Recent Progress in Perennial Buckwheat Development
Abstract
:1. Introduction
2. Taxonomy
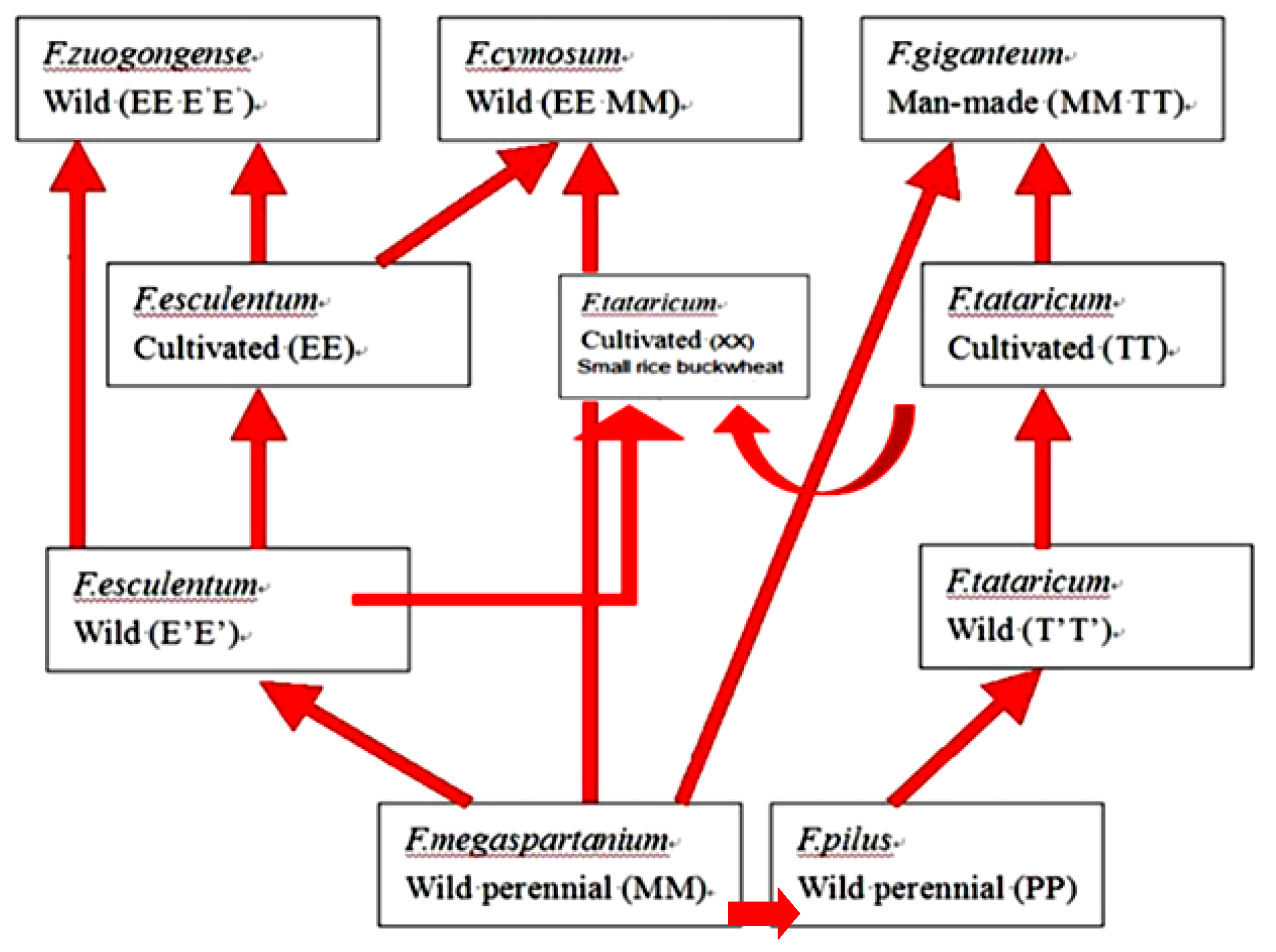
3. Genetics and Evolution
4. Natural Chemical Products and Pharmacological and Health Functions
5. Breeding of Perennial Buckwheat—the F.cymosum Complex
6. Product Development and Utilization
6.1. Food Products from Perennial Buckwheat Seeds
6.2. Perennial Buckwheat Drink
6.3. Golden Buckwheat Caudex Slices and Chewable Tablets
6.4. Commercial Medicines from Golden Buckwheat
6.5. Forage
7. Problems and Prospect
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, Q.F. (Ed.) Plant Sciences on Genus Fagopyrum; China Science Press: Beijing, China, 2012. [Google Scholar]
- Lin, R.F. (Ed.) Buckwheat in China; China Agricultural Science and Technology Press: Beijing, China, 1994. [Google Scholar]
- Liu, D.S.; Xu, R.Y.; Wang, Q.Q. Analysis of protein content and amino acid composition in buckwheat. Crop Germplasm Resour. 1997, 2, 26–28. [Google Scholar]
- Komori, A.; Naoto, I.; Kaori, F.; Shinya, K.; Asakazu, H. Measurement of rutin and quercetin in tartary buckwheat flour by ultraviolet-induced fluorescence. In Proceedings of the 10th International Symposium on Buckwheat, Yang Lin, China, 13–19 August 2007; pp. 403–409. [Google Scholar]
- Chen, Q.F. A study of development strategy about the agricultural production model of modern food crop. J. Mt. Agric. Biol. 2017, 36, 001–005. [Google Scholar]
- Chen, Q.F. A study of resources of Fagopyrum (Polygonaceae) native to China. Bot. J. Linn. Soc. 1999, 130, 53–64. [Google Scholar] [CrossRef]
- Chen, Q.F. Wide hybridization among Fagopyrum (Polygonaceae) species native to China. Bot. J. Linn. Soc. 1999, 131, 177–185. [Google Scholar] [CrossRef]
- Ohnishi, O.; Matsuoka, Y. Search for the wild ancestor of buckwheat. II. Taxonomy of Fagopyrum (Polygonaceae) species based on morphology, isozymes and cpDNA variability. Genes Genet. Syst. 1996, 71, 383–390. [Google Scholar] [CrossRef]
- Ohnishi, O. Discovery of the wild ancestor of common buckwheat. Fagopyrum 1991, 11, 5–10. [Google Scholar]
- Ohnishi, O. Search for the wild ancestor of buckwheat. I. Description of new Fagopyrum (polygonaceae) species and their distribution in China and the Himalayan hills. Fagopyrum 1998, 15, 18–28. [Google Scholar]
- Krotov, A.S.; Dranenko, E.T. An amphidiploid buckwheat, F. giganteum Krotov sp. nova. Byulletenl’ Vsesoyuznogo Ordena Lenina Instituta Rastenievodstva Imeni N.I. Vavilova 1973, 30, 41–45. [Google Scholar]
- Liu, J.N.; Tang, Y.; Xia, M.Z.; Shao, Q.R.; Cai, G.Z.; Luo, Q.; Sun, J.X. A new species of Chinese buckwheat (Polygonaceae)-Fagopyrum densovillosum Liu. Bull. Bot. Res. 2008, 28, 530–533. [Google Scholar]
