1. Introduction
Grain sorghum (
Sorghum bicolor) ranks fifth among the world’s cereal crops in area sown. As with all major cereals, sorghum cultivars currently in production all have an annual growth habit. Recent efforts to develop perennial grain sorghum germplasm are motivated by perennial crops’ potential for preventing and reversing soil degradation [
1,
2]. In the case of sorghum, such benefits could be realized across highly diverse landscapes, from the U.S. central plains, where sorghum grain is used primarily for livestock feed and biofuel production, to large regions of Africa and South Asia, where sorghum is grown primarily by smallholder farm families, with the grain used primarily for human consumption and vegetative material used to feed livestock.
The process of breeding perennial grain sorghum begins with controlled hybridization between annual grain sorghum and either of two wild perennial grasses,
S. halepense or
S. propinquum. The diploid (2
n = 20) species
S. bicolor and
S. propinquum diverged from a common ancestor approximately 1 to 2 million years BP [
3,
4].
Sorghum halepense was the result of later natural hybridization between
S. bicolor and
S. propinquum followed by a spontaneous doubling of the chromosome number to 40.
A highly invasive species,
S. halepense spread widely across the Eastern hemisphere, and, in the post-Columbian era, the Americas, while
S. propinquum is today confined to Southeast Asia [
3,
4]. Approximately 8000 year BP people living in the Sahel region domesticated
S. bicolor to produce grain sorghum [
5], and the grain eventually came to be distributed throughout Africa and beyond.
Grain sorghum is typically grown as an annual crop, producing a single harvest. However, like all grasses, grain sorghum can generate branches called tillers that sprout from the base of the plant near the soil surface, and most sorghum plants are able, under the right conditions, to sprout new tillers even after the original shoots have elongated, produced mature seed, and senesced. In tropical or semitropical environments, late tillering creates the potential for a second, or ratoon, crop of grain to be produced. The grain harvest from the ratoon stand, however, is much smaller than from the originally sown stand [
6], so ratooning of grain sorghum is infrequently practiced. Survival and productivity of a ratoon crop depends on an adequate supply of soil moisture during the ratoon growing season, which may extend well beyond the rainy season. Ratoon stands are not known to be maintained for more than two seasons.
Perennial
Sorghum species produce not only tillers but also basal subterranean stems known as rhizomes. Starchy and punctuated by meristematic buds, rhizomes extend downward and laterally into the soil. A new growing season begins when the rhizome buds sprout in response to temperature, moisture, or other signals. Drawing on the rhizome’s energy reserves, the resulting new shoots, called ramets, emerge and grow rapidly as the rhizome dies and degrades. Plants emerging from rhizomes rapidly produce vigorous new root systems and grow faster and larger than tillers emerging from aboveground nodes. In experimental plots, second-season grain yields from rhizomatous sorghum were similar to first-season yields [
7].
Rhizomes are necessary if adequate harvests of sorghum grain are to be obtained over several to many seasons from a single sowing. However, wild perennial Sorghum species, the sources of genes for rhizome development, are poorly suited for agriculture. They produce large numbers of thin, flexible culms (stems) and small quantities of very small, hulled seeds that drop to the ground when ripe. In contrast, a perennial grain sorghum will need to produce large, threshable seeds on large panicles (seed heads) supported by robust culms. In breeding perennial grain sorghum, it is first necessary to bring genes from grain sorghum that confer good grain-production traits into a common pool with genes from wild perennial species for rhizome development. That is accomplished by hybridizing the former with the latter to produce populations of plants that carry genes from both parents in various combinations.
