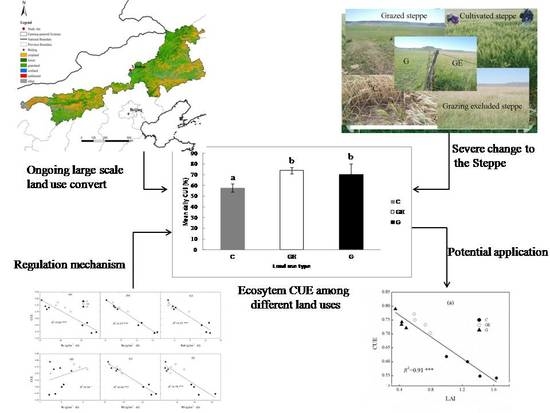
Comparison of Carbon-Use Efficiency Among Different Land-Use Patterns of the Temperate Steppe in the Northern China Pastoral Farming Ecotone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Community Characteristics and Composition of Biomass
2.4. Flux Measurements
2.5. Ecosystem CUE
2.6. Data Analysis
3. Results
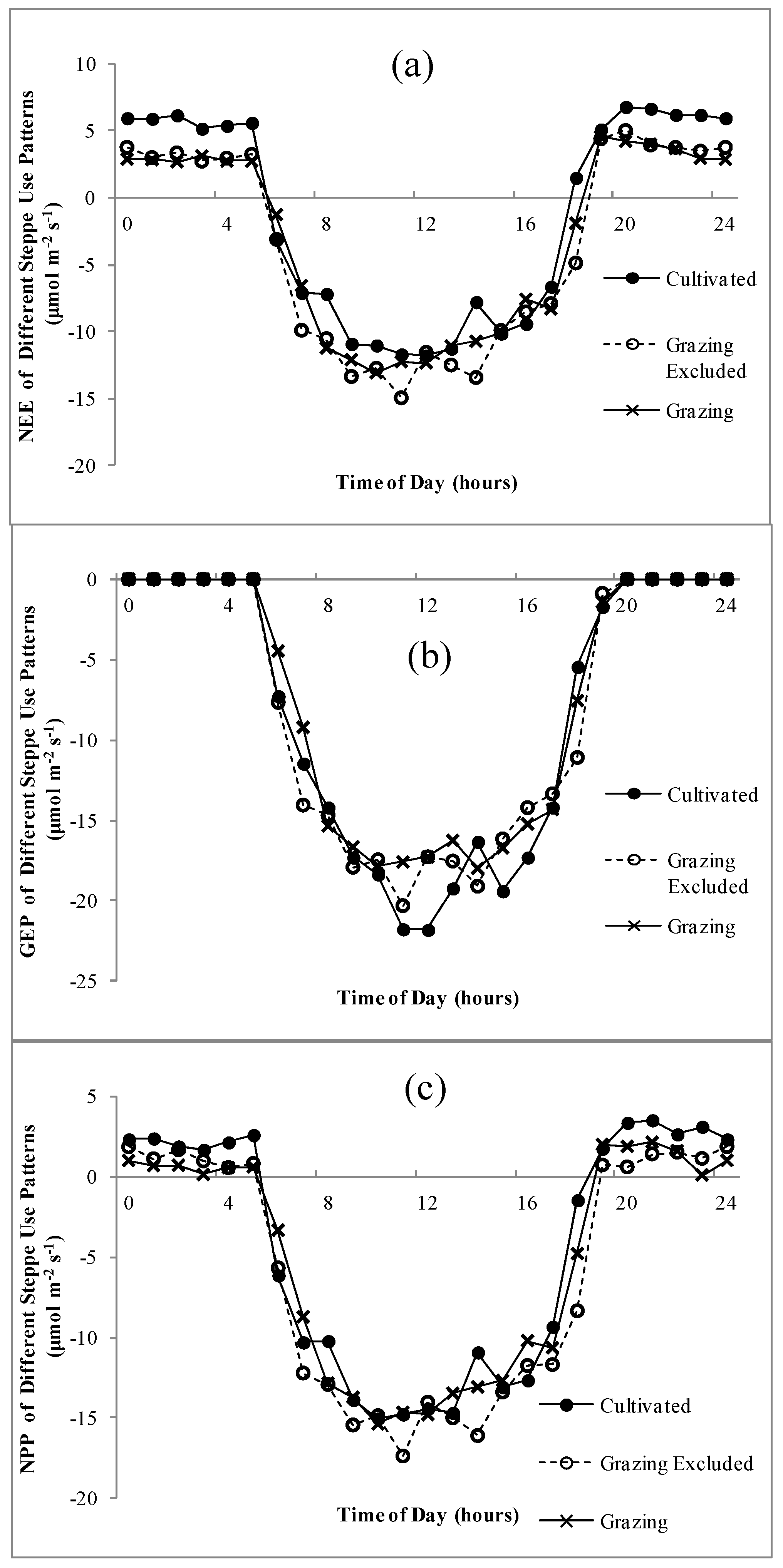
3.1. Photosynthetic and Carbon-Uptake Characteristics
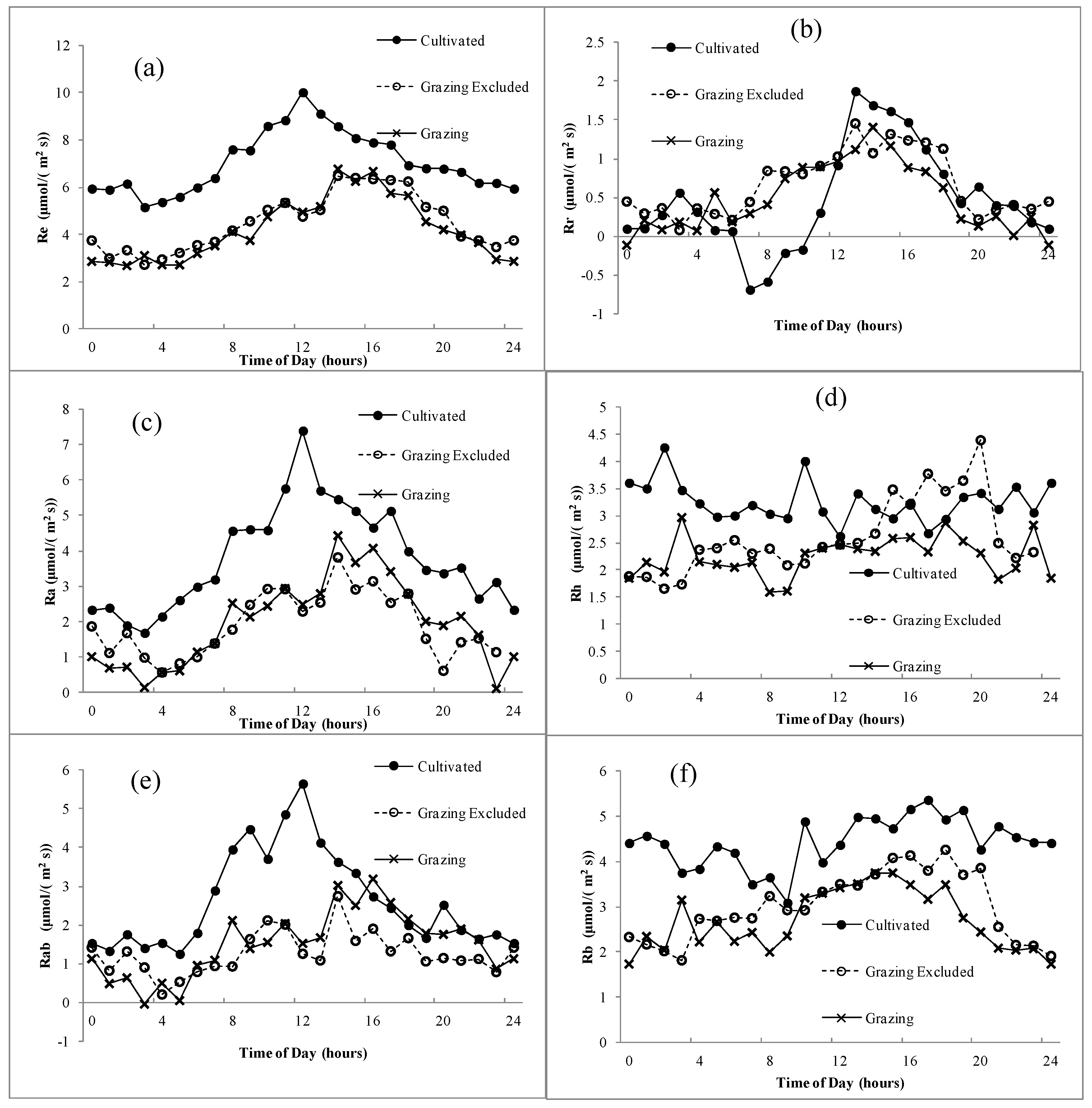
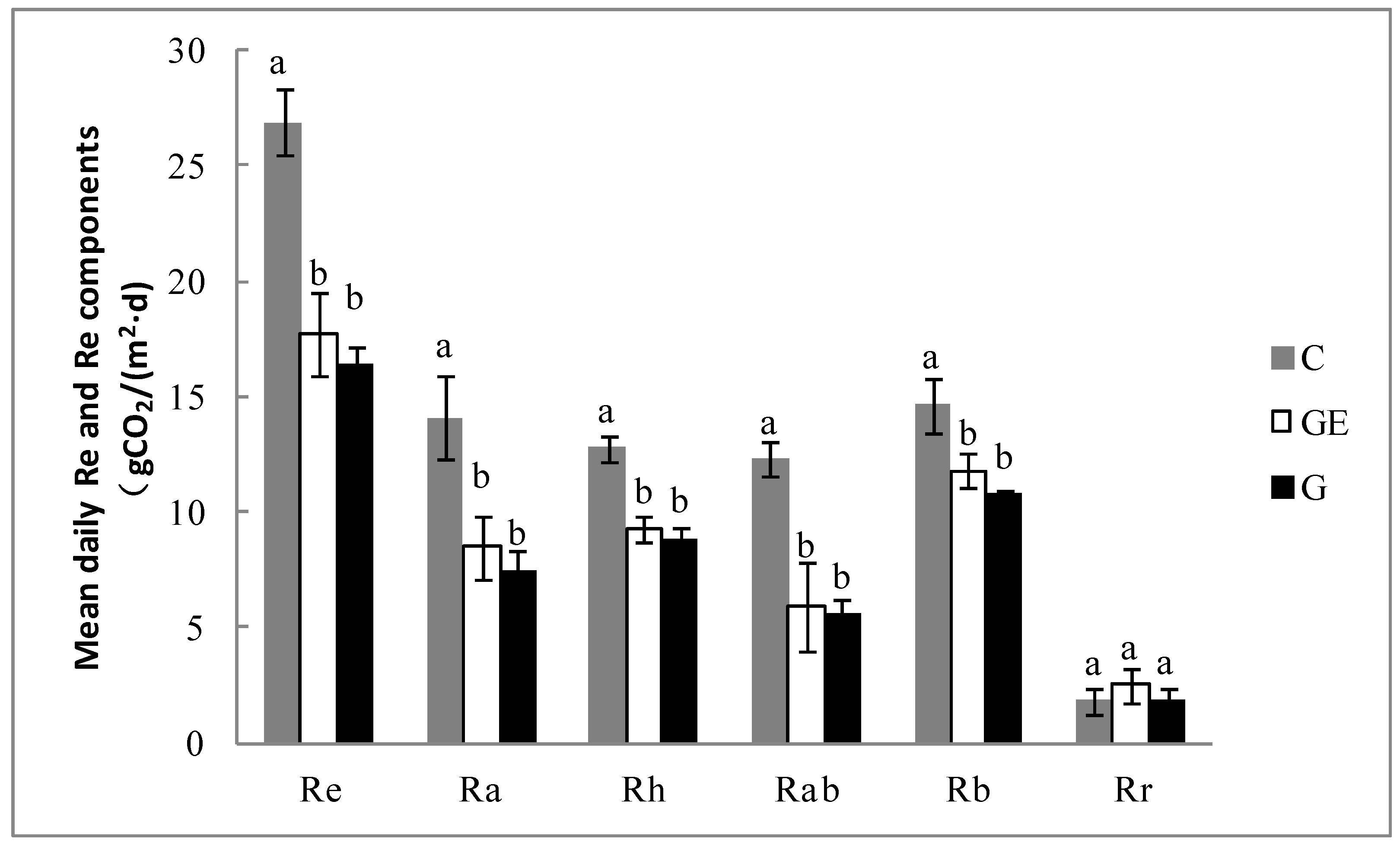
3.2. Ecosystem Respiration and Its Components
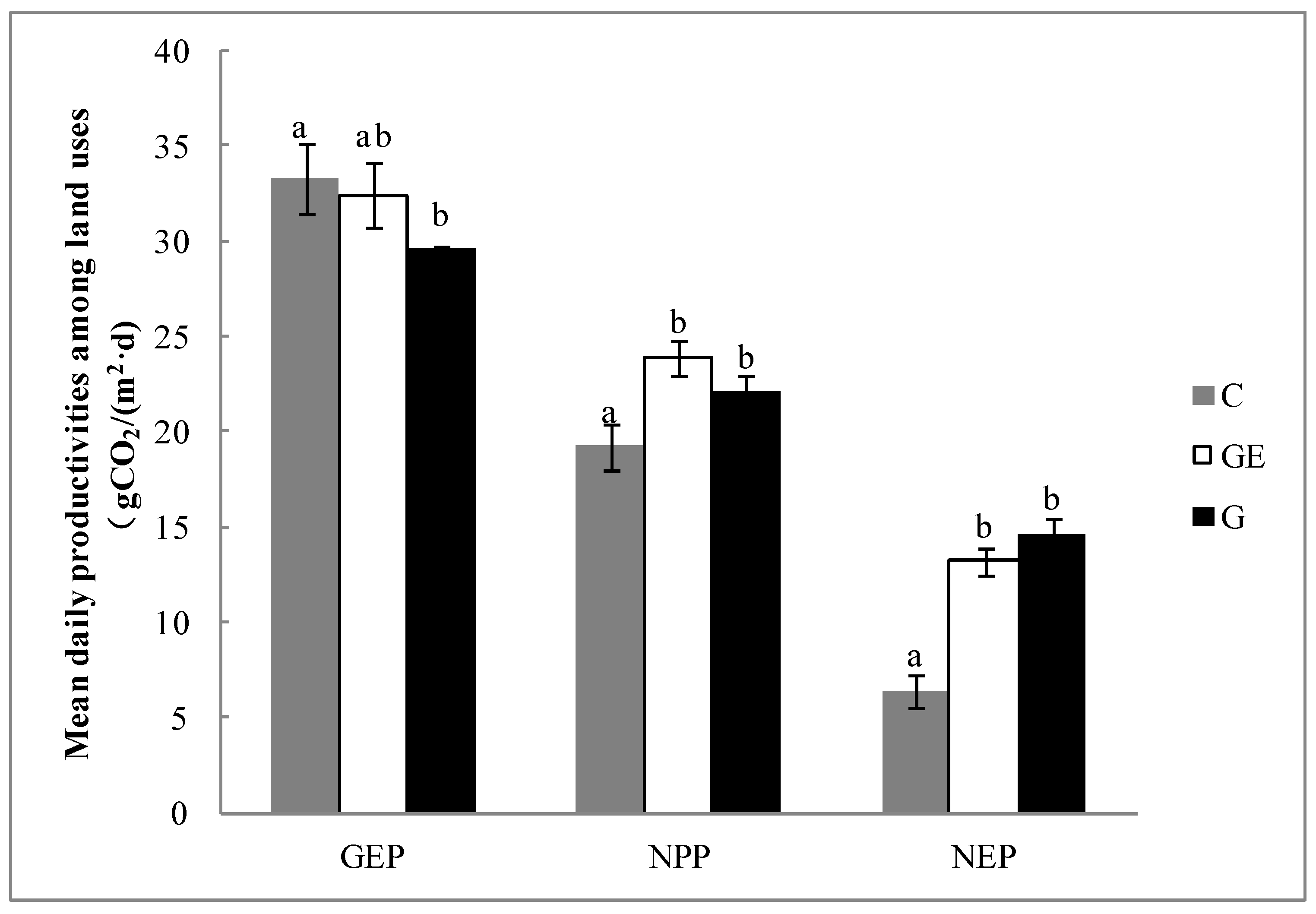
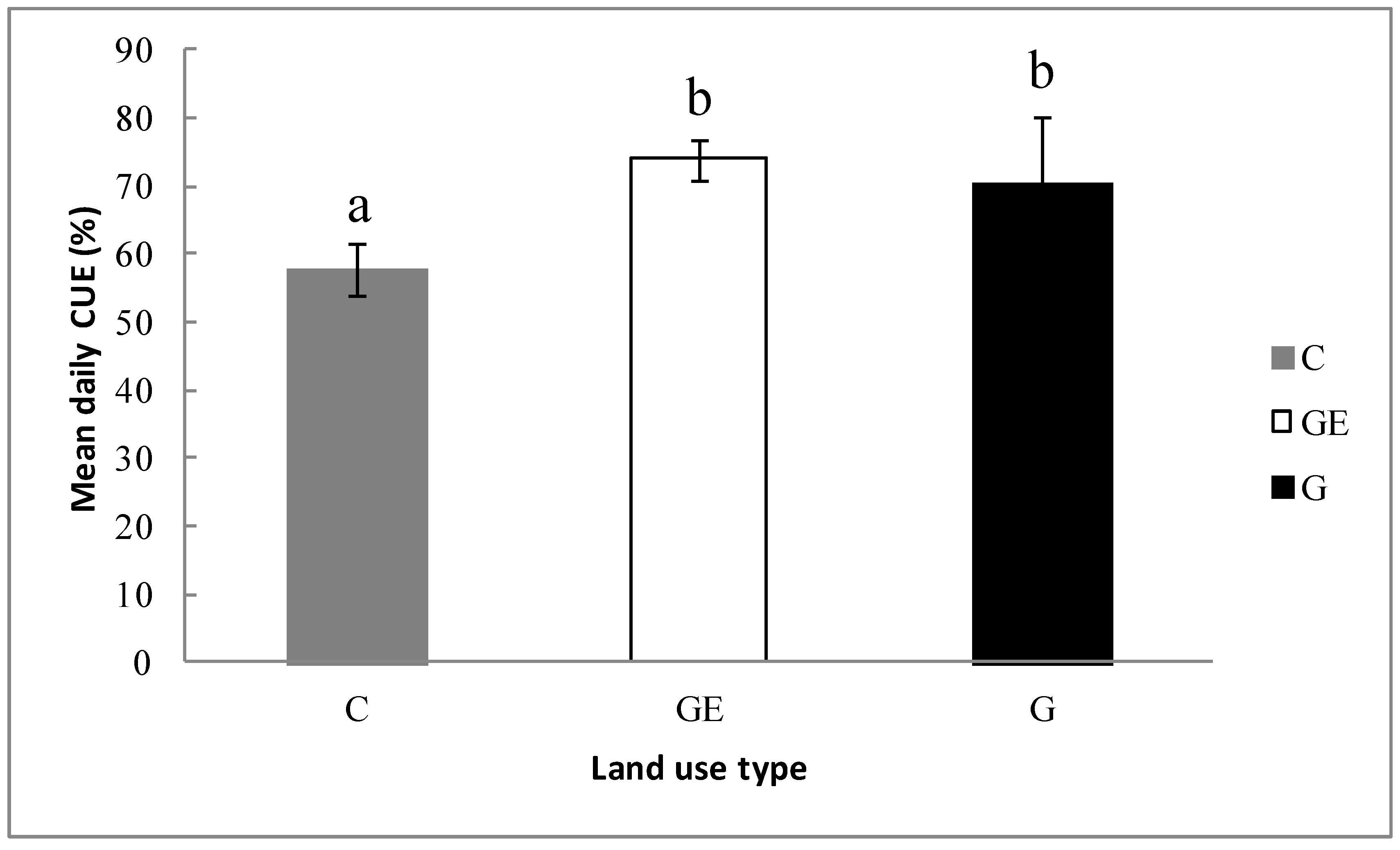
3.3. Carbon-Use Efficiency (CUE) among Steppe-Use Patterns
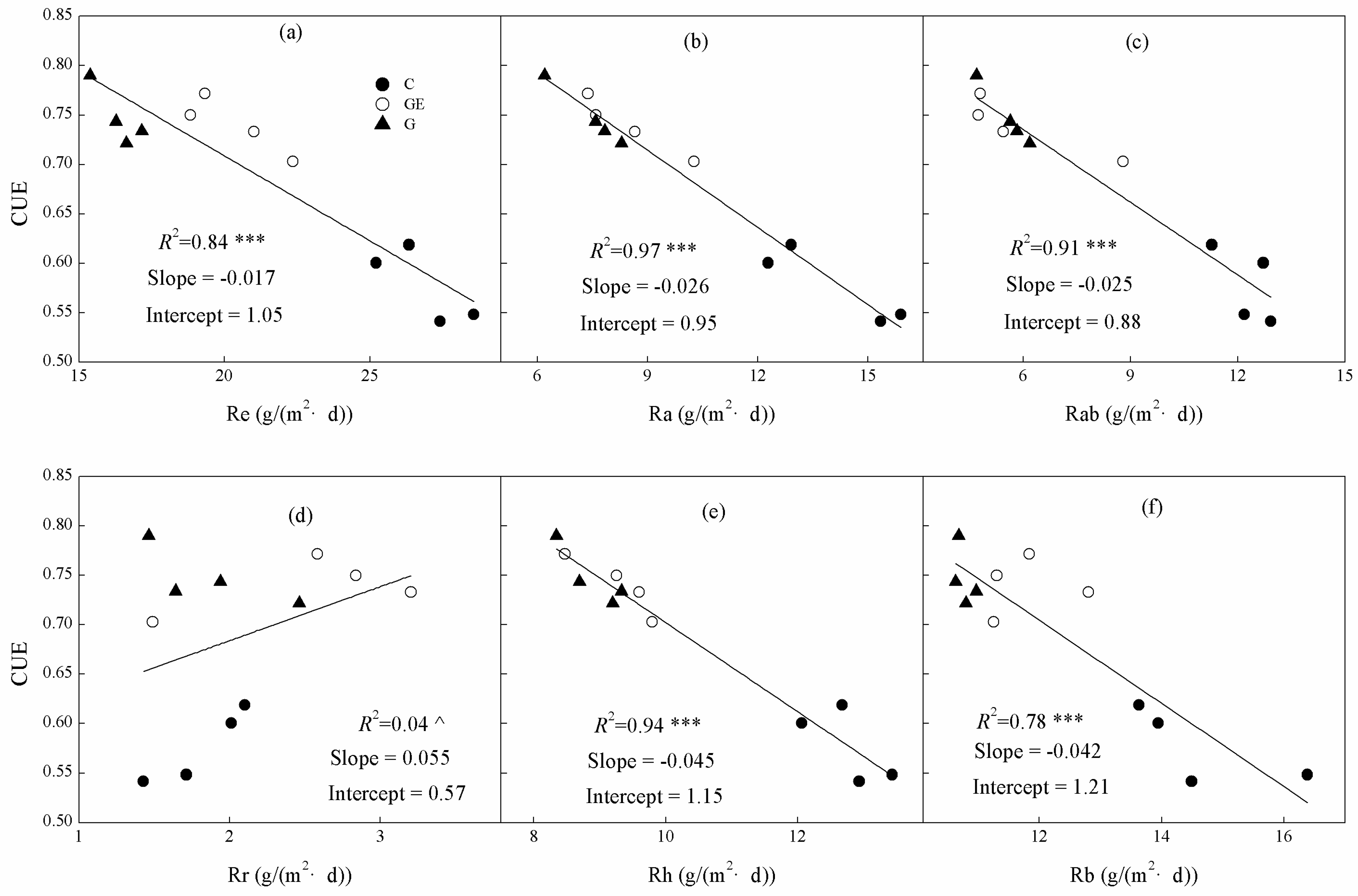
3.4. Correlations of CUE and Re Components
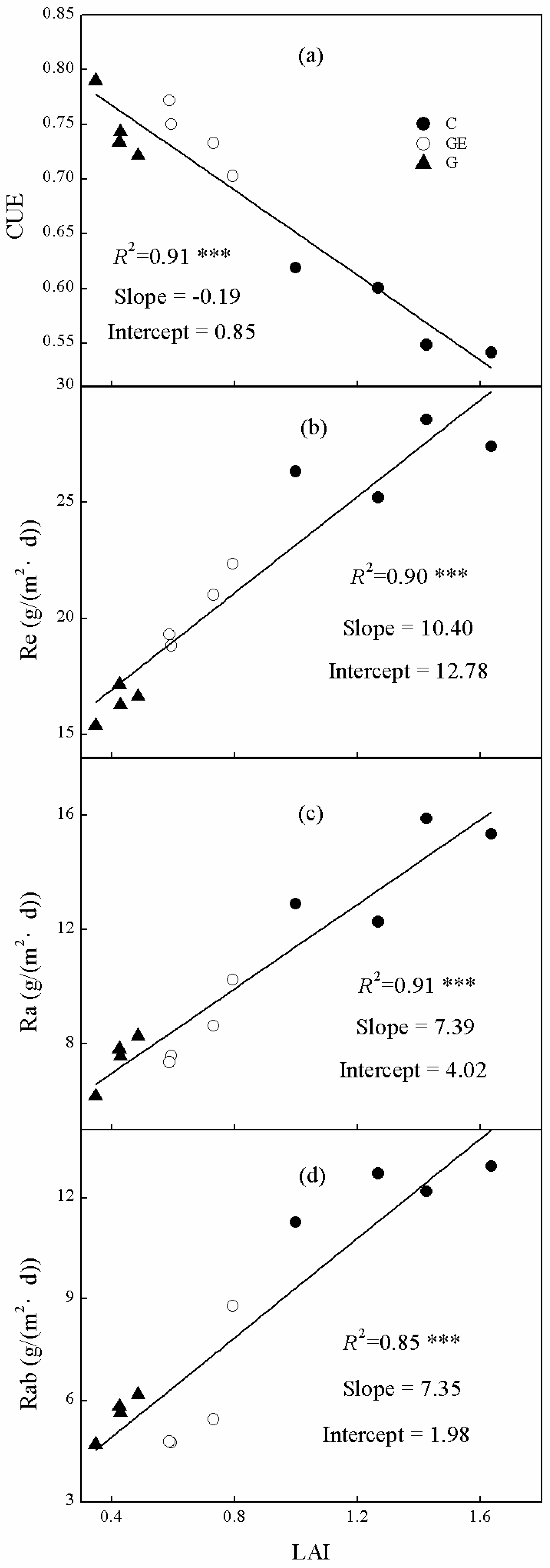
3.5. Correlations of CUE, Re Components and Leaf-Area Index (LAI)
4. Discussion
4.1. Effect of Different Land-Use Patterns on Ecosystem CUE
4.2. Underlying Mechanism Explaining the Effects of Land-Use Pattern on Ecosystem CUE
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Change, I.C. The Physical Science Basis. Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Novara, A.; Gristina, L.; Sala, G.; Galati, A.; Crescimanno, M.; Cerdà, A.; Badalamenti, E.; Mantia, T.L. Agricultural land abandonment in Mediterranean environment provides ecosystem services via soil carbon sequestration. Sci. Total Environ. 2017, 576, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Yanai, A.M.; Nogueira, E.M.; Pm, D.A.G.; Fearnside, P.M. Deforestation and Carbon Stock Loss in Brazil’s Amazonian Settlements. Environ. Manag. 2016, 59, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Twine, T.E.; Hill, J.; Noe, R.; Shi, J.; Li, M. Effects of Land Use Change for Crops on Water and Carbon Budgets in the Midwest USA. Sustainability 2017, 9, 225. [Google Scholar] [CrossRef]
