [Google Scholar] [CrossRef]
- Gañan, M.; Martínez-Rodríguez, A.J.; Carrascosa, A.V. Antimicrobial activity of phenolic compounds of wine against Campylobacter jejuni. Food Control 2009, 20, 739–742. [Google Scholar] [CrossRef]
- Silván, J.M.; Mingo, E.; Hidalgo, M.; de Pascual-Teresa, S.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Antibacterial activity of a grape seed extract and its fractions against Campylobacter spp. Food Control 2013, 29, 25–31. [Google Scholar] [CrossRef]
- Salaheen, S.; Nguyen, C.; Hewes, D.; Biswas, D. Cheap extraction of antibacterial compounds of berry pomace and their mode of action against the pathogen Campylobacter jejuni. Food Control 2014, 46, 174–181. [Google Scholar] [CrossRef]
- Kurekci, C.; Al Jassim, R.; Hassan, E.; Bishop-Hurley, S.L.; Padmanabha, J.; McSweeney, C.S. Effects of feeding plant-derived agents on the colonization of Campylobacter jejuni in broiler chickens. Poult. Sci. 2014, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.J.; Brooker, J.D.; Smyl, C. Growth rate of broiler chickens given condensed tannins extracted from grape seed. Aust. Poult. Sci. Symp. 2005, 17, 65–68. [Google Scholar]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Brenes, A.; Centeno, C.; Viveros, A.; Saura-Calixto, F.; Rebole, A.; Arija, I.; Estevez, R. Effect of dietary grape pomace and vitamin E on growth performance, nutrient digest digestibility, and susceptibility to meat lipid oxidation in chickens. Poult. Sci. 2007, 86, 508–516. [Google Scholar] [CrossRef] [PubMed]
| Starter | Grower/Finisher | |||||
|---|---|---|---|---|---|---|
| Control | L-DOC | H-DOC | L-OMWPE | H-OMWPE | ||
| Moisture % | 10.10 | 9.65 | 10.19 | 10.17 | 9.64 | 9.72 |
| Crude Protein % | 22.24 | 19.56 | 19.46 | 19.19 | 20.09 | 19.54 |
| NDF % | 17.93 | 22.14 | 22.16 | 23.52 | 23.58 | 22.50 |
| Starch % | 38.59 | 41.40 | 41.48 | 40.39 | 40.67 | 41.30 |
| Control | L-DOC | H-DOC | L-OMWPE | H-OMWPE | p Value | |
|---|---|---|---|---|---|---|