2. Establishment and Genetic Analysis of the Perennial Grain Sorghum Gene Pool
In regions where grain sorghum is cultivated, natural gene flow from
S. bicolor into
S. halepense is common [
8]. Controlled pollination of annual sorghum with
S. halepense,
S. propinquum, or
S. almum (a weedy perennial species produced by one or more spontaneous pollinations of the wild annual
S. arundinaceum by
S. halepense) has been used in the breeding of forage sorghums [
9,
10,
11]; however, hybridization for the purpose of developing perennial sorghum that produces grain for human consumption has been practiced only recently and not widely. Following publication of Jackson’s [
12] recommendation that a broad range of perennial grain crops, including perennial sorghum, be developed for use in food-producing polycultures, the first
S. bicolor ×
S. halepense hybridizations aimed at development of perennial grain sorghum were made by Kansas State University and The Land Institute (TLI) in Manhattan and Salina, Kansas, respectively, in the 1980s. The resulting populations were studied through the decade that followed [
13].
Meanwhile, at Texas A&M University, Paterson et al. [
14] conducted restriction fragment length polymorphism (RFLP) mapping of quantitative trait loci (QTL) that affect rhizome initiation and growth in a
S. bicolor ×
S. propinquum F
2 population, concluding that loci affecting those traits are scattered across nine of sorghum’s ten chromosome pairs. Comparative RFLP mapping showed correspondence between two major rhizome QTL in a rice (
Oryza sativa ×
O. longistaminata) population and rhizome QTL in
Sorghum [
15]. Results of those studies would later be built upon and extended by Kong et al. [
16], who used simple sequence repeat (SSR) markers to map rhizome QTL in a recombinant inbred line (RIL) population derived from the original
S. bicolor ×
S. propinquum F
2 population and Washburn et al. [
17], who mapped QTL for rhizome development and overwintering in an F
4 population from the
S. bicolor ×
S. propinquum cross.
Sorghum propinquum-derived populations hold potential for perennial sorghum development in the tropics and subtropics; however,
S. propinquum cannot survive winters in colder climates. Therefore, when TLI initiated a germplasm development program for perennial sorghum in 2002, parental sources of perenniality were limited to
S. halepense and
S. bicolor ×
S. halepense-derived perennial stocks that had been maintained in the years since Piper and Kulakow’s research [
7,
13].
Hybridization between tetraploid perennial
Sorghum plants and
S. bicolor is complicated by the difference in ploidy. Thirty-chromosome hybrid seeds produced by crosses between 20- and 40-chromosome sorghum parents are typically inviable; if a viable triploid hybrid is produced, it is generally highly sterile. Therefore, in TLI’s original
S. bicolor ×
S. halepense crosses colchicine-induced tetraploid (40-chromosome)
S. bicolor inbred lines were used as female parents [
13].
There is, however, a second route to producing
S. bicolor ×
S. halepense hybrids. Hadley [
18] demonstrated that predominantly tetraploid F
1 hybrids could be produced by fertilizing nuclear or cytoplasmic male-sterile
S. bicolor plants with
S. halepense pollen. This occurs because sorghum plants carrying nuclear or cytoplasmic/nuclear genes for male sterility produce, at a frequency of less than 1%, unreduced 20-chromosome female gametes. Interspecific hybrids produced in the TLI perennial sorghum program during the period 2002–2009 were all produced by pollinating male-sterile inbred lines. Since 2009, we have continued to use male-sterile parents but have also used several induced tetraploid sorghum lines as female parents. All perennial germplasm developed from these crosses to date has been tetraploid.
Perennial
Sorghum spp. have also been used in developing perennial germplasm for production of vegetative biomass. Perennial forage cultivars were released decades ago in India (‘Krish’ by [
19]) and Australia (‘Crooble’ by [
20]; ‘Silk’ by [
21]; and ‘Sucro’ by [
22]). More recently, Jessup et al. [
11] released the genetic stock PSH12TX09, a sorghum line perennial in the southern United States that is derived from a
S. bicolor ×
S. propinquum cross and is intended for use as a parent in breeding high-biomass cultivars to be used in production of forage or biofuel feedstock.