- Li, Y.; Fan, J.; Zhang, L.; Zhai, J.; Liu, G.; Li, J. The impact of different land use and management on community composition, species diversity and productivity in a typical temperate grassland. Acta Pratacult. Sin. 2013, 22, 1–9. [Google Scholar]
- Wang, J.; Xiao, X.; Qin, Y.; Dong, J.; Zhang, G.; Kou, W.; Jin, C.; Zhou, Y.; Zhang, Y. Mapping paddy rice planting area in wheat-rice double-cropped areas through integration of Landsat-8 OLI, MODIS, and PALSAR images. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Yokozawa, M.; Liu, J.; Zhang, Z. Climate change, land use change, and China’s food security in the twenty-first century: An integrated perspective. Clim. Chang. 2009, 93, 433–445. [Google Scholar] [CrossRef]
- Tao, F.; Zhang, S.; Zhang, Z. Changes in rice disasters across China in recent decades and the meteorological and agronomic causes. Reg. Environ. Chang. 2013, 13, 743–759. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, X.; Qin, Y.; Dong, J.; Zhang, G.; Kou, W.; Jin, C.; Wang, J.; Li, X. Mapping paddy rice planting area in rice-wetland coexistent areas through analysis of Landsat 8 OLI and MODIS images. Int. J. Appl. Earth Observ. Geoinf. 2016, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, M.; Zhuang, D.; Zhang, Z.; Deng, X. Study on spatial pattern of land-use change in China during 1995–2000. Sci. China Ser. D Earth Sci. 2003, 46, 373–384. [Google Scholar]
