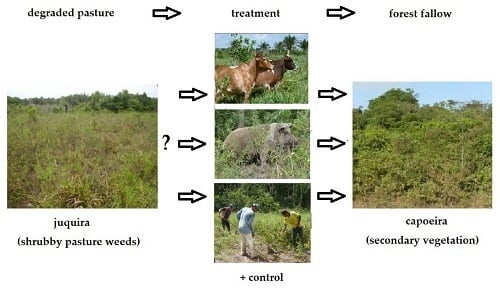
Accelerating Capoeira Regeneration on Degraded Pastures in the Northeastern Amazon by the Use of Pigs or Cattle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Vegetation Sampling
2.3. Soil Compaction
- µ = overall mean,
- at = effect of treatment (i = trt1, trt2, trt3, trt4),
- bf = block effect (j = farm1, farm2, farm3),
- ci = effect of time (k = time1, time2, time3),
- abtf = interaction of treatment and block effect,
- acti = interaction of treatment and time effect,
- dtfi = residual deviation.
2.4. Economic Costs and Benefits
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Serrão, E.A.S.; Falesi, I.C.; da Veiga, J.B.; Teixeira Neto, J.F. Productivity of Cultivated Pastures on Low Fertility Soils of the Amazon Brazil. In Pasture Production in Acid Soils of the Tropics; Sánchez, P.A., Tergas, L.E., Eds.; Centro Internacional de Agricultura Tropical: Cali, Colombia, 1979; pp. 195–225. [Google Scholar]
- Serrão, E.A.S.; Nepstad, D.C. Pastures on Amazonian forestlands: A review of environmental and economic performance. In Interdisciplinary Research on the Conservation and Sustainable Use of the Amazonian Rainforest and Its Information Requirements; Lieberei, R., Reisdorff, C., Dantas Machado, A., Eds.; SBCS: Geesthacht, Germany; Stern-Werke: Hamburg, Germany, 1996; pp. 221–239. ISBN 3-00-000909-4. [Google Scholar]
- Dias-Filho, M.B. Degradação de pastagens: Processos, causas e estratégias de recuperação, 4th ed.; MBDF: Belém, Brazil, 2015. [Google Scholar]
- Buschbacher, R.; Uhl, C.; Serrão, E.A.S. Abandoned pastures in Eastern Amazonia. II. Nutrient stocks in the soil and vegetation. J. Ecol. 1988, 76, 682–699. [Google Scholar] [CrossRef]
- Müller, M.M.L.; Guimarães, M.F.; Desjardins, T.; Mitja, D. The relationship between pasture degradation and soil properties in the Brazilian Amazon: A case study. Agric. Ecosyst. Environ. 2004, 103, 279–288. [Google Scholar] [CrossRef]
- Davidson, E.A.; Reis de Carvalho, C.J.; Figueira, A.M.; Ishida, F.Y.; Ometto, J.P.H.B.; Nardoto, G.B.; Sabá, R.T.; Hayashi, S.N.; Leal, E.C.; Vieira, I.C.G.; et al. Recuperation of nitrogen cycling in Amazonian forests following agricultural abandonment. Nature 2007, 447, 995–998. [Google Scholar] [CrossRef]
- Uhl, C.; Buschbacher, R.; Serrão, E.A.S. Abandoned pastures in Eastern Amazonia. I. Patterns of plant succession. J. Ecol. 1988, 76, 663–681. [Google Scholar] [CrossRef]
- Aide, T.M.; Zimmerman, J.K.; Pascarella, J.B.; Rivera, L.; Marcano-Vega, H. Forest Regeneration in a Chronosequence of Tropical Abandoned Pastures: Implications for Restoration Ecology. Rest. Ecol. 2000, 8, 328–338. [Google Scholar] [CrossRef]
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. Restoration of degraded tropical forest landscape. Science 2005, 310, 1628–1632. [Google Scholar] [CrossRef]
- Bruel, B.O.; Marques, M.C.M.; Britez, R.M. Survival and Growth of Tree Species under Two Direct Seedling Planting Systems. Rest. Ecol. 2010, 18, 414–417. [Google Scholar] [CrossRef]
- Bertacchi, M.I.F.; Amazonas, N.T.; Brancalion, P.H.; Brondani, G.E.; Oliveira, A.; Pascoa, M.A.; Rodrigues, R.R. Establishment of tree seedlings in the understory of restoration plantations: Natural regeneration and enrichment plantings. Rest. Ecol. 2015, 24, 100–108. [Google Scholar] [CrossRef]