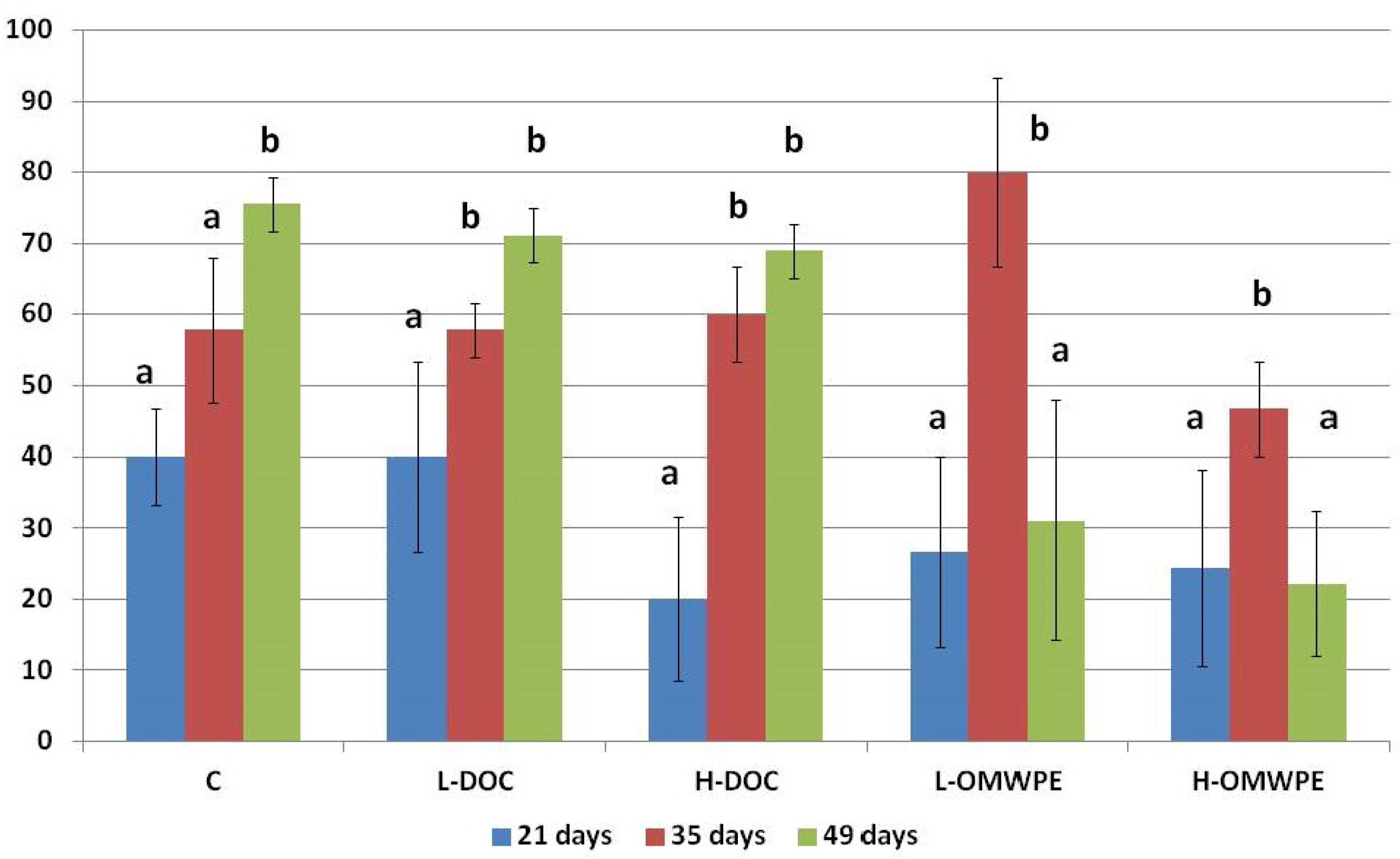
| 21 days | 40.00 ± 6.67 | 40.00 ±13.33 | 20.00 ± 11.55 | 26.27 ±13.33 | 24.44 ± 13.88 | 0.212 |
| 35 days | 57.78 ± 10.18ab | 57.78 ± 3.85ab | 60.00 ± 6.67b | 80.00 ± 13.33ab | 46.67 ± 6.67a | 0.012 |
| 49 days | 75.56 ± 3.85b | 71.11 ± 3.88b | 68.89 ± 3.85b | 31.11 ±16.78a | 20.00 ±6.67a | <0.001 |
| X2 | p Value | Odds Ratio | CI 95% Min | CI 95% Max | |
|---|---|---|---|---|---|
| CTR v L-DOC | 0.22 | 0.63 | 1.26 | 0.49 | 3.20 |
| CTR v H-DOC | 0.50 | 0.48 | 1.40 | 0.55 | 3.52 |
| CTR v L-OMWPE | 19.64 | <0.001 | 7.61 | 2.98 | 19.42 |
| CTR v H-OMWPE | 25.61 | <0.001 | 10.82 | 4.07 | 28.76 |
| CTR | L-DOC | H-DOC | L-OMWPE | H-OMWPE | SE | p Value | |
|---|---|---|---|---|---|---|---|
| Live weight | 1.88b | 2.02ab | 2.17a | 2.18a | 1.94ab | 0.059 | 0.017 |
| Carcass weight | 1.41b | 1.51ab | 1.58ab | 1.64a | 1.42b | 0.053 | 0.045 |
| Dressing percentage | 74.71 | 74.41 | 72.69 | 75.28 | 73.13 | 0.726 | 0.134 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branciari, R.; Ranucci, D.; Ortenzi, R.; Roila, R.; Trabalza-Marinucci, M.; Servili, M.; Papa, P.; Galarini, R.; Valiani, A. Dietary Administration of Olive Mill Wastewater Extract Reduces Campylobacter spp. Prevalence in Broiler Chickens. Sustainability 2016, 8, 837. https://doi.org/10.3390/su8090837
Branciari R, Ranucci D, Ortenzi R, Roila R, Trabalza-Marinucci M, Servili M, Papa P, Galarini R, Valiani A. Dietary Administration of Olive Mill Wastewater Extract Reduces Campylobacter spp. Prevalence in Broiler Chickens. Sustainability. 2016; 8(9):837. https://doi.org/10.3390/su8090837
Chicago/Turabian StyleBranciari, Raffaella, David Ranucci, Roberta Ortenzi, Rossana Roila, Massimo Trabalza-Marinucci, Maurizio Servili, Paola Papa, Roberta Galarini, and Andrea Valiani. 2016. "Dietary Administration of Olive Mill Wastewater Extract Reduces Campylobacter spp. Prevalence in Broiler Chickens" Sustainability 8, no. 9: 837. https://doi.org/10.3390/su8090837