- Fan, J.; Wang, K.; Harris, W.; Zhong, H.; Hu, Z.; Han, B.; Zhang, W.; Wang, J. Allocation of vegetation biomass across a climate-related gradient in the grasslands of Inner Mongolia. J. Arid Environ. 2009, 73, 521–528. [Google Scholar] [CrossRef]
- Hu, Z.; Yu, G.; Fan, J.; Zhong, H.; Wang, S.; Li, S. Precipitation-use efficiency along a 4500-km grassland transect. Glob. Ecol. Biogeogr. 2010, 19, 842–851. [Google Scholar]
- Oles, K.M.; Weixelman, D.A.; Lile, D.F.; Tate, K.W.; Snell, L.K.; Roche, L.M. Riparian Meadow Response to Modern Conservation Grazing Management. Environ. Manag. 2017, 60, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, W.; Fan, J. Soil organic carbon dynamics in Xilingol grassland of northern China induced by the Beijing-Tianjin Sand Source Control Program. Front. Earth Sci. 2016, 11, 407–415. [Google Scholar] [CrossRef]
- Fan, J.; Zhong, H.; Harris, W.; Yu, G.; Wang, S.; Hu, Z.; Yue, Y. Carbon storage in the grasslands of China based on field measurements of above-and below-ground biomass. Clim. Chang. 2008, 86, 375–396. [Google Scholar] [CrossRef]
- Egan, G.; Crawley, M.J.; Fornara, D.A. Effects of long-term grassland management on the carbon and nitrogen pools of different soil aggregate fractions. Sci. Total Environ. 2017, 613–614, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Scurlock, J.; Hall, D. The global carbon sink: A grassland perspective. Glob. Chang. Biol. 1998, 4, 229–233. [Google Scholar] [CrossRef]
- Rindfuss, R.R.; Walsh, S.J.; Turner Ii, B.; Fox, J.; Mishra, V. Developing a science of land change: Challenges and methodological issues. Proc. Natl. Acad. Sci. USA 2004, 101, 13976–13981. [Google Scholar] [CrossRef] [PubMed]
