Shrub Encroachment Shapes Soil Nutrient Concentration, Stoichiometry and Carbon Storage in an Abandoned Subalpine Grassland
Abstract
:1. Introduction
2. Methods
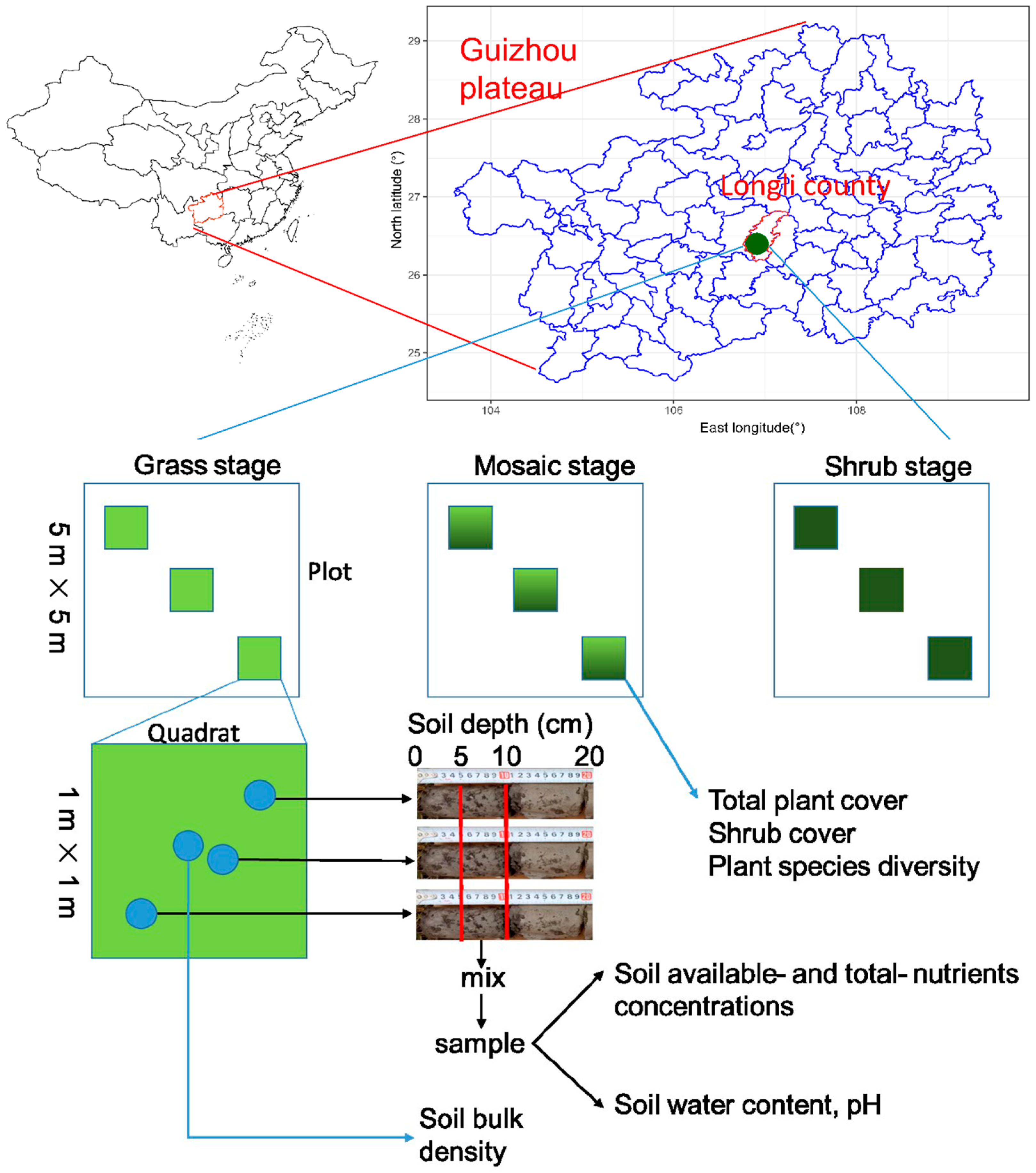
2.1. Site Description
2.2. Plant Quadrat Survey, Soil Sampling, and Determination
2.3. Data Analyses
3. Results
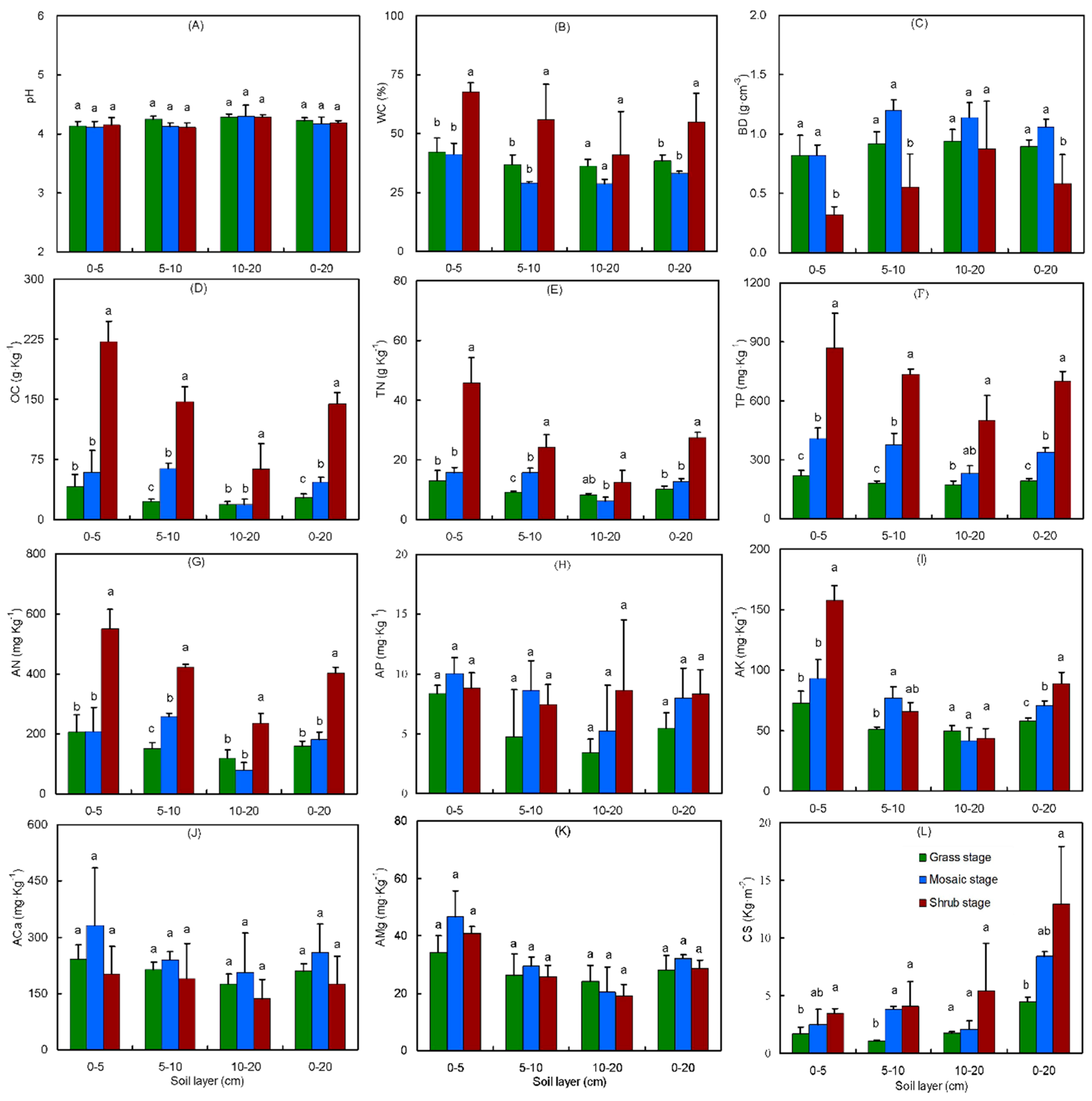
3.1. Shrub Encroachment Shaped the Soil Environment, Nutrient Concentration, and Carbon Storage
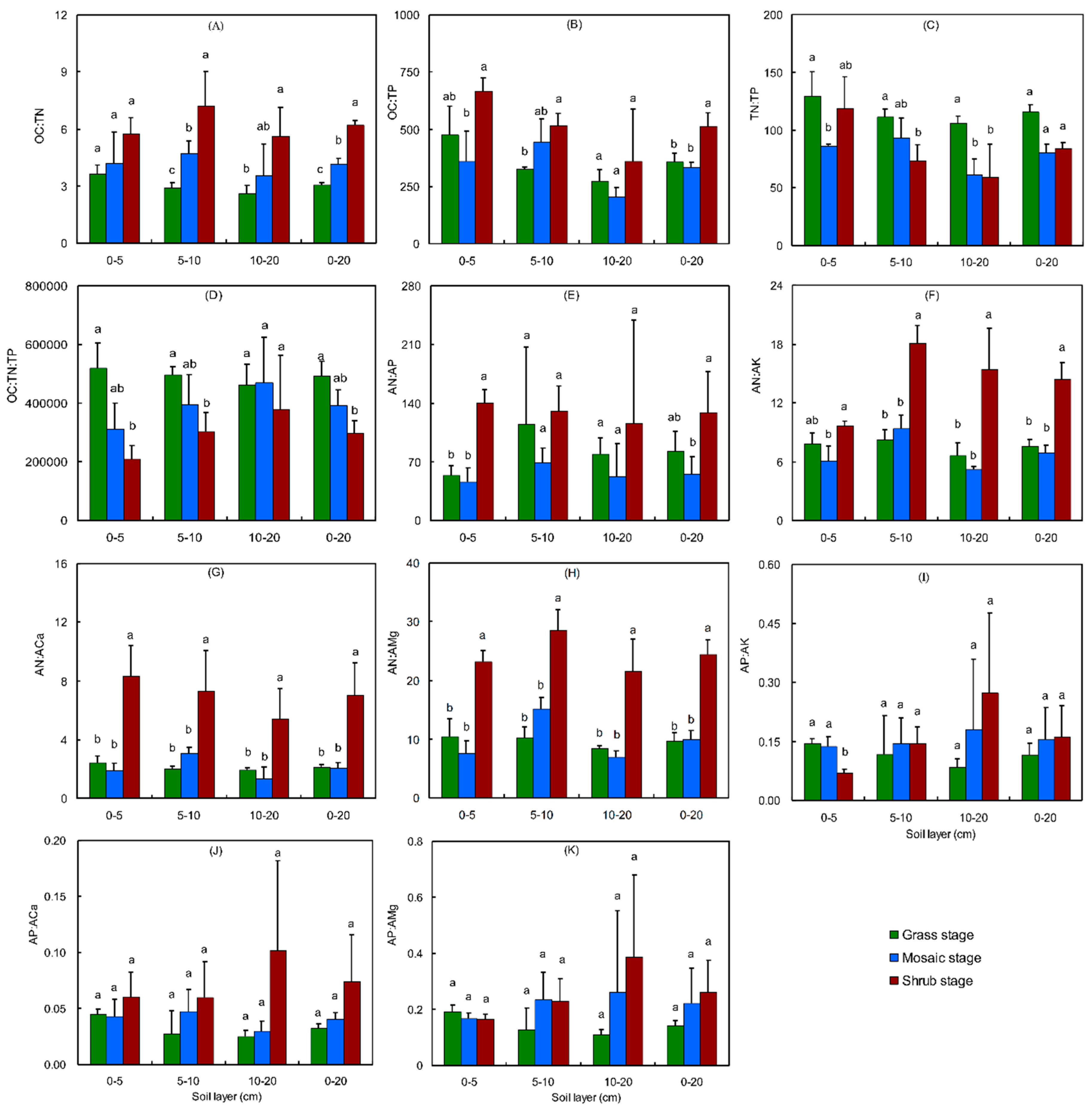
3.2. Shrub Encroachment Changed Soil Nutrient Stoichiometry
3.3. The Influence of Plant and Soil Factors on Soil Nutrients, Stoichiometry, and Carbon Storage
4. Discussion
4.1. Responses of Soil Nutrients, Carbon Storage, and Stoichiometry to Shrub Encroachment
4.2. SE in the Study Area May Not Indicate Land Degradation
4.3. The Main Factors Controlling Soil Nutrients Over Shrub Encroachment
4.4. The Main Factors Changing Soil Nutrient Stoichiometry Over Shrub Encroachment
4.5. The Main Factors Controlling Soil Carbon Storage over Shrub Encroachment
- (a)