3. Procedure for Developing Perennial Grain Sorghum Germplasm
At TLI from 2002 to the present, a simple methodology for developing perennial grain sorghum has been directed at introgressing genes for rhizome production and cold-weather survival from the perennial gene pool into a germplasm pool that has grain-production characteristics more similar to those of cultivated S. bicolor. The steps of the introgression cycle are as follows: (a) cross S. bicolor cultivars or breeding lines to perennial sorghum plants (i.e., S. bicolorn × S. halepense plants that have survived for more than one year in the field) or to S. halepense; (b) self-pollinate the F1 hybrids to produce large, diverse segregating populations of plants; (c) evaluate F2 populations and later-generation families in replicated field trials; (d) harvest seed for progeny testing from rhizomatous plants and families that have exhibited improved agronomic phenotypes, grain yield, and seed size; (e) leave evaluation plots undisturbed through the winter and dig the rhizomes of selected plants or families that emerge in spring, basing selection on the previous season’s data and field notes; (f) transplant the selected perennial plants into a crossing nursery and use them to pollinate S. bicolor parents; (g) go back to step b.
Note that this is not a closed cycle. When progeny of rhizomatous, but not necessarily cold-hardy, plants exit the cycle at step d, they can move into one of two streams: further testing on TLI’s experiment station or field testing by cooperating institutions in warmer climates. Rhizomatous lines that may not be able to survive winters in Kansas may be strongly perennial in the southeastern United States or in the tropics. Such populations have so far been field-tested in southern Texas, southern Alabama, northern Georgia, southern Florida, Puerto Rico, Uganda (
Figure 1), Mali, Kenya, the Republic of South Africa, Indonesia, and southwest China.
Starting in 2014–2015, many F
1 and F
2 plants with phenotypes very different from those observed in previous years began appearing in TLI nurseries. In previous experience, all F
1 hybrids and F
2 populations from annual × perennial
Sorghum crosses had phenotypes more similar to the perennial parent than to the
S. bicolor parent: more profuse tillering and branching, longer panicle branches, thinner culms, greater height, smaller seed, and more tenacious glumes. In contrast, the new, anomalous hybrid plants (comprising 129 of the 165 interploidy hybrids produced in 2013) were closer in phenotype to
S. bicolor. Further investigation showed that all of the 129 anomalous hybrids were not tetraploid as would be expected, but were in fact diploid [
23]. Only a single such diploid product of diploid × tetraploid
Sorghum hybridization had been reported previously [
24]. The timing and mechanism of chromosome elimination that leads to such hybrids is still under investigation; nevertheless, if perennial diploid plants can be selected from
S. bicolorn ×
S. halepense populations, complications of interploid hybridization and tetraploid segregation can be avoided, thereby streamlining perennial sorghum development.
4. Experimental Results and Progress in Germplasm Development
New crosses are made and newly generated F
1 hybrids are fed into the selection-backcrossing cycle each year. Populations currently under evaluation are derived from zero, one, two, three or four backcrosses to
S. bicolor. A three-year field trial, conducted by Nabukalu and Cox [
7] in 2011–2013, compared 27 perennial families from three stages in the germplasm development program: 2002 (when perennial plants were selected from Piper and Kulakow’s [
13] original population for use as parents), 2006 (when approximately 300 perennial plants were selected from crosses between the 2002 selections and
S. bicolor), and 2009 (after another turn of the selection cycle).
This retrospective study showed that grain yield and kernel weight had been improved by backcrossing and selection. Mean grain yield of the 2006 selections was 81% greater than that of the 2002 parental set (which was, in turn 120% greater than the yield of a S. halepense check entry). The mean yield of the 2009 selections was another 21% above that of the 2006 group, and the highest-yielding line in the 2009 group exceeded the 2006 mean by 73%. The highest-yielding experimental line overall, which belonged each year to the 2009 group, had mean yield that was 54% of the yield of a commercial hybrid grain sorghum check. Seed size did not improve as rapidly. The mean weight of individual kernels in the 2009 group, at 8.9 mg, was 150% greater than that of S. halepense but only 25% above the 2002 group mean. Dividing the mean seed yield of the 27 perennial lines by their mean single-seed weight to obtain a rough estimate of numbers of seeds produced, we concluded that the 2009 selections and the commercial hybrid check produced similar number of seeds per unit land area; the difference in their yields was entirely due to differences in weights of individual seeds.