- DeLucia, E.H.; Drake, J.E.; Thomas, R.B.; Gonzalez-Meler, M. Forest carbon use efficiency: Is respiration a constant fraction of gross primary production? Glob. Chang. Biol. 2007, 13, 1157–1167. [Google Scholar] [CrossRef]
- Dewar, R.C.; Medlyn, B.E.; McMurtrie, R.E. Acclimation of the respiration photosynthesis ratio to temperature: Insights from a model. Glob. Chang. Biol. 1999, 5, 615–622. [Google Scholar] [CrossRef]
- Dewar, R.C.; Medlyn, B.E.; McMurtrie, R.E. A mechanistic analysis of light and carbon use efficiencies. Plant Cell Environ. 1998, 21, 573–588. [Google Scholar] [CrossRef]
- Valentini, R.; Matteucci, G.; Dolman, A.; Schulze, E.D.; Rebmann, C.; Moors, E.; Granier, A.; Gross, P.; Jensen, N.; Pilegaard, K. Respiration as the main determinant of carbon balance in European forests. Nature 2000, 404, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, Y.; Xia, J.; Shi, Z.; Jiang, L.; Niu, S. Differential responses of ecosystem respiration components to experimental warming in a meadow grassland on the Tibetan Plateau. Agric. For. Meteorol. 2016, 220, 21–29. [Google Scholar] [CrossRef]
- Zhao, C.; Miao, Y.; Yu, C.; Zhu, L.; Wang, F.; Jiang, L.; Hui, D.; Wan, S. Soil microbial community composition and respiration along an experimental precipitation gradient in a semiarid steppe. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lin, G.; Huang, J.; Jenerette, G.D. Dependence of carbon sequestration on the differential responses of ecosystem photosynthesis and respiration to rain pulses in a semiarid steppe. Glob. Chang. Biol. 2009, 15, 2450–2461. [Google Scholar] [CrossRef]
- Chen, S.; Chen, J.; Lin, G.; Zhang, W.; Miao, H.; Wei, L.; Huang, J.; Han, X. Energy balance and partition in Inner Mongolia steppe ecosystems with different land use types. Agric. For. Meteorol. 2009, 149, 1800–1809. [Google Scholar] [CrossRef]