- Recent studies from both the laboratory and field indicated that nutrients or inorganic nutrients are a hidden cost for sequestering soil C [79,80,81], underscoring the constraining role of nutrients [82,83,84,85], especially available nutrients [79,81,84,86,87] in carbon gains in the soil [81,82,84,88], plants [83,89], regional [84,89], and global levels [82]. Our data found that N was not limited [90] in this land, as the OC:TN ratio in the grass (3.3), mosaic (4.5), and shrub (6.5) stage soils were lower than the value of 11.8 in global grassland soils [90]. However, the OC:TP ratio in the grass (400.7), mosaic (402.6), and shrub (592.3) stage soils were higher than the value of 64.3 in global grassland soils [90], indicating a P limitation in our study land. The soil OC concentration and CS increased with SE and was apparently not limited by the shortage of P, perhaps because a higher proportion of P was stored in the deeper layer (Figure S3), so the P limitation was reduced in the deeper soil layers (Figure 3B). This benefited shrubs, which have deeper roots. Furthermore, SE enhanced the proportion distribution pattern of the P storage (Figure S3). Therefore, SE could reduce nutrient limitation and promote soil carbon sequestration [2].
- (b)
- The traits of encroaching plants determine the functional outcome of encroachment [3]. Changes in soil C cycles might be impacted by vegetation types via differences in litter or root quality [91] and productivity. On the one hand, about 70% of the net primary productivity (NPP) of the natural ecosystem returns to the soil through litter [64], and SE increased the NPP [19], as a result of which the litter productivity increased. On the other hand, the accumulation of OC increased with the decrease in the C/N ratio of the litter [92], and shrub litter had a lower C/N ratio than grass [64,93]. The litter traits of shrubs were thus more conducive to the accumulation of soil OC than of that of grass.
- (c)
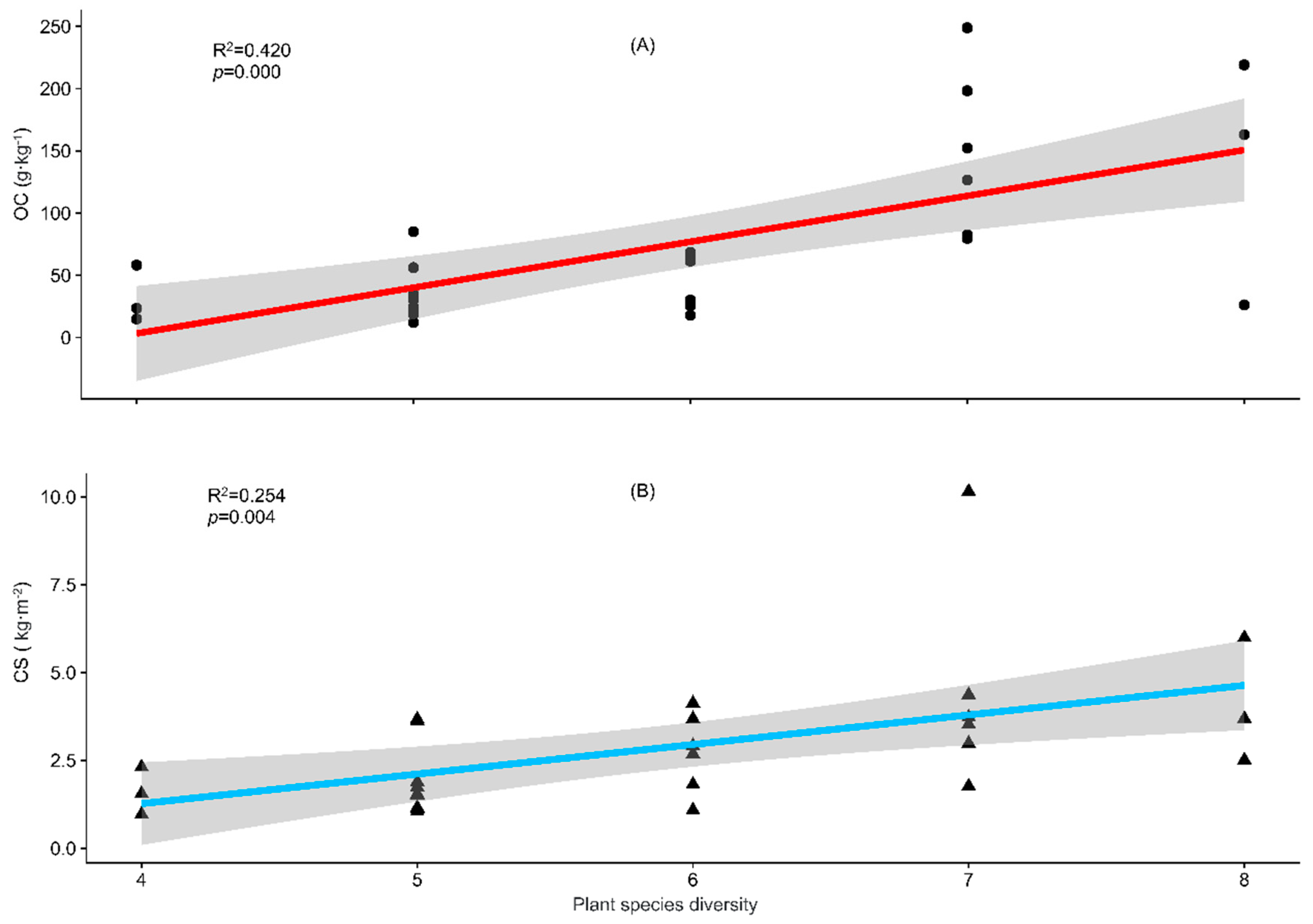
- Recent studies have found that a higher plant diversity leads to a greater soil CS [94,95]. Our regression analysis showed that the increasing plant species diversity significantly increased the soil OC concentration and CS (Figure 5), so our results supported this pathway only in terms of the plant species diversity.
