Tourist Traffic Significantly Affects Microbial Communities of Sandstone Cave Sediments in the Protected Landscape Area “Labské Pískovce” (Czech Republic): Implications for Regulatory Measures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Localities
2.2. Analyses of Attendance
2.3. Sampling
2.4. Microbial Analyses
2.4.1. Determination of PLFA
2.4.2. Determination of Microbial Activities in Sediments
2.5. Determination of Nutrients in Sediments
2.6. Statistical Analyses
3. Results
3.1. Cave Attendance
3.2. Cave and Sediment Characteristics
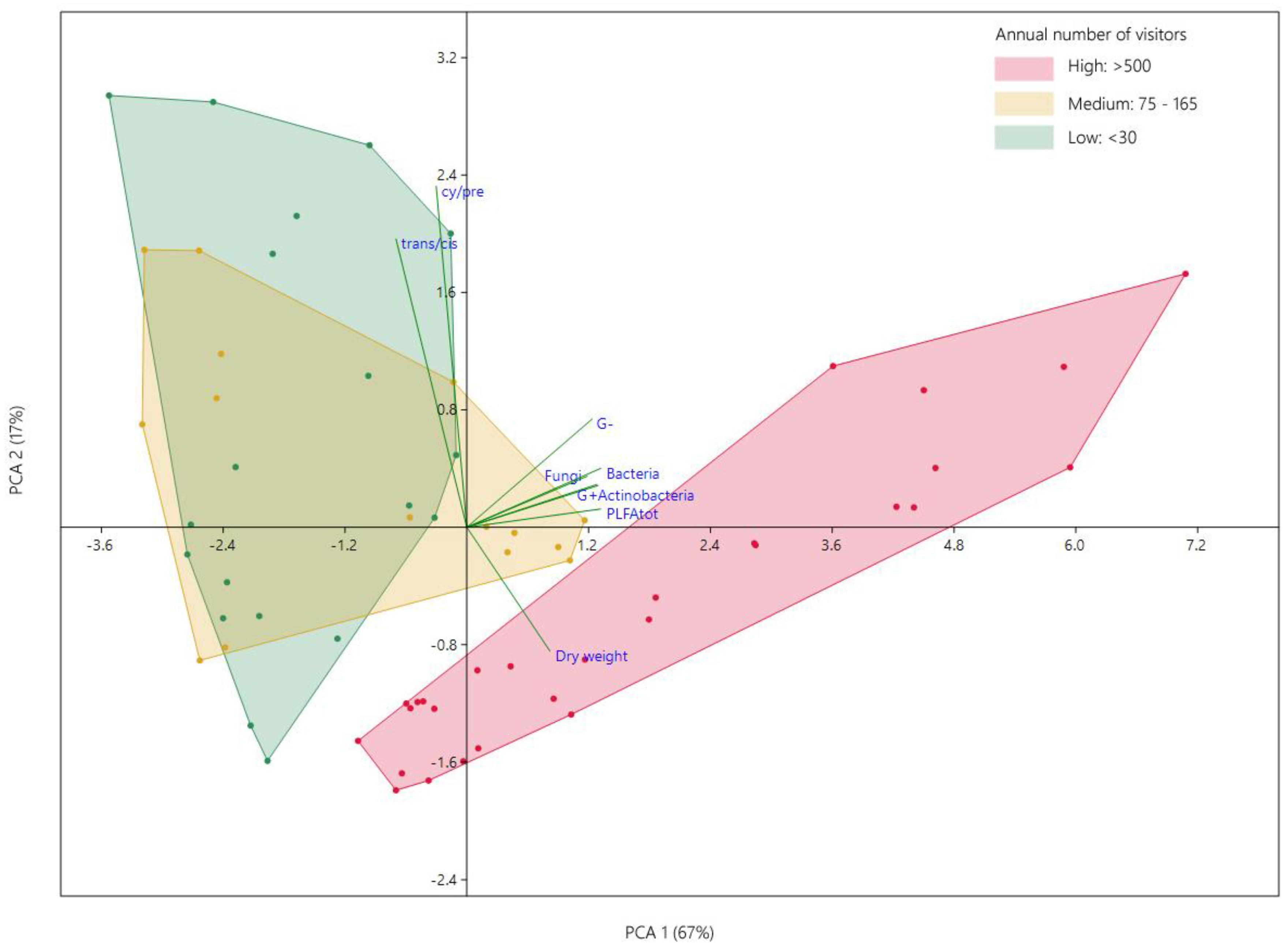
3.3. Effect of Attendance on Microbial Communities Composition
4. Discussion
- increased input of nutrients caused by visitors,
- input of external microorganisms.