- Miao, H.; Chen, S.; Chen, J.; Zhang, W.; Zhang, P.; Wei, L.; Han, X.; Lin, G. Cultivation and grazing altered evapotranspiration and dynamics in Inner Mongolia steppes. Agric. For. Meteorol. 2009, 149, 1810–1819. [Google Scholar] [CrossRef]
- Liu, H.; Zang, R.; Chen, H.Y. Effects of grazing on photosynthetic features and soil respiration of rangelands in the Tianshan Mountains of Northwest China. Sci. Rep. 2016. [Google Scholar] [CrossRef]
- Li, Y.; Fan, J.; Hu, Z.; Shao, Q.; Harris, W. Comparison of evapotranspiration components and water-use efficiency among different land use patterns of temperate steppe in the Northern China pastoral-farming ecotone. Int. J. Biometeorol. 2015, 60, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.M.; Hoilett, N.; Lorenz, N.; Dick, R.P.; Liles, M.R.; Ramsier, C.; Kloepper, J.W. Glyphosate effects on soil rhizosphere-associated bacterial communities. Sci. Total Environ. 2016, 543, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Sherry, R.A.; Zhou, X.; Luo, Y. Ecosystem carbon fluxes in response to warming and clipping in a tallgrass prairie. Ecosystems 2013, 16, 948–961. [Google Scholar] [CrossRef]
- Raich, J.; Schlesinger, W. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 1992, 44, 81–99. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Allison, S.D.; Mayes, M.A.; Luo, Y. Soil carbon sensitivity to temperature and carbon use efficiency compared across microbial-ecosystem models of varying complexity. Biogeochemistry 2014, 119, 67–84. [Google Scholar] [CrossRef]
- DeLucia, E.H.; Schlesinger, W.H. Resource—Use efficiency and drought tolerance in adjacent Great Basin and sierran plants. Ecology 1991, 72, 51–58. [Google Scholar] [CrossRef]