- (d)
- Here, for the first time, we integrated the plant factors, soil environment factors, and soil nutrition factors and stoichiometry ratios in order to explore the driving factors of soil carbon storage over shrub encroachment. Our RDA model showed that the soil stoichiometry ratios (OC:TN, TN:TP, OC:TN:TP, AP:ACa) and soil environment factors (BD and pH) explained 87.78% of the total variation of the soil carbon storage (Figure 4E). The plant factors, especially the plant species diversity, and the soil nutrition factors were not as important as we expected. Instead, the soil stoichiometry ratios and soil environment factors were the first two important factors that influenced the soil carbon storage. Our results supported a new pathway of SE control over soil carbon storage.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saixiyala; Yang, D.; Zhang, S.; Liu, G.; Yang, X.; Huang, Z.; Ye, X. Facilitation by a Spiny Shrub on a Rhizomatous Clonal Herbaceous in Thicketization-Grassland in Northern China: Increased Soil Resources or Shelter from Herbivores. Front. Plant Sci. 2017, 8, 809. [Google Scholar] [CrossRef] [PubMed]
- Blaser, W.J.; Shanungu, G.K.; Edwards, P.J.; Olde Venterink, H. Woody encroachment reduces nutrient limitation and promotes soil carbon sequestration. Ecol. Evol. 2014, 4, 1423–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eldridge, D.J.; Bowker, M.A.; Maestre, F.T.; Roger, E.; Reynolds, J.F.; Whitford, W.G. Impacts of shrub encroachment on ecosystem structure and functioning: Towards a global synthesis. Ecol. Lett. 2011, 14, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Du, B.M.; Ji, H.W.; Peng, C.; Liu, X.J.; Liu, C.J. Altitudinal patterns of leaf stoichiometry and nutrient resorption in Quercus variabilis in the Baotianman Mountains, China. Plant Soil 2016, 413, 193–202. [Google Scholar] [CrossRef]
- Ratajczak, Z.; Nippert, J.B.; Collins, S.L. Woody encroachment decreases diversity across North American grasslands and savannas. Ecology 2012, 93, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, N.; Lehmann, C.E.; Murphy, B.P.; Durigan, G. Savanna woody encroachment is widespread across three continents. Glob. Chang. Biol. 2017, 23, 235–244. [Google Scholar] [CrossRef]
- Wigley, B.J.; Bond, W.J.; Hoffman, M.T. Thicket expansion in a South African savanna under divergent land use: Local vs. global drivers? Glob. Chang. Biol. 2010, 16, 964–976. [Google Scholar] [CrossRef]
- Barger, N.N.; Archer, S.R.; Campbell, J.L.; Huang, C.-Y.; Morton, J.A.; Knapp, A.K. Woody plant proliferation in North American drylands: A synthesis of impacts on ecosystem carbon balance. J. Geophys. Res. 2011, 116. [Google Scholar] [CrossRef]
- Morgan, J.A.; Milchunas, D.G.; LeCain, D.R.; West, M.; Mosier, A.R. Carbon dioxide enrichment alters plant community structure and accelerates shrub growth in the shortgrass steppe. Proc. Natl. Acad. Sci. USA 2007, 104, 14724–14729. [Google Scholar] [CrossRef] [Green Version]
- Throop, H.L.; Lajtha, K.; Kramer, M. Density fractionation and 13C reveal changes in soil carbon following woody encroachment in a desert ecosystem. Biogeochemistry 2013, 112, 409–422. [Google Scholar] [CrossRef]
- Soliveres, S.; Maestre, F.T.; Eldridge, D.J.; Delgado-Baquerizo, M.; Quero, J.L.; Bowker, M.A.; Gallardo, A. Plant diversity and ecosystem multifunctionality peak at intermediate levels of woody cover in global drylands. Glob. Ecol. Biogeogr. A J. Macroecol. 2014, 23, 1408–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulmatiski, A.; Beard, K.H. Woody plant encroachment facilitated by increased precipitation intensity. Nat. Clim. Chang. 2013, 3, 833–837. [Google Scholar] [CrossRef]
- Anadon, J.D.; Sala, O.E.; Turner, B.L., II; Bennett, E.M. Effect of woody-plant encroachment on livestock production in North and South America. Proc. Natl. Acad. Sci. USA 2014, 111, 12948–12953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalle, G.; Maass, B.L.; Isselstein, J. Encroachment of woody plants and its impact on pastoral livestock production in the Borana lowlands, southern Oromia, Ethiopia. Afr. J. Ecol. 2006, 44, 237–246. [Google Scholar] [CrossRef]
- Blaum, N.; Rossmanith, E.; Popp, A.; Jeltsch, F. Shrub encroachment affects mammalian carnivore abundance and species richness in semiarid rangelands. Acta Oecol. 2007, 31, 86–92. [Google Scholar] [CrossRef]