4.1. Implications for Regulation
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A—Brief History of Cave Visiting in the Area
Appendix B–Demographic Data of Cave Visitors
References
- Cílek, V. The origin and development of sandstone landforms. In Sandstone Landscapes; Härtel, H., Cílek, V., Herben, T., Jackson, A., Williams, R., Eds.; Academia: Praha, Czech Republic, 2007. [Google Scholar]
- Holec, M.; Kadora, T.; Holcová, D. Spiders of selected sandstone caves of National Nature Reserve Kaňon Labe (the Protected Landscape Area Labské pískovce, the Czech Republic). Stud. Oecol. 2010, 4, 153–158. [Google Scholar]
- Chapman, P. Caves and Cave Life; HarperCollins: London, UK, 1993. [Google Scholar]
- Benda, P.; Chvátal, P. Bats Fibernating in Fault Caves of the Elbe River Canyon in the Elbe Sandstones in 1995–2010; The North Bohemian Museum in Liberec: Liberec, Czech Republic, 2011. [Google Scholar]
- Zhou, J.P.; Gu, Y.Q.; Zou, C.S.; Mo, M.H. Phylogenetic diversity of bacteria in an earth-cave in Guizhou province, southwest of China. J. Microbiol. 2007, 45, 105–112. [Google Scholar] [PubMed]
- Van Beynen, P.; Ford, D.; Schwarcz, H. Seasonal variability in organic substances in surface and cave waters at Marengo cave, Indiana. Hydrol. Process. 2000, 14, 1177–1197. [Google Scholar] [CrossRef]
- Adetutu, E.M.; Thorpe, K.; Shahsavari, E.; Bourne, S.; Cao, X.S.; Fard, R.M.N.; Kirby, G.; Ball, A.S. Bacterial community survey of sediments at Naracoorte caves, Australia. Int. J. Speleol. 2012, 41, 137–147. [Google Scholar] [CrossRef]
- Ikner, L.A.; Toomey, R.S.; Nolan, G.; Neilson, J.W.; Pryor, B.M.; Maier, R.M. Culturable microbial diversity and the impact of tourism in Kartchner Caverns, Arizona. Microb. Ecol. 2007, 53, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Chelius, M.K.; Beresford, G.; Horton, H.; Quirk, M.; Selby, G.; Simpson, R.T.; Horrocks, R.; Moore, J.C. Impacts of alterations of organic inputs on the bacterial community within the sediments of Wind cave, South Dakota, USA. Int. J. Speleol. 2009, 38, 1–10. [Google Scholar] [CrossRef]
- Cigna, A.A. Modern trend in cave monitoring. Acta Carsol. 2002, 31. [Google Scholar] [CrossRef]
- Demek, J. Geomorfologie Českých Zemí; Nakladatelství Československé akademie věd: Prague, Czech Republic, 1965. [Google Scholar]
- Kuncová, J. Ústecko. In Chráněná území ČR, Svazek I; Mackovčin, P., Sedláček, M., Eds.; Nature Conservation Agency of the Czech Republic and EcoCenter Brno: Prague, Czech Republic, 1999; p. 352. [Google Scholar]
- Culek, M.; Grulich, V.; Laštůvka, Z.; Divíšek, J. Biogeografické Regiony České Republiky; Masarykova Univerzita: Brno, Czech Republic, 2013. [Google Scholar]