- Chambers, J.Q.; Tribuzy, E.S.; Toledo, L.C.; Crispim, B.F.; Higuchi, N.; dos Santos, J.; Araujo, A.C.; Kruijt, B.; Nobre, A.D.; Trumbore, S.E. Respiration from a tropical forest ecosystem: Partitioning of sources and low carbon use efficiency. Ecol. Appl. 2004, 14, S72–S88. [Google Scholar] [CrossRef]
- Marthews, T.R.; Malhi, Y.; Girardin, C.A.; Silva Espejo, J.E.; Aragão, L.E.; Metcalfe, D.B.; Rapp, J.M.; Mercado, L.M.; Fisher, R.A.; Galbraith, D.R. Simulating forest productivity along a neotropical elevational transect: Temperature variation and carbon use efficiency. Glob. Chang. Biol. 2012, 18, 2882–2898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Mu, J.E.; Wein, A.M.; McCarl, B.A. Land use and management change under climate change adaptation and mitigation strategies: A US case study. Mitig. Adapt. Strateg. Glob. Chang. 2015, 20, 1041–1054. [Google Scholar] [CrossRef]
- Jilani, T.; Hasegawa, T.; Matsuoka, Y. The future role of agriculture and land use change for climate change mitigation in Bangladesh. Mitig. Adapt. Strateg. Glob. Chang. 2015, 20, 1289–1304. [Google Scholar] [CrossRef]
- Metcalfe, D.B.; Meir, P.; Aragão, L.E.; Lobo-do-Vale, R.; Galbraith, D.; Fisher, R.; Chaves, M.M.; Maroco, J.; da Costa, A.C.L.; de Almeida, S.S. Shifts in plant respiration and carbon use efficiency at a large-scale drought experiment in the eastern Amazon. New Phytol. 2010, 187, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, M.; Tenhunen, J.; Roupsard, O.; Ourcival, J.M.; Rambal, S.; Dore, S.; Valentini, R. Ecosystem respiration in two Mediterranean evergreen Holm Oak forests: Drought effects and decomposition dynamics. Funct. Ecol. 2002, 16, 27–39. [Google Scholar] [CrossRef]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Chang. Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Reichstein, M.; Bahn, M.; Mahecha, M.D.; Kattge, J.; Baldocchi, D.D. Linking plant and ecosystem functional biogeography. Proc. Natl. Acad. Sci. USA 2014, 111, 13697–13702. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.L.; Bell, J.; Pendall, E.; Ogle, K. Does declining carbon—Use efficiency explain thermal acclimation of soil respiration with warming? Glob. Chang. Biol. 2013, 19, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wan, S.; Hui, D.; Wallace, L.L. Acclimatization of soil respiration to warming in a tall grass prairie. Nature 2001, 413, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; van Dijk, A.I.; de Jeu, R.A.; Canadell, J.G.; McCabe, M.F.; Evans, J.P.; Wang, G. Recent reversal in loss of global terrestrial biomass. Nat. Clim. Chang. 2015, 5, 470–474. [Google Scholar] [CrossRef]
- Lloyd, J.; Taylor, J. On the temperature dependence of soil respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Suyker, A.E.; Verma, S.B. Gross primary production and ecosystem respiration of irrigated and rainfed maize–soybean cropping systems over 8 years. Agric. For. Meteorol. 2012, 165, 12–24. [Google Scholar] [CrossRef]
- Piao, S.; Friedlingstein, P.; Ciais, P.; de Noblet-Ducoudré, N.; Labat, D.; Zaehle, S. Changes in climate and land use have a larger direct impact than rising CO2 on global river runoff trends. Proc. Natl. Acad. Sci. USA 2007, 104, 15242–15247. [Google Scholar] [CrossRef] [PubMed]
- Regina, K.; Sheehy, J.; Myllys, M. Mitigating greenhouse gas fluxes from cultivated organic soils with raised water table. Mitig. Adapt. Strateg. Glob. Chang. 2015, 20, 1529–1544. [Google Scholar] [CrossRef]