- Brandt, J.S.; Haynes, M.A.; Kuemmerle, T.; Waller, D.M.; Radeloff, V.C. Regime shift on the roof of the world: Alpine meadows converting to shrublands in the southern Himalayas. Biol. Conserv. 2013, 158, 116–127. [Google Scholar] [CrossRef]
- Koch, B.; Edwards, P.J.; Blanckenhorn, W.U.; Walter, T.; Hofer, G. Shrub encroachment affects the diversity of plants, butterflies, and grasshoppers on two swiss subalpine pastures. Arct. Antarct. Alp. Res. 2015, 47, 345–357. [Google Scholar] [CrossRef]
- Hector, A.; Báez, S.; Collins, S.L. Shrub invasion decreases diversity and alters community stability in Northern Chihuahuan Desert plant communities. PLoS ONE 2008, 3, e2332. [Google Scholar]
- Lett, M.S.; Knapp, A.K.; Briggs, J.M.; Blair, J.M. Influence of shrub encroachment on aboveground net primary productivity and carbon and nitrogen pools in a mesic grassland. Can. J. Bot. 2004, 82, 1363–1370. [Google Scholar] [CrossRef]
- Grace, J.; Jose, J.S.; Meir, P.; Miranda, H.S.; Montes, R.A. Productivity and carbon fluxes of tropical savannas. J. Biogeogr. 2006, 33, 387–400. [Google Scholar] [CrossRef]
- Du Toit, J.; Kock, R.; Deutsch, J. Wild Rangelands: Conserving Wildlife WhileMaintaining Livestock in Semi-Arid Ecosystems; Blackwell Publishing Ltd.: Oxford, UK, 2010. [Google Scholar]
- Ward, D.; Trinogga, J.; Wiegand, K.; Du Toit, J.; Okubamichael, D.; Reinsch, S.; Schleicher, J. Large shrubs increase soil nutrients in a semi-arid savanna. Geoderma 2018, 310, 153–162. [Google Scholar] [CrossRef]
- Ridolfi, L.; Laio, F.; D’Odorico, P. Fertility Island Formation and Evolution in Dryland Ecosystems. Ecol. Soc. 2008, 13, 5. [Google Scholar] [CrossRef]
- Guidi, C.; Vesterdal, L.; Gianelle, D.; Rodeghiero, M. Changes in soil organic carbon and nitrogen following forest expansion on grassland in the Southern Alps. For. Ecol. Manag. 2014, 328, 103–116. [Google Scholar] [CrossRef]
- Qiu, L.; Wei, X.; Zhang, X.; Cheng, J.; Gale, W.; Guo, C.; Long, T. Soil organic carbon losses due to land use change in a semiarid grassland. Plant Soil 2012, 355, 299–309. [Google Scholar] [CrossRef]
- Alberti, G.; Leronni, V.; Piazzi, M.; Petrella, F.; Mairota, P.; Peressotti, A.; Piussi, P.; Valentini, R.; Gristina, L.; La Mantia, T.; et al. Impact of woody encroachment on soil organic carbon and nitrogen in abandoned agricultural lands along a rainfall gradient in Italy. Reg. Environ. Chang. 2011, 11, 917–924. [Google Scholar] [CrossRef] [Green Version]
- Jackson, R.B.; Banner, J.L.; Jobbágy, E.G.; Pockman, W.T.; Wall, D.H. Ecosystem carbon loss with woody plant invasion of grasslands. Nature 2002, 418, 623–626. [Google Scholar] [CrossRef]
- Gómez-Rey, M.X.; Madeira, M.; Gonzalez-Prieto, S.J.; Coutinho, J. Soil C and N dynamics in a Mediterranean oak woodland with shrub encroachment. Plant Soil 2013, 371, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Hagos, M.G.; Smit, G.N. Soil enrichment by Acacia mellifera subsp. detinens on nutrient poor sandy soil in a semi-arid southern African savanna. J. Arid Environ. 2005, 61, 47–59. [Google Scholar] [CrossRef]
- Dong, X.W.; Zhang, X.K.; Bao, X.L.; Wang, J.K. Spatial distribution of soil nutrients after the establishment of sand-fixing shrubs on sand dune. Plant Soil Environ. 2009, 55, 288–294. [Google Scholar] [CrossRef] [Green Version]
- Throop, H.L.; Archer, S.R. Shrub (Prosopis velutina) encroachment in a semidesert grassland: Spatial-temporal changes in soil organic carbon and nitrogen pools. Glob. Chang. Biol. 2008, 14, 2420–2431. [Google Scholar] [CrossRef]
- Mills, A.; Fey, M. Transformation of thicket to savanna reduces soil quality in the Eastern Cape, South Africa. Plant Soil 2004, 265, 153–163. [Google Scholar] [CrossRef]
- McKinley, D.C.; Blair, J.M. Woody Plant Encroachment by Juniperus virginiana in a Mesic Native Grassland Promotes Rapid Carbon and Nitrogen Accrual. Ecosystems 2008, 11, 454–468. [Google Scholar] [CrossRef]
- Su, Y.Z.; Zhao, H.L.; Li, Y.L.; Cui, J.Y. Influencing Mechanisms of Several Shrubs on Soil Chemical Properties in Semiarid Horqin Sandy Land, China. Arid Land Res. Manag. 2004, 18, 251–263. [Google Scholar] [CrossRef]
- Zhao, H.-L.; Zhou, R.-L.; Su, Y.-Z.; Zhang, H.; Zhao, L.-Y.; Drake, S. Shrub facilitation of desert land restoration in the Horqin Sand Land of Inner Mongolia. Ecol. Eng. 2007, 31, 1–8. [Google Scholar] [CrossRef]