Strength of perenniality, while not as strong as that of
S. halepense in the 2002 germplasm, did not decline over selection cycles and remained adequate. Most experimental entries were not as strongly rhizomatous as the
S. halepense check, but they were rhizomatous enough to be perennial. Mimicking
S. halepense and committing up to 70% of its photosynthetic resources to rhizome growth [
4] would be an unnecessary and unproductive waste of resources for a perennial grain sorghum. Despite past predictions that perennial crops will have lower grain yields than annual counterparts (a concern that has been allayed by DeHaan et al. [
25]), there was no significant association between strength of perenniality and grain yield in the 2011–2013 experiments. The 2009 perennial selections did, however, continue to display other traits of the wild progenitor that are undesirable: excessive tillering and branching throughout the growing season that resulted in non-synchronous flowering and maturity and waste of resources; excessive plant height; and small, hulled, brown kernels. Some trait associations, such as between rhizomatousness and aboveground branching, have a logical anatomical basis, and QTL mapping has shown that some chromosomal regions in
S. propinquum increase both rhizome development and tillering and/or axillary branching; however, two other QTL were found to affect rhizomes only [
16]. In a study of TLI perennial sorghum germplasm conducted by Nakasagga [
26] in Uganda, rhizome number had statistically significant but not strong positive correlations with tiller number (0.35), head number (0.37), and plant height (0.27), suggesting that it may be possible to select rhizomatous plants that do not have excessive vegetative growth. Other undesirable trait associations in the pre-2009 populations, such as between winter survival ability and small-seededness, had no obvious anatomical or physiological explanation; they may have resulted from tight genetic linkage, which, we anticipated, could be broken.
Backcrossing and selection have continued in the years since 2009. Further retrospective studies have not been done, but based on phenotypes of selected plants and data collected since 2016, there is reason to expect that frequencies of “domestication” alleles continue to increase in the perennial gene pool. Although excessive aboveground branching remains a problem, perennial plants with shorter stature; fewer, thicker culms; broader leaves; larger, more compact panicles; and most importantly, larger seeds are becoming more frequent.
To be suitable for human food, sorghum grain should be relatively large, with a white seed coat. All of the 2002–2009 winter-hardy selections evaluated in 2011–2013 [
7] produced brown seed (which have not been tested for tannins), with mean individual seed weights ranging from 5.8 to 9.9 mg, compared with weights of 23 to 28 mg for
S. bicolor checks. These weights were typical; of approximately 2000 winter-hardy plants selected and grown to maturity during the years 2006 through 2013, none had individual seed weights exceeding 10 mg. Seed sizes have recently improved, however. Among more than 650 surviving plants selected from nurseries in spring 2016, more than 40 had seed weights exceeding 10 mg; 24 had seeds larger than 14 mg, 11 larger than 16 mg, and four larger than 18 mg.
Perennial populations under selection by the TLI grain sorghum program have also been evaluated for biomass production. Among 97 perennial lines evaluated in a trial in southern Italy, ten had higher biomass yield than a commercial forage-type check hybrid, and three exceeded the yield of a sterile biomass-type check hybrid; fiber concentrations in the perennial lines were either superior or comparable to those of the check hybrids [
27].