- Liu, J.N.; Tang, Y.; Xia, M.Z.; Shao, Q.R.; Cai, G.Z.; Luo, Q.; Sun, J.X. A new species of Sichuan polygonaceae buckwheat in China-Fagopyrum crispatifolium Liu. J. Syst. Evol. 2008, 46, 929–932. [Google Scholar]
- Chen, Q.F. Recent progresses on interspecific crossbreeding of genus Fagopyrum Mill. In Proceedings of the 13th International Symposium on Buckwheat (ISB), Cheongju, Korea, 9–11 September 2016. [Google Scholar]
- Li, F.L.; Zeller, F.J.; Huang, K.F.; Shi, T.X.; Chen, Q.F. Improvement of fluorescent chromosome in situ PCR and its application in the phylogeny of the genus Fagopyrum Mill. using nuclear genes of chloroplast origin (cpDNA). Plant Syst. Evol. 2013, 299, 1679–1691. [Google Scholar] [CrossRef]
- Morris, M.R. Cytogenetic studies on buckwheat. J. Hered. 1951, 42, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.S.; Lin, R.F.; Li, Y.Q.; Niu, D.K. Studies of chromosome on different types of buckwheat. Chin. J. Cell Biol. 1984, 6, 130–131. [Google Scholar]
- Chen, Q.F. Karyotype analysis of five buckwheat species (Fagopyrum) native to China. Guihaia 2001, 21, 107–110. [Google Scholar]
- Chen, Q.F.; Hsam, S.L.K.; Zeller, F. A study of cytology, isozyme and interspecific hybridization on the big-achene group of buckwheat species (Fagopyrum, Polygonaceae). Crop Sci. 2004, 44, 1511–1518. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Chen, Q.F. The study of peroxidase isozyme in three leaf stages of genus Fagopyrum. J. Wuhan Bot. Res. 2008, 26, 213–217. [Google Scholar]
- Zhang, Y.Z.; Chen, Q.F. Study on the esterase isozyme of leaf of genus Fagopyrum. J. Wuhan Bot. Res. 2008, 26, 428–432. [Google Scholar]
- Zhang, Y.Z.; Chen, Q.F. The study of the isozyme of the glutamatic transaminase on the germplasm resources of genus Fagopyrum. Seeds 2008, 27, 39–46. [Google Scholar]
- Zhang, Y.Z.; Chen, Q.F. The study of seed peroxidase isozyme of germplasm resources of genus Fagopyrum. Guihaia 2008, 28, 553–557. [Google Scholar]
- Zhang, Y.Z.; Chen, Q.F. Studies on esterase isozyme of young leaves in wild buckwheat plants. Seeds 2009, 28, 41–43. [Google Scholar]
- Guo, Y.Z.; Chen, Q.F.; Yang, L.Y.; Huang, Y.H. Analyses of the seed protein contents on the cultivated and wild buckwheat resources. Genet. Resour. Crop Evol. 2007, 54, 1465–1472. [Google Scholar] [CrossRef]
- Li, J.H.; Chen, Q.F.; Zeller, F.J. Variation in seed protein subunits among species of the genus Fagopyrum Mill. Plant Syst. Evol. 2008, 273, 192–202. [Google Scholar] [CrossRef]