- Denich, M. Estudo da importância de uma vegetação secundária nova para o incremento da produtividade do sistema de produção na Amazônia oriental brasileira, 2nd ed.; GTZ, EMBRAPA/CPATU: Eschborn, Germany; Belém, Brazil, 1991. [Google Scholar]
- Chazdon, R.L. Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation; University of Chicago Press: Chicago, IL, USA, 2014. [Google Scholar]
- Holl, K.D. Factors Limiting Tropical Rain Forest Regeneration in Abandoned Pasture: Seed Rain, Seed Germination, Microclimate, and Soil. Biotropica 1999, 31, 229–242. [Google Scholar] [CrossRef]
- Feldpausch, T.R.; Rondon, M.A.; Fernandes, E.C.M.; Riha, S.J.; Wandelli, E. Carbon and nutrient accumulation in secondary forests regenerating on pastures in central Amazonia. Ecol. Appl. 2004, 14, 164–176. [Google Scholar] [CrossRef]
- De Rouw, A. The fallow period as a weed-break in shifting cultivation (tropical wet forests). Agric. Ecosyst. Environ. 1995, 54, 31–43. [Google Scholar] [CrossRef]
- Nepstad, D.C.; Uhl, C.; Pereira, C.A.; Cardoso da Silva, J.M. A comparative study of tree establishment in abandoned pasture and mature forest of eastern Amazonia. Oikos 1996, 76, 25–39. [Google Scholar] [CrossRef]
- Uhl, C.; Nepstad, D.; da Silva, J.M.C.; Vieira, I. Restauração da floresta em pastagens degradadas. Ciência Hoje 1991, 13, 23–31. [Google Scholar]
- Aide, T.M.; Cavelier, J. Barriers to lowland tropical forest restoration in the Sierra Nevada de Santa Marta Colombia. Rest. Ecol. 1994, 2, 219–229. [Google Scholar] [CrossRef]
- Da Silva, J.M.C.; Uhl, C.; Murray, G. Plant Succession, Landscape Management, and the Ecology of Frugivorous Birds in Abandoned Amazonian Pastures. Conserv. Biol. 1996, 10, 491–543. [Google Scholar] [CrossRef]
- Muscarella, R.; Fleming, T.H. The role of frugivorous bats in tropical forest succession. Biol. Rev. 2007, 82, 573–590. [Google Scholar] [CrossRef]
- Reid, J.L.; Holl, K.D.; Zahawi, R.A. Seed dispersal limitations shift over time in tropical forest restoration. Ecol. Appl. 2015, 25, 1072–1082. [Google Scholar] [CrossRef] [Green Version]
- Aide, T.M.; Zimmerman, J.K.; Herrera, L.; Rosario, M.; Serrano, M. Forest recovery in abandoned tropical. pastures in Puerto Rico. For. Ecol. Man. 1995, 77, 77–86. [Google Scholar] [CrossRef]
- Engel, V.L.; Parrotta, J.A. An evaluation of direct seeding for reforestation of degraded lands in central São Paulo state Brazil. For. Ecol. Man. 2001, 152, 169–181. [Google Scholar] [CrossRef]
- Zahawi, R.A.; Augspurger, C.K. Early plant succession in abandoned pastures in Ecuador. Biotropica 1999, 31, 540–552. [Google Scholar] [CrossRef]
- Aide, T.M.; Zimmerman, J.K.; Rosario, M.; Marcano, H. Forest recovery in abandoned cattle pastures along an elevational gradient in northeastern Puerto Rico. Biotropica 1996, 28, 537–548. [Google Scholar] [CrossRef]
- Uhl, C. Factors controlling succession following slash-and-burn agriculture in Amazonia. J. Ecol. 1987, 75, 377–407. [Google Scholar] [CrossRef]
- Lindell, C.A.; Reid, J.L.; Cole, R.J. Planting design effects on avian seed dispersers in a tropical forest restoration experiment. Rest. Ecol. 2013, 21, 515–522. [Google Scholar] [CrossRef]