A set of 196 post-2009 rhizomatous TLI selections were field-tested at two locations over two growing seasons in Uganda in 2015 [
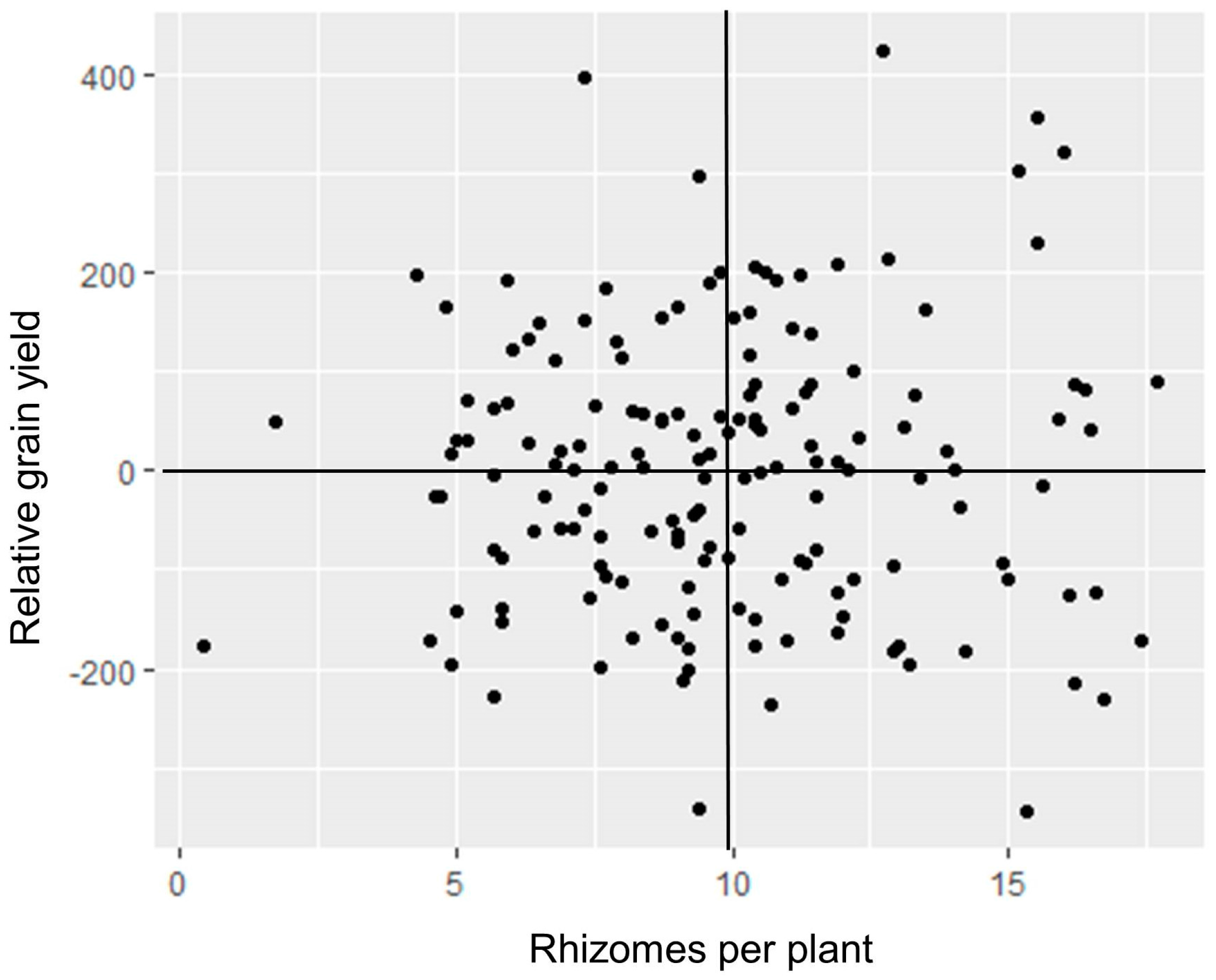
28]. The replicated experiment was sown in April, and harvest was completed by August. Plants were allowed to regrow in September after a short dry season, with a second harvest in December. Plants produced 88% more rhizomes in the second (regrowth) season than in the first season, and rhizomes spread further from the base of the plant. Forty-seven percent of families were strongly rhizomatous in the first season, and that share rose to 91% in the second season, as many of the weakly rhizomatous plants did not survive. Heavy late-season disease incidence prevented collection of reliable grain yield data; however, correlations between rhizome numbers of 164 of the families tested in Uganda and grain yields of the same set of families in 2015 replicated trials in Kansas were close to zero, with 24% of the families above the mean for both traits (
Figure 2).