- Ohsako, T.; Ohnishi, O. Intra- and interspecific phylogeny of wild Fagopyrum (Polygonaceae) species based on nucleotide sequences of noncoding regions in chloroplast DNA. Am. J. Bot. 2000, 87, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.J.; Chen, Q.F. Study on RAPD of genus Fagopyrumresources. Seed 2009, 28, 37–48. [Google Scholar]
- Yang, X.Y.; Wu, Z.F.; Chen, H.; Shao, J.-R.; Wu, Q. Karyotype and genetics relationship based on RAPD markers of six wild buckwheat species (Fagopyrum ssp.) from southwest of China. Genet. Resour. Crop Evol. 2010, 57, 649–656. [Google Scholar] [CrossRef]
- Zhang, C.P.; He, P.; He, J.X.; Lei, S.T.; Hu, S.J. ISSR Analysis on genetic diveristy of Fagopyrum cymosum collected from eight wild populations. Chin. Tradit. Herb. Drugs 2010, 41, 1519–1523. [Google Scholar]
- Wu, C.S.; Wang, Y.N.; Liu, S.M.; Chaw, S.M. Chloroplast genome (cpDNA) of Cycastaitungensis and 56 cp protein-coding genes of gnetumparvifolium: Insights into cpDNA evolution and phylogeny of extant seed slants. Mol. Biol. Evol. 2007, 24, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yu, S.; Chen, Q.F. Sequence analysis of F 3′ 5′ H from Fagopyrum cymosum. Seed 2014, 33, 6–10. [Google Scholar]
- Li, G.; Yu, S.; Chen, Q.F. Molecular cloning and sequences analysis of TC from Fagopyrum cymosum. Seed 2015, 34, 1–4. [Google Scholar]
- Meng, H.; Li, C.L.; Wu, Q.; Shao, J.R.; Chen, H. Cloning and sequence analysis of the chalcone synthase gene (CHS) from Fagopyrum dibotrys. Acta Pratacult. Sin. 2010, 19, 162–169. [Google Scholar]
- Bu, X.X.; Luo, X.P.; Bai, Y.C.; Li, C.L.; Chen, H.; Wu, Q. Gene cloning of anthocyanin synthase in Fagopyrumdibotrys and correlation between its expression level and anthocyan in content. Chin. Tradit. Herb. Drugs 2014, 45, 985–989. [Google Scholar]
- Li, C.L.; Feng, Z.Y.; Bai, Y.C.; Chen, H.; Zhao, H.X.; Wu, Q. Molecular cloning and prokaryotic expression of phenylalanine ammonialyase gene FdPAL from Fagopyrum dibotrys. China J. Chin. Mater. Med. 2011, 36, 3238–3243. [Google Scholar]
- Liang, C.G.; Chen, Q.Q.; Shi, T.X.; Chen, Q.J.; Chen, Q.F. Sequence analysis of MAPK gene fragment and phylogenetic relationship of genus Fagopyrum. Acta Agric. Zhejiangensis 2016, 28, 1631–1636. [Google Scholar]
- Li, R.Y.; Pan, F.; Chen, Q.F.; Shi, T.X. Excavation and polymorphism analysis of EST-SSR from transcriptorne of tartary buckwheat. J. Agric. Sci. Technol. 2015, 17, 42–52. [Google Scholar]
- Yasui, Y.; Hirakawa, H.; Matsui, K.; Katsube-Tanaka, T.; Yang, S.J.; Aii, J.; Sato, S.; Mori, M. Construction of buckwheat genome database (BGDB). In Proceedings of the 13th International Symposium on Buckwheat (ISB), Cheongju, Korea, 9–11 September 2016; pp. 205–206. [Google Scholar]