- Nepstad, D.C.; Uhl, C.; Serrão, E.A.S. Surmounting barriers to forest regeneration in abandoned, highly degraded pastures: A case study from Paragominas, Pará, Brazil. In Alternatives to Deforestation: Steps toward Sustainable Use of the Amazon Rain Forest; Anderson, A.B., Ed.; Columbia University Press: New York, NY, USA, 1990; pp. 215–229. [Google Scholar]
- Nepstad, D.C.; Uhl, C.; Serrão, E.A.S. Restoration of degraded Amazonian landscape: Forest recovery and agricultural restoration. Ambio 1991, 20, 248–255. [Google Scholar]
- Zahawi, R.A.; Holl, K.D. Comparing the performance of tree stakes and seedlings to restore abandoned tropical pastures. Rest. Ecol. 2009, 17, 854–864. [Google Scholar] [CrossRef]
- Zahawi, R.A.; Holl, K.D.; Cole, R.J.; Reid, J.L. Testing applied nucleation as a strategy to facilitate tropical forest recovery. J. Appl. Ecol. 2013, 50, 88–96. [Google Scholar] [CrossRef]
- Hohnwald, S.; Rischkowsky, B.; Camarão, A.P.; Schultze-Kraft, R.; Rodrigues Filho, J.A.; King, J.M. Integrating cattle into the slash-and-burn cycle on smallholdings in the Eastern Amazon: Grass-capoeira pasture versus grass-legume pasture. Agric. Ecosyst. Environ. 2006, 117, 266–276. [Google Scholar] [CrossRef]
- Hohnwald, S.; Trautwein, J.; Camarão, A.P.; Wollny, C.B.A. Relative palatability and growth performance of capoeira species as supplementary forages in the NE-Amazon. Agric. Ecosyst. Environ. 2016, 218, 107–115. [Google Scholar] [CrossRef]
- Vieira, D.L.M.; Holl, K.D.; Peneireiro, F.M. Agro-Successional Restoration as a Strategy to Facilitate Tropical Forest Recovery. Rest. Ecol. 2009, 17, 451–459. [Google Scholar] [CrossRef]
- Hohnwald, S.; Rischkowsky, B.; Schultze-Kraft, R.; Rodrigues-Filho, J.A.; Camarão, A.P. Experiences with legumes as part of a ley pasture in a low input farming system of North-Eastern Pará, Brazil. Pasturas Trop. 2005, 27, 2–12. [Google Scholar]
- Baar, R.; dos Reis Cordeiro, M.; Denich, M.; Fölster, H. Floristic inventory of secondary vegetation in agricultural systems of East-Amazonia. Biodivers. Conserv. 2004, 13, 501–528. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Lima, R.A.F.; Gandolfi, S.; Nave, A. On the restoration of high diversity forests: 30 years of experience in the Brazilian Atlantic Forest. Biol. Conserv. 2009, 142, 1242–1251. [Google Scholar] [CrossRef]
- Brown, S.; Lugo, A.E. Tropical secondary forests. J. Trop. Ecol. 1990, 6, 1–32. [Google Scholar] [CrossRef]
- Denich, M.; Vielhauer, K.; Kato, M.D.A.; Block, A.; Kato, O.R.; de Sá Abreu, T.D.; Lücke, W.; Vlek, P.L.G. Mechanized land preparation in forest-based fallow systems: The experience from Eastern Amazonia. Agric. Syst. 2004, 61, 91–106. [Google Scholar] [CrossRef]
- Kato, M.S.A.; Kato, O.R.; Denich, M.; Vlek, P.L.G. Fire-free alternatives to slash and-burn for shifting cultivation in the eastern Amazon region: The role of fertilizers. Field Crops Res. 1999, 62, 225–237. [Google Scholar] [CrossRef]
- Sommer, R.; Vlek, P.L.G.; de Sá, T.D.A.; Vielhauer, K.; Rodrigues Coelho, R.F.; Fölster, H. Nutrient balance of shifting cultivation by burning or mulching in the Eastern Amazon—Evidence for subsoil nutrient accumulation. Nutr. Cycl. Agroecosyst. 2004, 68, 257–271. [Google Scholar] [CrossRef]