- Tomášek, M. Půdy České Republiky; Česká Geologická Služba: Prague, Czech Republic, 2014. [Google Scholar]
- Zelles, L.; Bai, Q.Y.; Rackwitz, R.; Chadwick, D.; Beese, F. Determination of phospholipid-derived and lipopolysaccharide-derived fatty-acids as an estimate of microbial biomass and community structures in soils. Biol. Fertil. Soils 1995, 19, 115–123. [Google Scholar] [CrossRef]
- International Organization for Standardization. Soil Quality—Determination of Soil Microbial Diversity—Part 2: Method by Phospholipid Fatty Acid Analysis (PLFA) Using the Simple PLFA Extraction Method; International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- Troegl, J.; Pavlorkova, J.; Packova, P.; Sejak, J.; Kuran, P.; Popelka, J.; Pacina, J. Indication of importance of including soil microbial characteristics into biotope valuation method. Sustainability 2016, 8, 253. [Google Scholar] [CrossRef]
- Troegl, J.; Jirkova, I.; Kuran, P.; Akhmetshina, E.; Brovdyova, T.j.; Sirotkin, A.; Kirilina, T. Phospholipid fatty acids as physiological indicators of Paracoccus denitrificans encapsulated in silica sol-gel hydrogels. Sensors 2015, 15, 3426–3434. [Google Scholar] [CrossRef] [PubMed]
- Kuran, P.; Troegl, J.; Novakova, J.; Pilarova, V.; Danova, P.; Pavlorkova, J.; Kozler, J.; Novak, F.; Popelka, J. Biodegradation of spilled diesel fuel in agricultural soil: Effect of humates, zeolite, and bioaugmentation. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Federici, E.; Giubilei, M.A.; Cajthaml, T.; Petruccioli, M.; D’Annibale, A. Lentinus (Panus) tigrinus augmentation of a historically contaminated soil: Matrix decontamination and structure and function of the resident bacterial community. J. Hazard. Mater. 2011, 186, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Chaudhary, A.; Choudhary, R.; Kaushik, R. Phospholipid fatty acid—A bioindicator of environment monitoring and assessment in soil ecosystem. Curr. Sci. 2005, 89, 1103–1112. [Google Scholar]
- Moore-Kucera, J.; Dick, R.P. PLFA profiling of microbial community structure and seasonal shifts in soils of a Douglas-fir chronosequence. Microb. Ecol. 2008, 55, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P.; Trogl, J.; Frouz, J.; Snajdr, J.; Valaskova, V.; Merhautova, V.; Cajthaml, T.; Herinkova, J. Enzyme activities and microbial biomass in topsoil layer during spontaneous succession in spoil heaps after brown coal mining. Soil Biol. Biochem. 2008, 40, 2107–2115. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich-3 soil test extractant: A modification of Mehlich-2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- International Organization for Standardization. Soil Quality—Determination of pH; International Standard Organization: Geneva, Switzerland, 2005. [Google Scholar]