- Zhang, L.J.; Li, X.X.; Ma, B.; Gao, Q.; Du, H.; Han, Y.; Li, Y.; Cao, Y.; Qi, M.; Zhu, Y.; et al. The tartary buckwheat genome provides Insights into rutin biosynthesis and abiotic stress tolerance. Mol. Plant 2017, 10, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.S.; Yi, F. A review of researches on chemical components and active substances of wild resource plant Fagopyrum cymosum (Trev.) Meism. J. Anhui Agric. Sci. 2013, 41, 3379–3381. [Google Scholar]
- Chen, X.F.; Gu, Z.L. Study of anti-tumor effect on Fagopyrum cymosum. Chin. Tradit. Herb. Drugs 2000, 31, 715–718. [Google Scholar]
- Liu, Y.L.; Fang, Q.N.; Zhang, X.Q.; Feng, X.X.; Zhang, L.F.; He, X.W. Studies on the active ingredients of Fagopyrum cymosum. Chin. Pharm. J. 1980, 15, 40–41. [Google Scholar]
- Zhang, W.J.; Li, X.C.; Liu, Y.Q.; Yao, R.C.; Nonaka, G.I. Phenolic constituents from Fagopyrum dibotrys. Acta Botanica Yunnanica. 1994, 16, 354–356. [Google Scholar]
- Shao, M.; Yang, Y.H.; Gao, H.Y.; Wu, B.; Wang, L.B.; Wu, L.J. Chemical constituents from Elsholtzia blanda. China J. Chin. Mater. Med. 2005, 30, 1591–1593. [Google Scholar]
- Shao, M.; Yang, Y.H.; Gao, H.Y.; Wu, B.; Wang, L.B. Study on the chemical constituents of Fagopyrum cymosum. J. Shenyang Pharm. Univ. 2005, 22, 100–102. [Google Scholar]
- Wang, K.J.; Zhang, Y.J.; Yang, C.R. Antioxidant phenolic constituents from Fagopyrum dibotrys. J. Ethnopharmacol. 2005, 99, 259. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Z.; Zhou, J.Y.; Pan, H.L. Study on bacteriostasis of Fagopyrum cymosum Meisn. Chin. J. Microecol. 2005, 8, 330–331. [Google Scholar]
- Wu, H.Z.; Zhou, J.Y.; Pan, H.L. Study on chemical constituents of Fagopyrum dibotrys (D.Don) Hara. Chin. Hosp. Pharm. J. 2008, 28, 1829–1831. [Google Scholar]
- Bai, Z.Z.; Sun, H.; Cao, F.; Mu, F.K. Analysis of volatile oil from Fagopyrum dibotrys (D.Don) Hara. by GC/MS. Chin. J. Pharm. Anal. 2007, 11, 1832–1835. [Google Scholar]
- Huang, X.Y.; Wang, J.Y.; Chen, Q.F. Golden buckwheat (Fagopyrum cymosum) leaf tea function and mechanism of resistance to type II diabetes. Lishizhen Med. Mater. Med. Res. 2014, 6, 1334–1337. [Google Scholar]
- Huang, X.Y.; Huang, S.; Chen, Q.F. Antineoplastic function and mechanism of golden buckwheat leaf fermented tea in the mice of H22 tumor. J. Anhui Agric. Univ. 2015, 42, 854–859. [Google Scholar]
- Huang, X.Y.; Huang, S.; Chen, Q.F. Antineoplastic function and mechanism of golden buckwheat leaf fermented tea. Mod. Tradit. Chin. Med. Mater. Med. World Sci. Technol. 2015, 5, 981–984. [Google Scholar]
- Huang, S.; Wang, J.Y.; Chen, Q.F.; Huang, X.Y. Antioxidant activity of the active components of the buckwheat leaf tea. Food Oil 2016, 2, 30–32. [Google Scholar]