- Denich, M.; Vlek, P.L.G.; de Sá, T.D.A.; Vielhauer, K.; Lücke, W. A concept for the development of fire-free fallow management in the Eastern Amazon, Brazil. Agric. Ecosyst. Environ. 2005, 110, 43–58. [Google Scholar] [CrossRef]
- Hohnwald, S. Bird Records from the Rural Landscape of Igarapé-Açu Municipality, Northeastern Pará. Boletim do Museu Paraense Emílio Goeldi. Ciências Naturais 2009, 4, 119–131. [Google Scholar]
- Hohnwald, S.; Acioli de Abréu, E.; Krummel, T.; da Veiga, J.B.; Wollny, C.B.A.; Calandrini de Azevedo, C.M.B.; Gerold, G. Degraded Pasture Expansion and Woody Enrichment Strategies for Pasture Fertility Preservation in the Bragantina Region, North-Eastern Amazon. Erdkunde 2010, 64, 17–31. [Google Scholar] [CrossRef]
- Hohnwald, S.; Rischkowsky, B.; King, J.M.; Camarão, A.P.; Rodrigues Filho, J.A.; Zeppenfeld, T. Intensive Cattle Browsing Did Not Prevent Fallow Recuperation on Smallholder Grass-Capoeira Pastures in the NE-Amazon. Agric. Syst. 2015, 89, 813–828. [Google Scholar] [CrossRef]
- Staver, C. The Role of Weeds in the Productivity of Amazonian Bush Fallow Agriculture. Exp. Agric. 1991, 27, 287–304. [Google Scholar] [CrossRef]
- Dias-Filho, M.B. Germination and emergence of Stachytarpheta cayennensis and Ipomoea asarifolia. Planta Daninha 1996, 14, 118–126. [Google Scholar] [CrossRef]
- Krummel, T.; Hohnwald, S.; Gerold, G. Biologisch degradierte Rinderweiden und ihre agrarökologischen Regradationsmöglichkeiten für Kleinbauern in Nordost-Amazonien. Innsbr. Geogr. Stud. 2011, 38, 43–56. [Google Scholar]
- Vieira, I.C.G.; Uhl, C.; Nepstad, D.C. The role of shrub Cordia multispicata Cham. as a ‘succession facilitator’ in an abandoned pasture, Paragominas, Amazônia. Vegetatio 1994, 115, 91–99. [Google Scholar]
- Holl, K.D.; Loik, M.E.; Lin, E.H.V.; Samuels, I.A. Tropical Montane Forest Restoration in Costa Rica: Overcoming Barriers to Dispersal and Establishment. Rest. Ecol. 2000, 8, 339–349. [Google Scholar] [CrossRef]
- Reid, J.L.; Holl, K.D. Arrival ≠ Survival. Rest. Ecol. 2013, 21, 153–155. [Google Scholar] [CrossRef]
- Peña-Domene, M.; Martínez-Garza, C.; Palmas-Pérez, S.; Rivas-Alonso, E.; Howe, H.F. Roles of Birds and Bats in Early Tropical-Forest Restoration. PLoS ONE 2014, 9, e104656. [Google Scholar] [CrossRef]
- Posada, J.M.; Aide, T.M.; Cavalier, J. Cattle and Weedy Shrubs as Restoration Tools of Tropical Montane Rainforest. Rest. Ecol. 2000, 8, 370–379. [Google Scholar] [CrossRef]
- Elledge, A.E.; McAlpine, C.A.; Murray, P.J.; Gordon, I.J. Modelling habitat preferences of feral pigs for rooting in lowland rainforest. Biol. Invasion 2013, 15, 1523–1535. [Google Scholar] [CrossRef]
- Nogueira-Filho, S.L.G.; Nogueira, S.S.C.; Fragoso, J.M.V. Ecological impacts of feral pigs in the Hawaiian Islands. Biodivers. Conserv. 2009, 18, 3677–3683. [Google Scholar] [CrossRef]
- Spatz, G.; Mueller-Dombois, D. Succession patterns after pig diggings in grassland communities on Mauna Loa, Hawaii. Phytocoenologia 1975, 3, 346–373. [Google Scholar]
- Censo Agropecuário 2006 and Produção da Pecuária Municipal-2014 of Igarapé-Açu; IBGE: Brasilia, Brazil, 2017. Available online: http://cod.ibge.gov.br/2358p (accessed on 15 December 2017).