- Gazol, A.; Tamme, R.; Takkis, K.; Kasari, L.; Saar, L.; Helm, A.; Pärtel, M. Landscape- and small-scale determinants of grassland species diversity: Direct and indirect influences. Ecography 2012, 35, 944–951. [Google Scholar] [CrossRef]
- Briggs, J.M.; Knapp, A.K.; Blair, J.M.; Heisler, J.L.; Hoch, G.A.; Lett, M.S.; Mccarron, J.K. An ecosystem in transition: Causes and consequences of the conversion of mesic grassland to shrubland. Bioscience 2005, 55, 243–254. [Google Scholar] [CrossRef]
- Buitenwerf, R.; Bond, W.J.; Stevens, N.; Trollope, W.S.W. Increased tree densities in South African savannas: >50 years of data suggests CO2 as a driver. Glob. Chang. Biol. 2012, 18, 675–684. [Google Scholar] [CrossRef]
- Cabral, A.C.; Miguel, J.M.; Rescia, A.J.; Schmitz, M.F.; Pineda, F.D. Shrub encroachment in argentinean savannas. J. Veg. Sci. 2010, 14, 145–152. [Google Scholar] [CrossRef]
- Asner, G.P.; Mosier, A.R. Effects of Woody Vegetation Encroachment on Soil Nitrogen Oxide Emissions in a Temperate Savanna. Ecol. Appl. 2003, 13, 897–910. [Google Scholar]
- Chen, L.; Li, H.; Zhang, P.; Zhao, X.; Zhou, L.; Liu, T.; Hu, H.; Bai, Y.; Shen, H.; Fang, J. Climate and native grassland vegetation as drivers of the community structures of shrub-encroached grasslands in Inner Mongolia, China. Landsc. Ecol. 2014, 30, 1627–1641. [Google Scholar] [CrossRef]
- Lett, M.S.; Knapp, K.A. Consequences of Shrub Expansion in Mesic Grassland: Resource Alterations and Graminoid Responses. J. Veg. Sci. 2003, 14, 487–496. [Google Scholar] [CrossRef]
- Li, H.; Shen, H.; Chen, L.; Liu, T.; Hu, H.; Zhao, X.; Zhou, L.; Zhang, P.; Fang, J. Effects of shrub encroachment on soil organic carbon in global grasslands. Sci. Rep. 2016, 6, 28974. [Google Scholar] [CrossRef] [Green Version]
- Jiao, F.; Wen, Z.M.; An, S.S.; Yuan, Z. Successional changes in soil stoichiometry after land abandonment in Loess Plateau, China. Ecol. Eng. 2013, 58, 249–254. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Pan, F.; Li, D.; Chen, H.; Wang, K. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest china. Plant Soil 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, Y.; Fang, Y.; Ma, R.; Lal, R.; An, S.; Huang, Y. Impact of vegetation restoration on plants and soil C:N:P stoichiometry on the Yunwu Mountain Reserve of China. Ecol. Eng. 2017, 109, 92–100. [Google Scholar] [CrossRef]
- Urbina, I.; Grau, O.; Sardans, J.; Ninot, J.; Penuelas, J. Plant-soil stoichiometric changes along the succession from subalpine grasslands to shrublands in the Pyrenees. Geophys. Res. Abstr. 2018, 20, 19126. [Google Scholar]
- Bui, E.N.; Henderson, B.L. C:N:P stoichiometry in Australian soils with respect to vegetation and environmental factors. Plant Soil 2013, 373, 553–568. [Google Scholar] [CrossRef]
- Hu, C.; Li, F.; Xie, Y.H.; Deng, Z.M.; Chen, X.S. Soil carbon, nitrogen, and phosphorus stoichiometry of three dominant plant communities distributed along a small-scale elevation gradient in the East Dongting Lake. Phys. Chem. Earth Parts A/B/C 2017, 103, 28–34. [Google Scholar] [CrossRef]
- Yong, Z.; Boutton, T.W.; Ben, W.X. Soil c:n:p stoichiometry responds to vegetation change from grassland to woodland. Biogeochemistry 2018, 140, 341–357. [Google Scholar]
- Song, Z.; Liu, H.; Zhao, F.; Xu, C. Ecological stoichiometry of N:P:Si in China’s grasslands. Plant Soil 2014, 380, 165–179. [Google Scholar] [CrossRef]
- Rong, Q.; Liu, J.; Cai, Y.; Lu, Z.; Zhao, Z.; Yue, W.; Xia, J. “Fertile island” effects of Tamarix chinensis Lour. on soil N and P stoichiometry in the coastal wetland of Laizhou Bay, China. J. Soils Sediments 2016, 16, 864–877. [Google Scholar] [CrossRef]
- Feng, D.; Bao, W. Shrub encroachment alters topsoil C:N:P stoichiometric ratios in a high-altitude forest cutover. Iforest Biogeosci. For. 2018, 11, 594–599. [Google Scholar] [CrossRef]
- Schwob, G.; Roy, M.; Manzi, S.; Pommier, T.; Fernandez, M.P. Green alder (Alnus viridis) encroachment shapes microbial communities in subalpine soils and impacts its bacterial or fungal symbionts differently. Environ. Microbiol. 2017, 19, 3235–3250. [Google Scholar] [CrossRef]
- Forestry, G.A.O. Classification and Code of Guizhou Vascular Plants. Available online: http://gzslky.freetable.cn/index.php?m=Index&c=Content&a=index&cid=31&aid=329 (accessed on 27 January 2018).