- Minami, M.; Gomi, M.; Ujihara, A. Pollen tube growth in interspecific crosses in Fagopyrum. J. Fac. Agric. Shinshu Univ. 1992, 29, 129–135. [Google Scholar]
- Hirose, T.; Ujihara, A.; Kitayashi, H.; Minami, M. Interspecific cross-compatibility in Fagopyrum according to pollen tube growth. Breed. Sci. 1994, 44, 307–314. [Google Scholar] [CrossRef]
- Hirose, T.; Ujihara, A.; Kitayashi, H.; Minami, M. Pollen tube behaviour related to self-incompatibility in interspecific crosses of Fagopyrum. Breed. Sci. 1995, 45, 65–70. [Google Scholar]
- Kachonpadungkitti, Y.; Mangkita, W.; Romchatngoen, S.; Hasegawa, K.; Hisajima, S. Possibility of cross breeding of buckwheat (Fagopyrum esculentum Moench) in vitro. Shokubutsu Kojo Gakkaishi 2003, 15, 98–101. [Google Scholar] [CrossRef]
- Ujihara, A.Y.; Nakamura, Y.; Minami, M. Interspecific hybridization in genus Fagopyrum. Properties of hybrids (F. esculentum × F. cymosum) through ovule culture. Gamma Field Symp. 1990, 29, 33–45. [Google Scholar]
- Samimy, C. Barrier to interspecific crossing of Fagopyrum esculentum with F. tataricum. I. Site of pollen tube arrest. II. Organogenesis from immature embryos of F. tataricum. Euphytica 1991, 54, 215–219. [Google Scholar]
- Lee, B.S.; Ujihara, A.; Minami, M.; Hirose, T. Breeding of interspecific hybrids in genus Fagopyrum. 4. Production of interspecific hybrid ovules culture among F. esculentum, F. tataricum and F. cymosum. Breed. Sci. 1994, 44 (Suppl. 1), 183. [Google Scholar]
- Nóra, M.D.; Andrew, J.C.; Judit, D. Progress and prospects for interspecific hybridization in buckwheat and the genus Fagopyrum. Biotechnol. Adv. 2013, 31, 1768–1775. [Google Scholar]
- Feng, X.Y.; Chen, Q.F. Determination of total flavones content in different organs of F. megaspartanium. Guizhou Agric. Sci. 2007, 35, 15–16. [Google Scholar]
- Liu, N.; Zeller, F.J.; Chen, Q.F. The flavonoid content in leaves and inflorescences of the wild perennial Fagopyrum cymosum complex. Genet. Resour. Crop Evol. 2013, 60, 825–838. [Google Scholar] [CrossRef]
- Li, G.; Yu, S.; Zhou, Y.H.; Chen, Q.F. Spectrophotometric determination of flavonoids content in leaves of Fagopyrum cymosum complex. Asian J. Chem. 2013, 25, 7575–7578. [Google Scholar]
- Li, G.; Yu, S.; Zhou, Y.H.; Chen, Q.F. Analysis of the antioxidant related substances in leaves of the Fagopyrum cymosum complex and common buckwheat plants. Guangdong Agric. Sci. 2013, 40, 7–11. [Google Scholar]
- Li, G.; Yu, S.; Chen, Q.F. Research status of antioxidative substances from buckwheat. Ningxia J. Agric. For. Sci. Technol. 2014, 2, 87–89. [Google Scholar]