- International Organization for Standardization. Soil Quality—Determination of Organic Carbon in Soil by Sulfochromic Oxidation; International Standard Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in Organischen körpern. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Zbíral, J.; Honsa, I.; Malý, S.; Čižmár, D. Jednotné Pracovní Postupy. Analýza Půd III; Central Institute for Supervising and Testing in Agriculture: Brno, Czech Republic, 2011. [Google Scholar]
- International Organization for Standardization. Soil Quality—Determination of Nitrate, Nitrite and Ammonium in Field-Moist Soils by Extraction with Potassium Chloride Solution—Part 1: Manual Method; International Organization for Standardization: Geneva, Switzerland, 2003. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Essington, M.E. Soil and Water Chemistry: An Integrative Approach; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Bailey, V.L.; Peacock, A.D.; Smith, J.L.; Bolton, H. Relationships between soil microbial biomass determined by chloroform fumigation-extraction, substrate-induced respiration, and phospholipid fatty acid analysis. Soil Biol. Biochem. 2002, 34, 1385–1389. [Google Scholar] [CrossRef]
- Urbanova, M.; Kopecky, J.; Valaskova, V.; Sagova-Mareckova, M.; Elhottova, D.; Kyselkova, M.; Moenne-Loccoz, Y.; Baldrian, P. Development of bacterial community during spontaneous succession on spoil heaps after brown coal mining. FEMS Microbiol. Ecol. 2011, 78, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Epure, L.; Meleg, I.N.; Munteanu, C.M.; Roban, R.D.; Moldovan, O.T. Bacterial and fungal diversity of quaternary cave sediment deposits. Geomicrobiol. J. 2014, 31, 116–127. [Google Scholar] [CrossRef]
- Frostegard, A.; Tunlid, A.; Baath, E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011, 43, 1621–1625. [Google Scholar] [CrossRef]
- Kukla, J. Stopy historie objevování a návštěvnosti jeskyní Labských pískovců. In Minulosti Českého Švýcarska-sborník příspěvků ze semináře 2014; Belisová, N., Ed.; The National Park Bohemian Switzerland: Krásná Lípa, Czech Republic, 2015. [Google Scholar]
- Glassheim, E. National mythologies and ethnic cleansing: The expulsion of Czechoslovak Germans in 1945. Cent. Eur. Hist. 2000, 33, 463–486. [Google Scholar] [CrossRef]
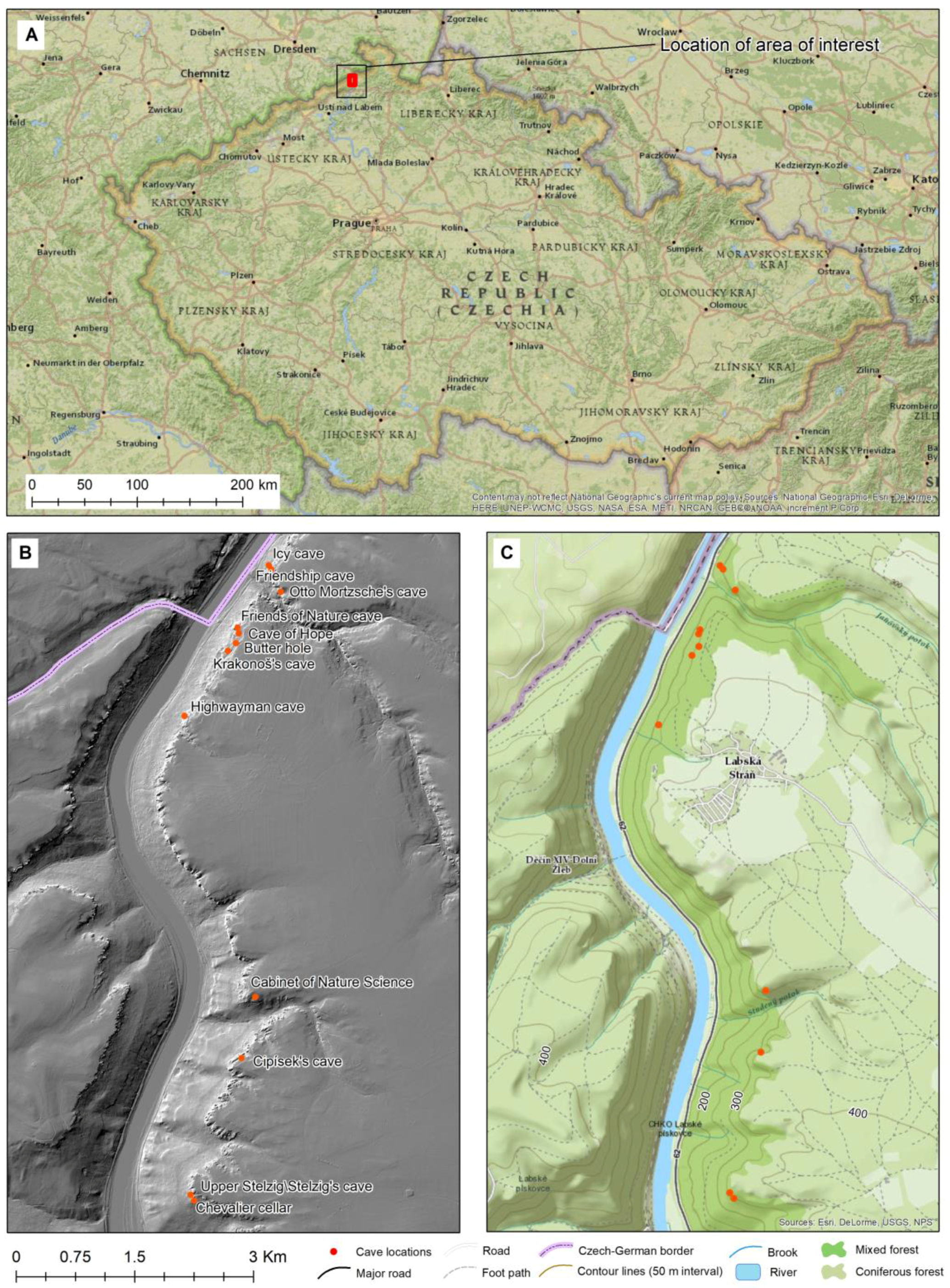
| Cave | GPS | Annual Attendance |