- Pachêco, N.A.; Bastos, T.X. Boletim Agrometeorológico de 2007 para Igarape-Açu, PA. In Documentos 354; Embrapa Amazônia Oriental: Belém, Brazil, 2009. [Google Scholar]
- Pachêco, N.A.; Bastos, T.X. Boletim Agrometeorológico de 2008 para Igarape-Açu, PA. In Documentos 372; Embrapa Amazônia Oriental: Belém, Brazil, 2011. [Google Scholar]
- Baena, A.R.C.; Falesi, I.C.; Dutra, S. Características Físio-Químicas do Solo em Diferentes Agroecossistemas na Região Bragantina do Nordeste Paraense; Embrapa Amazônia Oriental: Belém, Brazil, 1998. [Google Scholar]
- R: A Language and Environment for Statistical Computing; R Core Team R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 17 January 2018).
- Oanda cooperation; Currency converter. 2019. Available online: https://www.oanda.com/currency/converter/ (accessed on 15 February 2019).
- Kotanen, P.M. Responses of vegetation to a changing regime of disturbance: Effects of feral pigs in a Californian coastal prairie. Ecography 1995, 18, 190–198. [Google Scholar] [CrossRef]
- Aplet, G.H.; Anderson, S.J.; Stone, C.P. Association between feral pig disturbance and the composition of some alien plant assemblages in Hawaii Volcanoes National Park. Plant Ecol. 1991, 95, 55–62. [Google Scholar] [CrossRef]
- Sharrow, S.H. Soil compaction by grazing livestock in silvopastures as evidenced by changes in soil physical properties. Agric. Syst. 2007, 71, 215–223. [Google Scholar] [CrossRef]
- Veloso, H.P. As communidades e as estações botânicas de Teresópolis, Estado do Rio de Janeiro. Boletim do Museu Nacional. Botânica. 1945, 3, 1–95. [Google Scholar]
- Albuquerque, N.I.; Guimarães, D.A.; Tavares Dias, H.L.; le Pendu, Y.; Reis Kahwage, P.; Rossetto Garcia, A. Intensive production system of collared peccary (Pecari tajacu) in Brazilian Amazon. Adv. Anim. Biosci. 2010, 1, 480–481. [Google Scholar] [CrossRef]
- Garnero, A.D.V.; Marcondes, C.R.; Albuquerque, N.I.; Araújo, R.O.; de Pendu, Y.; Guimarães, D.A. Growth curve of female collared peccaries (Pecari tajacu) raised in captivity in the Brazilian Amazon Region. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 2013, 65, 961–966. [Google Scholar] [CrossRef]
- Haines-Young, R.; Potschin-Young, M. Revision of the Common International Classification for Ecosystem Services (CICES V5. 1): A Policy Brief. One Ecosyst. 2018, 3, e27108. [Google Scholar] [CrossRef]
- Díaz, S.; Pascual, U.; Stenseke, M.; Martín-López, B.; Watson, R.T.; Molnár, Z.; Hill, R.; Chan, K.M.; Baste, I.A.; Brauman, K.A. Assessing nature’s contributions to people. Science 2018, 359, 270–272. [Google Scholar] [CrossRef]