- Li, G.; Yu, S.; Deng, Y.; Zhou, Y.H.; Chen, Q.F. Study on extraction technology of flavonoids from Golden Buckwheat leaves. Jiangsu Agric. Sci. 2013, 41, 264–266. [Google Scholar]
- Shi, T.X.; Gu, L.L.; Chen, Z.L.; Chen, Q.F. Analysis of flavonoids, soluble protein and soluble sugar content in leaves of Fagopyrum tataricum. Jiangsu Agric. Sci. 2014, 42, 252–255. [Google Scholar]
- Huang, M.J.; Tang, H.M. The preliminary study of Fagopyrum dibotrys scented tea’s manufacture crafts. Food Res. Dev. 2015, 6, 53–56. [Google Scholar]
- Gu, L.L.; Chen, Q.F. A study on brewing condition of golden buckwheat functional leaf tea. Food Res. Dev. 2014, 3, 59–64. [Google Scholar]
- Gu, L.G.; Huang, X.Y.; Li, Y.; Chen, Q.F. Production technology optimization on fermented tea of golden buckwheat. Guizhou Agric. Sci. 2014, 3, 59–64. [Google Scholar]
- Gu, L.L.; Chen, Q.F. Variation of flavonoid and GABA content in leaves among different golden buckwheat accessions. Southwest China J. Agric. Sci. 2014, 27, 582–586. [Google Scholar]
- Liu, N.; Chen, Q.F. Research on the making technology of golden buckwheat green tea. J. Yunnan Agric. Univ. (Nat. Sci.) 2008, 23, 76–79. [Google Scholar]
- He, M.S.; Qian, B.H.; Wang, Z.L.; Wang, Z.Y.; Yan, Y.M. Quality standard for Jinqiaomai Tablets. Chin. Tradit. Pat. Med. 2010, 32, 779–782. [Google Scholar]
- Hu, T.; Huang, K.F.; Huang, X.Y.; Chen, Q.F. Preparation process of chewable tablet of a fermented tea from golden buckwheat (Fagopyrum cymosum) comeplex leaf. J. Anhui Agric. Sci. 2017, 44, 27–32. [Google Scholar]
- Xu, Y.; Tong, Y.H.; Qiu, H.G. Preparation of buckwheat capsule and quality standards. Lishizhen Med. Mater. Med. Res. 2004, 15, 753–754. [Google Scholar]
- Huang, J.W.; Zhao, D. Analysis of the efficacy of Weimaining capsule combined with chemotherapy in the advanced non-small cell lung cancer. Asia-Pac. Tradit. Med. 2015, 11, 125–126. [Google Scholar]
- Zhang, M.; Gao, Z.; Yang, Q.J.; Qian, Y.; Li, D.; Tan, G.F.; Zhang, Y. Clinical observation of Weimaining capsule combined with bronchial artery infusion chemotherapy in the treatment of advanced non-small cell lung cancer. Chin. J. Clin. Oncol. Rehabilit. 2013, 10, 1114–1116. [Google Scholar]
- Zhao, S.Q.; Xu, C.Q.; Chen, X.H. Effect of Weimaining capsule in treating non-small cell lung cancer and its effect on serum epidermal growth factor receptor. Chin. J. Gerontol. 2014, 18, 5070–5071. [Google Scholar]
- Tao, Z.Q.; Gao, X.; Tang, Y.F.; Sun, J.; Zhang, Y.; You, J.S.; Ni, W.B.; Yu, H.Z. Effect of buckwheat liquid on serum cytokines and lung function in patients with chronic obstructive pulmonary disease. Liaoning J. Tradit. Chin. Med. 2008, 35, 332–333. [Google Scholar]