- Franzluebbers, K.; Franzluebbers, A.; Jawson, M. Environmental controls on soil and whole-ecosystem respiration from a tallgrass prairie. Soil Sci. Soc. Am. J. 2002, 66, 254–262. [Google Scholar] [CrossRef]
- Dong, J.; Liu, J.; Tao, F.; Xu, X.; Wang, J. Spatio-temporal changes in annual accumulated temperature in China and the effects on cropping systems, 1980s to 2000. Clim. Res. 2009, 40, 37–48. [Google Scholar] [CrossRef]
- Dong, J.; Xiao, X.; Chen, B.; Torbick, N.; Jin, C.; Zhang, G.; Biradar, C. Mapping deciduous rubber plantations through integration of PALSAR and multi-temporal Landsat imagery. Remote Sens. Environ. 2013, 134, 392–402. [Google Scholar] [CrossRef]
- Niu, S.; Wu, M.; Han, Y.; Xia, J.; Li, L.; Wan, S. Water—Mediated responses of ecosystem carbon fluxes to climatic change in a temperate steppe. New Phytol. 2008, 177, 209–219. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Fan, J.; Hu, Z. Comparison of Carbon-Use Efficiency Among Different Land-Use Patterns of the Temperate Steppe in the Northern China Pastoral Farming Ecotone. Sustainability 2018, 10, 487. https://doi.org/10.3390/su10020487
Li Y, Fan J, Hu Z. Comparison of Carbon-Use Efficiency Among Different Land-Use Patterns of the Temperate Steppe in the Northern China Pastoral Farming Ecotone. Sustainability. 2018; 10(2):487. https://doi.org/10.3390/su10020487
Chicago/Turabian StyleLi, Yuzhe, Jiangwen Fan, and Zhongmin Hu. 2018. "Comparison of Carbon-Use Efficiency Among Different Land-Use Patterns of the Temperate Steppe in the Northern China Pastoral Farming Ecotone" Sustainability 10, no. 2: 487. https://doi.org/10.3390/su10020487