| Species Name | Plant (Sub) Family | Life-Form |
|---|---|---|
| a) Spontaneous Legume Species | ||
| Zornia latifolia Sm. | Papilionoideae | herb |
| Mimosa pudica L. | Mimosoideae | herb |
| Stylosanthes gracilis Kunth | Papilionoideae | shrub |
| Senna chrysocarpa (Desv.) H.S. Irwin & Barneby | Caesalpinioideae | shrub |
| Mimosa quadrivalvis L. | Mimosoideae | herb |
| Machaerium madeirense Pittier | Papilionoideae | liana |
| Machaerium froesii Rudd | Papilionoideae | liana |
| Desmodium barbatum (L.) Benth. | Papilionoideae | herb |
| Desmodium canum (J.F. Gmel.) Schinz & Thell. | Papilionoideae | herb |
| Bauhinia guianensis Aubl. | Caesalpinioideae | liana |
| b) Capoeira | ||
| Vismia guianensis (Aubl.) Choisy | Clusiaceae | tree |
| Lacistema pubescens Mart. | Lacistemataceae | tree |
| Myrcia sylvatica (G. Mey.) DC. | Myrtaceae | shrub |
| Myrcia deflexa (Poir.) DC. | Myrtaceae | shrub |
| Myrcia bracteata (Rich.) DC. | Myrtaceae | shrub |
| Banara guianensis Aubl. | Connaraceae | tree |
| Lecythis lurida (Miers) S.A. Mori | Lecythidaceae | tree |
| Abarema cochleata (Willd.) Barneby & J.W. Grimes | Mimosoideae | tree |
| c) Juquira | ||
| Borreria verticillata (L.) G. Mey. | Rubiaceae | shrub |
| Myrciaria tenella (DC.) O. Berg | Myrtaceae | shrub |
| Borreria latifolia (Aubl.) K. Schum. | Rubiaceae | shrub |
| Stachytarpheta cayennensis (Rich.) M. Vahl | Verbenaceae | shrub |
| Paspalum maritimum Trin. | Poaceae | herb |
| Paspalum conjugatum P.J. Bergius | Poaceae | herb |
| Panicum pilosum Sw. | Poaceae | herb |
| Scleria pterota C. Presl | Cyperaceae | herb |
| Rourea ligulata Baker | Connaraceae | liana |
| Rolandra argentea Rottb. | Asteraceae | shrub |
| Rollinia exsucca (DC. ex Dunal) A. DC. | Annonaceae | tree |
| Hyptis atrorubens Poit. | Lamiaceae | herb |
| Imperata brasiliensis Trin. | Poaceae | herb |
| Emilia sonchifolia (L.) DC. | Asteraceae | herb |
| Ipomoea asarifolia (Desr.) Roem. & Schult. | Verbenaceae | liana |
| Borreria suaveolens G. Mey. | Rubiaceae | shrub |
| Andropogon bicornis L. | Poaceae | herb |
| Andropogon leucostachyus Kunth | Poaceae | herb |
| Capoeira Trees time1 [#] | Capoeira Trees time2 [#] | Mean Tree Heights time1 [m] | Mean Tree Heights time2 [m] | Capoeira Saplings time3 [#] | |
|---|---|---|---|---|---|
| n = 1440 | n = 1129 | n = 960 | |||
| trt1 | 5.84 (0.47) ab1 | 6.13 (0.54) a1 | 1.0 a1 (0.6) | 1.1 a1 (0.4) | 2.56 (2.8) a |
| trt2 | 7.07 (0.45) a1 | 6.42 (0.41) a1 | 1.3 b1 (0.7) | 1.3 b1 (0.6) | 2.64 (2.8) a |
| trt3 | 5.52 (0.40) b1 | 4.55 (0.45) b1 | 0.9 a1 (0.7) | 1.3 b2 (0.4) | 2.84 (3.0) a |