- Flora of China Editorial Committee. Flora of China; Science Press & Missouri Botanical Garden Press: Beijing, China; St. Louis, MO, USA, 2006; Volume 22, pp. 1–733. [Google Scholar]
- Walker, L.R.; Wardle, D.A.; Bardgett, R.D.; Clarkson, B.D. The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 2010, 98, 725–736. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.-L.; Liu, S.-J.; Ye, Y.-Y.; Zhang, W.; Wang, K.-L.; Su, Y.-R. Effects of environmental factors on soil organic carbon under natural or managed vegetation restoration. Land Degrad. Dev. 2018, 29, 387–397. [Google Scholar] [CrossRef]
- Belay, L.; Kebede, F. The Impact of Woody Plants Encroachment on Soil Organic Carbon and Total Nitrogen Stocks in Yabello District, Borana Zone, Southern Ethiopia. J. Drylands 2010, 3, 234–240. [Google Scholar]
- Bao, D.S. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Liu, Y.; Dang, Z.-Q.; Tian, F.-P.; Wang, D.; Wu, G.-L. Soil Organic Carbon and Inorganic Carbon Accumulation Along a 30-year Grassland Restoration Chronosequence in Semi-arid Regions (China). Land Degrad. Dev. 2017, 28, 189–198. [Google Scholar] [CrossRef]
- Liu, X.; Ma, J.; Ma, Z.-W.; Li, L.-H. Soil nutrient contents and stoichiometry as affected by land-use in an agro-pastoral region of northwest China. Catena 2017, 150, 146–153. [Google Scholar] [CrossRef]
- SPSS. SPSS Two Way ANOVA—Basics Tutorial. Available online: https://www.spss-tutorials.com/spss-two-way-anova-basics-tutorial/ (accessed on 2 February 2018).
- Liu, S.; Zhang, W.; Wang, K.; Pan, F.; Yang, S.; Shu, S. Factors controlling accumulation of soil organic carbon along vegetation succession in a typical karst region in Southwest China. Sci. Total Environ. 2015, 521–522, 52–58. [Google Scholar] [CrossRef]
- Mcculley, R.L.; Archer, S.R.; Boutton, T.W.; Hons, F.M.; Zuberer, D.A. Soil respiration and nutrient cycling in wooded communities developing in grassland. Ecology 2004, 85, 2804–2817. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Wang, K.; Hou, Y. Soil organic carbon and total nitrogen as affected by land use types in karst and non-karst areas of northwest Guangxi, China. J. Sci. Food Agric. 2011, 92, 1086–1093. [Google Scholar] [CrossRef]
- Schade, J.D.; Hobbie, S.E. Spatial and temporal variation in islands of fertility in the Sonoran Desert. Biogeochemistry 2005, 73, 541–553. [Google Scholar] [CrossRef]
- Yu, Z.P.; Wang, M.H.; Huang, Z.Q.; Lin, T.C.; Vadeboncoeur, M.A.; Searle, E.B.; Chen, H.Y.H. Temporal changes in soil c-n-p stoichiometry over the past 60 years across subtropical china. Glob. Chang. Biol. 2017, 24, 1308–1320. [Google Scholar] [CrossRef]
- Nidzgorski, D.A.; Hobbie, S.E. Urban trees reduce nutrient leaching to groundwater. Ecol. Appl. A Publ. Ecol. Soc. Am. 2016, 26, 1566–1580. [Google Scholar] [CrossRef]
- Qiao, L.; Zou, C.B.; Stebler, E.; Will, R.E. Woody plant encroachment reduces annual runoff and shifts runoff mechanisms in the tallgrass prairie, USA. Water Resour. Res. 2017, 4838. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Finlay, J.C.; Janke, B.D.; Nidzgorski, D.A.; Millet, D.B.; Baker, L.A. Contrasting nitrogen and phosphorus budgets in urban watersheds and implications for managing urban water pollution. Proc. Natl. Acad. Sci. USA 2017, 114, 4177–4182. [Google Scholar] [CrossRef] [Green Version]
- Jobbágy; Esteban, G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar]
- Maestre, F.T.; Bowker, M.A.; Puche, M.D.; Belen Hinojosa, M.; Martinez, I.; Garcia-Palacios, P.; Castillo, A.P.; Soliveres, S.; Luzuriaga, A.L.; Sanchez, A.M.; et al. Shrub encroachment can reverse desertification in semi-arid Mediterranean grasslands. Ecol. Lett. 2009, 12, 930–941. [Google Scholar] [CrossRef]
- Knapp, A.K.; Briggs, J.M.; Collins, S.L.; Archer, S.R.; Bret-Harte, M.S.; Ewers, B.E.; Peters, D.P.; Young, D.R.; Shaver, G.R.; Pendall, E.; et al. Shrub encroachment in North American grasslands: Shifts in growth form dominance rapidly alters control of ecosystem carbon inputs. Glob. Chang. Biol. 2008, 14, 615–623. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Pilmanis, A.M. Plant-Soil Interactions in Deserts. Biogeochemistry 1998, 42, 169–187. [Google Scholar] [CrossRef]
- Zhao, H.L.; Liu, R.T. The “bug island” effect of shrubs and its formation mechanism in Horqin Sand Land, Inner Mongolia. Catena 2013, 105, 69–74. [Google Scholar] [CrossRef]
- Qu, L.; Wang, Z.; Huang, Y.; Zhang, Y.; Song, C.; Ma, K. Effects of plant coverage on shrub fertile islands in the upper minjiang river valley. Sci. China Life Sci. 2018, 61, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Kirkby, C.A.; Banerjee, S.; Kirkegaard, J.A. The inorganic nutrient cost of building soil carbon. Carbon Manag. 2014, 5, 265–268. [Google Scholar] [CrossRef]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Batten, G.D.; Blanchard, C.; Kirkegaard, J.A. Carbon-nutrient stoichiometry to increase soil carbon sequestration. Soil Biol. Biochem. 2013, 60, 77–86. [Google Scholar] [CrossRef]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Conyers, M.; Kirkegaard, J.A. Inorganic Nutrients Increase Humification Efficiency and C-Sequestration in an Annually Cropped Soil. PLoS ONE 2016, 11, e0153698. [Google Scholar] [CrossRef] [PubMed]