- He, Y.K.; Zhao, W.; Ma, J.; Mian, J.Y.; Cheng, N.; Deng, L.S.; Zhao, M.Y. Optimization of high yield cultivation technique in Fagopyrum dibotrys. Guizhou Agric. Sci. 2016, 44, 52–55. [Google Scholar]
- Deng, R.; Zhang, D.H.; Wang, A.N.; Xiang, Q.H.; Luo, Q.H.; Zhang, L. Study on silage experiment of Golden buckwheat. Caoye Yu Xumu 2012, 9, 5–7. [Google Scholar]
- Xu, L.X.; Xiong, K.N.; Yang, S.M.; Guo, W.; Liu, K.X. The effect of quality of lactobacillus and sucrose on the silage of golden buckwheat. Anim. Husb. Vet. Med. 2016, 48, 54–59. [Google Scholar]
- Zhang, J.; Deng, R.; Xu, Z.H.; Li, L.N.; Zhang, D.H. Gaining effect of bean zangxiang with fresh stem leaves of Qian Fagopyrum dibotrys. Swine Prod. 2017, 3, 17–18. [Google Scholar]
- Ruan, Y.; Ji, X.Q.; Xia, X.L.; Wen, M.; Li, S.J.; Liu, Y.J.; Chen, J.Q. Effect of golden buckwheat on the immunity effect of duck plague seedlings. Heilongjiang Anim. Sci. Vet. Med. 2015, 10, 203–205. [Google Scholar]
- Ruan, Y.; Ji, X.Q.; Xia, X.L.; Wen, M.; Li, S.J.; Liu, T.J.; Chen, J.Q. Effects of Fago-con immune effects of avian influenza vaccine for ducks. Guangdong Agric. Sci. 2015, 13, 119–122. [Google Scholar]










| Section | Species | Key Distribution | Annual/Perennial | Ploidy | Genome | Properties |
|---|---|---|---|---|---|---|
| Big-achene group | 1. Common buckwheat, F.esculentum Moench | Worldwide | Annual | 2x = 16, 4x = 32 | 2x = 16, EE 4x = 32, EEEE | Big flower and achene, cross-pollination |
| 2. Tartary buckwheat, F.tataricum (Linnaeus) Gaertner | Southwest China | Annual | 2x = 16, 4x = 32 | 2x = 16, TT; 4x = 32, TTTT | Small green flower and bigger achene, self-pollination | |
| 3. F.megaspartanium Chen | South China | Perennial | 2x = 16 | 2x = 16, MM | F.cymosum complex. Big flower and achene, cross-pollination, bulbous caudex | |
| 4. F.pilus Chen | Tibet, China | Perennial | 2x = 16, | 2x = 16, PP | F.cymosum complex. Bigger flower and achene, cross-pollination, bulbous caudex | |
| 5. F.cymosum (Trev.) Meisner | Southwest China | Perennial | 4x = 32 | 4x = 32, MMXX | F.cymosum complex. Bigger flower and achene, cross-pollination, with subsurface transverse stem | |
| 6. F.zengongense QF Chen | Tibet, China | Annual | 4x = 32 | 4x = 32, EEE’E’ | Bigger flower and achene, self or cross-pollination | |
| 7. F.giganteum Krotov | Man-made | Annual | 4x = 32 | 4x = 32, TTXX | Bigger flower and big achene, cross or self-pollination | |
| 8. F.tatari-cymosum Chen | Man-made | Perennial | 4x = 32 | 4x = 32, TTXX | Bigger flower and big achene, self-pollination | |