| trt4 | 6.52 (0.41) ab1 | 3.77 (0.29) b2 | 1.1 a1 (0.6) | 1.5 c2 (0.5) | 1.33 (1.8) b |
| Legume Cover time1 [%] | Legume Cover time2 [%] | |
|---|---|---|
| n = 1360 | ||
| trt1 | 6.9 a1 (11.0) | 14.4 a2 (19.4) |
| trt2 | 15.6 b1 (20.6) | 15.4 a1 (18.5) |
| trt3 | 20.3 bc1 (25.1) | 29.2 b2 (27.0) |
| trt4 | 24.0 c1 (28.4) | 14.4 a2 (19.7) |
| time1 | ||||
| Depths | trt1 | trt2 | trt3 | trt4 |
| 2.5 cm | 1.88 (0.13) a | 1.95 (0.13) a | 1.71 (0.14) a | 2.05 (0.14) a |
| 5 cm | 2.07 (0.13) ab | 2.21 (0.13) ab | 1.82 (0.15) a | 2.25 (0.12) b |
| 10 cm | 2.21 (0.13) ab | 2.37 (0.11) a | 1.90 (0.16) b | 2.43 (0.10) a |
| 15 cm | 2.18 (0.13) ab | 2.32 (0.16) ab | 1.90 (0.16) a | 2.40 (0.11) b |
| 20 cm | 2.12 (0.14) a | 2.24 (0.18) a | 1.68 (0.12) b | 1.98 (0.14) ab |
| 40 cm | 1.56 (0.09) a | 1.34 (0.17) a | 1.38 (0.11) a | 1.40 (0.05) a |
| time2 | ||||
| Depths | trt1 | trt2 | trt3 | trt4 |
| 2.5 cm | 2.95 (0.18) b | 3.06 (0.19) bc | 2.63 (0.19) bc | 2.44 (0.15) ac |
| 5 cm | 3.04 (0.15) cd | 3.51 (0.19) bcd | 3.43 (0.20) c | 2.84 (0.17) bd |
| 10 cm | 3.06 (0.14) c | 3.77 (0.15) c | 4.20 (0.18) c | 4.01 (0.19) c |
| 15 cm | 3.26 (0.15) c | 3.86 (0.17) c | 4.05 (0.25) c | 4.67 (0.14) c |
| 20 cm | 3.34 (0.20) c | 3.61 (0.19) c | 3.45 (0.27) c | 3.42 (0.29) c |
| 40 cm | 2.51 (0.16) b | 3.26 (0.22) ab | 4,29 (0.19) b | 4.21 (0.21) b |
| Number of Pigs | Mean Pig Weights [kg/pig] | Total Pig Weight before [kg/0.5 ha] | Total Pig Weight after [kg/0.5 ha] | Total Weight Gain [kg; %] | Economic Gain from Pigs [US$/ha] | |
|---|---|---|---|---|---|---|
| farm1 | 14 | 20.1 | 281 (20.0) | 572 (25.0) | 291; +104% | 727.50 |
| farm2 | 11 | 24.0 | 264 (17.3) | 467 (18.7) | 203; +77% | 507.50 |
| farm3 | 10 | 29.8 | 298 (15.5) | 624 (21.6) | 326; +109% | 815.00 |
| mean | 11.7 | 24.6 | 281.0 | 554.3 | 273.3 | 683.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hohnwald, S.; Kato, O.R.; Walentowski, H. Accelerating Capoeira Regeneration on Degraded Pastures in the Northeastern Amazon by the Use of Pigs or Cattle. Sustainability 2019, 11, 1729. https://doi.org/10.3390/su11061729
Hohnwald S, Kato OR, Walentowski H. Accelerating Capoeira Regeneration on Degraded Pastures in the Northeastern Amazon by the Use of Pigs or Cattle. Sustainability. 2019; 11(6):1729. https://doi.org/10.3390/su11061729
Chicago/Turabian StyleHohnwald, Stefan, Osvaldo Ryohei Kato, and Helge Walentowski. 2019. "Accelerating Capoeira Regeneration on Degraded Pastures in the Northeastern Amazon by the Use of Pigs or Cattle" Sustainability 11, no. 6: 1729. https://doi.org/10.3390/su11061729