- van Groenigen, J.W.; van Kessel, C.; Hungate, B.A.; Oenema, O.; Powlson, D.S.; van Groenigen, K.J. Sequestering soil organic carbon: A nitrogen dilemma. Environ. Sci. Technol. 2017, 51, 4738–4739. [Google Scholar] [CrossRef] [PubMed]
- Oren, R.; Ellsworth, D.S.; Johnsen, K.H.; Phillips, N.; Ewers, B.E.; Maier, C.; Schäfer, K.V.R.; McCarthy, H.; Hendrey, G.; McNulty, S.G.; et al. Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 2001, 411, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Van Groenigen, K.J.; Six, J.; Hungate, B.A.; de Graaff, M.A.; van Breemen, N.; van Kessel, C. Element interactions limit soil carbon storage. Proc. Natl. Acad. Sci. USA 2006, 103, 6571–6574. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.; Blagodatskaya, E.; Kuzyakov, Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Glob. Chang. Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef]
- Gosling, P.; van der Gast, C.; Bending, G.D. Converting highly productive arable cropland in Europe to grassland: -a poor candidate for carbon sequestration. Sci. Rep. 2017, 7, 10493. [Google Scholar] [CrossRef] [PubMed]
- Hartman, W.H.; Ye, R.Z.; Horwath, W.R.; Tringe, S.G. A genomic perspective on stoichiometric regulation of soil carbon cycling. ISME J. 2017, 11, 2652–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Passioura, J.B.; Batten, G.D.; Blanchard, C.; Kirkegaard, J.A. Nutrient availability limits carbon sequestration in arable soils. Soil Biol. Biochem. 2014, 68, 402–409. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Nadelhoffer, K.J.; Högberg, P. A synthesis: The role of nutrients as constraints on carbon balances in boreal and Arctic regions. Plant Soil 2002, 242, 163–170. [Google Scholar] [CrossRef]
- Wang, W.; Sardans, J.; Zeng, C.; Zhong, C.; Li, Y.; Peñuelas, J. Responses of soil nutrient concentrations and stoichiometry to different human land uses in a subtropical tidal wetland. Geoderma 2014, 232–234, 459–470. [Google Scholar] [CrossRef]
- Pan, P.; Zhao, F.; Ning, J.; Zhang, L.; Ouyang, X.; Zang, H. Impact of understory vegetation on soil carbon and nitrogen dynamic in aerially seeded Pinus massoniana plantations. PLoS ONE 2018, 13, e0191952. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, R.; Huang, C.; Wang, B.; Cao, H.; Koopal, L.K.; Tan, W. Effect of different vegetation cover on the vertical distribution of soil organic and inorganic carbon in the Zhifanggou Watershed on the loess plateau. Catena 2016, 139, 191–198. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Perez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef]
- Steinbeiss, S.; BeßLer, H.; Engels, C.; Temperton, V.M.; Buchmann, N.; Roscher, C.; Kreutziger, Y.; Baade, J.; Habekost, M.; Gleixner, G. Plant diversity positively affects short-term soil carbon storage in experimental grasslands. Glob. Chang. Biol. 2008, 14, 2937–2949. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vazquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | Grass Stage | Mosaic Stage | Shrub Stage |
|---|---|---|---|
| Latitude 1 | 26°19′44″ N | 26°19′49″ N | 26°19′48″ N |
| Longitude 2 | 106°51′9″ E | 106°51′6″ E | 106°51′7″ E |
| Altitude a. s. l. 3 | 1610.7 m | 1610.5 m | 1610.2 m |
| Dominant species | Eulalia pallens (Hack.) Kuntze;Arundinella anomala Steud. | Eulalia pallens (Hack.) Kuntze;Arundinella anomala Steud.;Vaccinium fragile Franch. | Vaccinium fragile Franch.; pallens (Hack.) Kuntze |
| Plant functional types | Subalpine grassland with dense and tall herbaceous plants and grasses with few shrubs | Patchy shrubs interspaced by subalpine grassland | Dense shrubs stand with few herbaceous plants and grasses |
| Total plant cover (%) | 91.67 ± 2.89 a | 86.67 ± 4.16 a | 85.00 ± 5.00 a |
| Shrub relative cover (%) | 6.53 ± 0.91 c | 34.38 ± 4.23 b | 83.10 ± 1.74 a |
| Plant species richness per plot | 10 | 12 | 15 |
| Plant species richness per quadrat | 4.67 ± 0.57 b | 5.67 ± 0.57 b | 7.33 ± 0.57 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Wang, P.; Zhang, W.; Zhang, Y.; Li, S.; Wei, X.; Chen, X.; Zhang, Y.; Yang, F. Shrub Encroachment Shapes Soil Nutrient Concentration, Stoichiometry and Carbon Storage in an Abandoned Subalpine Grassland. Sustainability 2019, 11, 1732. https://doi.org/10.3390/su11061732
Ding L, Wang P, Zhang W, Zhang Y, Li S, Wei X, Chen X, Zhang Y, Yang F. Shrub Encroachment Shapes Soil Nutrient Concentration, Stoichiometry and Carbon Storage in an Abandoned Subalpine Grassland. Sustainability. 2019; 11(6):1732. https://doi.org/10.3390/su11061732
Chicago/Turabian StyleDing, Leilei, Puchang Wang, Wen Zhang, Yu Zhang, Shige Li, Xin Wei, Xi Chen, Yujun Zhang, and Fuli Yang. 2019. "Shrub Encroachment Shapes Soil Nutrient Concentration, Stoichiometry and Carbon Storage in an Abandoned Subalpine Grassland" Sustainability 11, no. 6: 1732. https://doi.org/10.3390/su11061732