In the Uganda study, some regrowth came from elongated rhizomes and other came from extremely short rhizomes growing downward from the base of the plant and then turning upward in many plants. Such shoots developed into more vigorous adult plants than did ratoon shoots from aboveground tillers [
27]. This type of regrowth has been demonstrated more extensively in another tropical crop, rice. A recently developed cultivar of perennial rice (
O. sativa ×
O. longistaminata), PR23 [
29], regrows from belowground nodes and consequently does not experience the yield decline characteristic of ratoon crops. In trials of 22 perennial rice lines maintained through four growing seasons in southern China and Laos, PR23 had the highest mean grain yield and consistently high regrowth [
29].
5. Future Lines of Research
The backcross–selection–backcross cycle is continuing at TLI, with heightened emphasis on increasing seed size and reducing branching while retaining perenniality. Output of rhizomatous selections will continue in two streams: lines that exhibit strong winter survival and lines that express perenniality only under warm temperate or tropical conditions.
The Uganda field studies suggested no impediments to selecting for perenniality in sorghum populations under tropical conditions. However, perennial germplasm that has been developed under very different temperate conditions is poorly adapted to tropical photoperiods, pests, and pathogens. In East Africa, high-latitude germplasm will be useful primarily in crosses with locally adapted germplasm to produce populations from which perennial lines can be selected [
29]. An interim outcome in developing a tropical perennial sorghum would be an improved ratooning grain sorghum. Aims would be for more vigorous regrowth and higher yields in the second (and possibly subsequent) season than are achieved by current annual cultivars when ratooned [
30].
Meanwhile, analyses of phenotypic and genomic data from approximately 250 BC
1F
1-derived lines of a
S. bicolor × (
S. bicolor ×
S. halepense) population studied during 2013–2017 in Georgia and Kansas [
31] are identifying genetic markers for perennial habit and other traits. Marker-assisted selection could increase the efficiency of the germplasm-development program, especially if it can be used in breaking possible tight linkage between loci governing rhizome development and loci affecting important agronomic traits such as seed size.
Introgression of
S. halepense germplasm into the grain sorghum gene pool may open up opportunities that go well beyond perenniality. The interspecific hybridity and polyploidy of
S. halepense, along with its dispersal and adaptation to an extraordinary variety of environments on every continent, suggests that its gene pool may harbor many alleles useful for improving
S. bicolor for a broad range of traits [
3,
4].
Diploid progeny recently produced by hybridization between diploid annual and tetraploid perennial parents could potentially be useful for developing diploid perennial sorghum for both temperate and tropical environments. More intensive genetic and cytological studies and more extensive field testing, however, are just beginning. Questions include the following: Does chromosome elimination occur during formation of gametes in the male parent or after fertilization? Are some chromosomes eliminated preferentially, and if so, which ones? Most importantly, do the diploid populations identified to date segregate for perenniality, and, if not, can perennial diploids be produced by hybridizing male-sterile inbred lines with the most strongly perennial S. halepense accessions?
Fifteen years of hybridization and selection have produced a large, diverse sorghum gene pool that segregates for perenniality and many other plant traits. Breeding will continue, aimed at simultaneously increasing the frequencies of gene complexes conferring perenniality and those conferring high productivity and improved human-food quality. Evaluation in a broader range of environments and development of a marker-assisted selection system will be emphasized.