| Small-achene group | 1. F.gracilipes (Hemsley) Dammer ex Diels | Southwest China | Annual | 4x = 32 | / | small flower and achene, self-pollination |
| 2. F.leptopodum (Diels) Hedberg | Southwest China | Annual | 2x = 16 | / | small flower and achene, cross or self-pollination | |
| 3. F.gilesii (Hemsley) Hedberg | Southwest China | Annual | 2x = 16 | / | small flower and achene, cross or self-pollination | |
| 4. F.lineare (Samuelsson) Haraldson | Yunnan, China | Annual | 2x = 16 | / | small flower and achene, cross or self-pollination | |
| 5. F.urophyllum (Bureau et Franch) Gross | Southwest China | Perennial | 2x = 16 | / | small flower and achene, cross pollination, perennial sub-shrubs | |
| 6. F.statice (Lev.) Gross | Southwest China | Perennial | 2x = 16 | / | small flower and achene, cross pollination, perennial herbs | |
| 7. F.caudatum (Samuelsson) Li | Southwest China | Annual | / | / | small flower and achene, cross or self-pollination | |
| 8. F.pleioramosum Ohnishi | Southwest China | Annual | 2x = 16 | / | small flower and achene, cross or self-pollination | |
| 9. F.capillatum Ohnishi | Southwest China | Annual | / | / | small flower and achene, cross or self-pollination | |
| 10. F.calliansum Ohnishi | Southwest China | Annual | / | / | small flower and achene, cross or self-pollination | |
| 11. F.macrocarpum Ohsako & Ohnishi | Southwest China | Annual | 2x = 16 | / | small flower and achene, cross or self-pollination | |
| 12. F.gracilipedoides Ohsako& Ohnishi | Southwest China | Annual | / | / | small flower and achene, cross or self-pollination | |
| 13. F.jinshaense Ohsako& Ohnishi | Southwest China | Annual | / | / | small flower and achene, cross or self-pollination | |
| 14. F.rubifolium Ohsako& Ohnishi | Southwest China | Annual | / | / | small flower and achene, cross or self-pollination | |
| 15. F.crispatifolium Liu | Southwest China | Annual | / | / | small flower and achene, cross or self-pollination | |
| 16. F.densiovillosumLiu | Southwest China | Annual | 16 | / | small flower and achene, cross or self-pollination |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.-F.; Huang, X.-Y.; Li, H.-Y.; Yang, L.-J.; Cui, Y.-S. Recent Progress in Perennial Buckwheat Development. Sustainability 2018, 10, 536. https://doi.org/10.3390/su10020536
Chen Q-F, Huang X-Y, Li H-Y, Yang L-J, Cui Y-S. Recent Progress in Perennial Buckwheat Development. Sustainability. 2018; 10(2):536. https://doi.org/10.3390/su10020536
Chicago/Turabian StyleChen, Qing-Fu, Xiao-Yan Huang, Hong-You Li, Li-Juan Yang, and Ya-Song Cui. 2018. "Recent Progress in Perennial Buckwheat Development" Sustainability 10, no. 2: 536. https://doi.org/10.3390/su10020536