|---|---|---|
| Cipískova jeskyně (Cipísek’s cave) | 50°49′44.4″ N, 14°14′03.7″ E | 19.40 ± 6.73 |
| Horní Stelzig (Upper Stelzig) | 50°49′08.1″ N, 14°13′51.0″ E | <10 |
| Jeskyně nadějí (Cave of Hope) | 50°51′32.6″N, 14°13′38.3″ E | 88.00 ± 27.00 |
| Jeskyně Otto Mortzsche (Otto Mortzsche’s cave) | 50°51′43.5″ N, 14°13′53.3″ E | 20.20 ± 3.90 |
| Jeskyně přátel přírody (Friends of Nature cave) | 50°51′33.0″ N, 14°13′38.1″ E | 162.80 ± 67.88 |
| Jeskyně Přátelství (Friendship cave) | 50°51′48.9″ N, 14°13′48.3″ E | 534.60 ± 175.84 |
| Kabinet přírodovědy (Cabinet of Nature Science) | 50°50′00.3″ N, 14°14′05.8″ E | <10 |
| Krakonošova jeskyně (Krakonoš’s cave) | 50°51′26.7″ N, 14°13′35.5″ E | 21.40 ± 8.17 |
| Ledová jeskyně (Icy cave) | 50°51′49.8″ N, 14°13′47.0″ E | 116.60 ± 46.82 |
| Loupežnická jeskyně (Highwayman cave) | 50°51′09.7″ N, 14°13′23.0″ E | 649.60 ± 47.69 |
| Máslová díra (Butter hole) | 50°51′29.0″ N, 14°13′38.3″ E | 27.20 ± 15.55 |
| Rytířský sklep (Chevalier cellar) | 50°49′06.7″ N, 14°13′52.7″ E | 28.60 ± 21.30 |
| Parameter | Avg ± Std.Dev. | Median | Lower Quartile | Upper Quartile | Min | Max | n |
|---|---|---|---|---|---|---|---|
| Nutrients | |||||||
| Dry weight | 0.96 ± 0.04 | 0.97 | 0.95 | 0.99 | 0.82 | 1.00 | 45 |
| pH (CaCl2) | 4.10 ± 0.94 | 3.84 | 3.66 | 4.11 | 3.23 | 6.67 | 18 |
| P 1 (mg/kg dwt) | 264 ± 353 | 103 | 53 | 238 | 30 | 1241 | 18 |
| K 1 (mg/kg dwt) | 118 ± 148 | 81 | 50 | 124 | 15 | 688 | 18 |
| Ca 1 (mg/kg dwt) | 2151 ± 3228 | 270 | 184 | 1889 | 64 | 10413 | 18 |
| Mg 1 (mg/kg dwt) | 151 ± 159 | 83 | 67 | 188 | 31 | 661 | 18 |
| Cox (% dwt) | 0.70 ± 0.41 | 0.75 | 0.32 | 0.94 | 0.09 | 1.53 | 18 |
| humus (% dwt) | 1.20 ± 0.70 | 1.29 | 0.55 | 1.62 | 0.16 | 2.54 | 18 |
| Ntot (% dwt) | 0.07 ± 0.05 | 0.06 | 0.03 | 0.1 | 0.01 | 0.23 | 18 |
| N-NH4 (mg/kg dwt) | 28 ± 26 | 18 | 13 | 25 | 9 | 113 | 18 |
| N-NO3 (mg/kg dwt) | 381 ± 520 | 82 | 11 | 626 | 4 | 2031 | 18 |
| N-NO2 (mg/kg dwt) | < LOD | - | - | - | - | - | 18 |
| Microbial Properties (PLFA) | |||||||
| PLFAtot (mg/kg dwt) | 5.20 ± 4.74 | 4.51 | 1.82 | 6.64 | 0.37 | 28.16 | 121 |
| F/B | 0.06 ± 0.08 | 0.05 | 0 | 0.09 | 0 | 0.42 | 121 |
| G+/G- | 1.04 ± 0.57 | 0.89 | 0.66 | 1.34 | 0.00 | 2.92 | 121 |
| Actinobacteria (%) | 21 ± 13 | 20 | 12 | 26 | 1 | 75 | 121 |
| trans/cis | 0.20 ± 0.35 | 0.13 | 0.00 | 0.24 | 0 | 2.94 | 120 |
| cy/pre | 1.47 ± 1.48 | 1.02 | 0.43 | 1.96 | 0.05 | 8.06 | 120 |
| Enzyme Activities | |||||||
| phosphatases (μU/g dwt) | 30 ± 38 | 20 | 9 | 33 | 0 | 221 | 180 |
| glucosidases (μU/g dwt) | 5.4 ± 10.0 | 2.1 | 0.6 | 4.6 | 0 | 60.1 | 180 |
| proteases (μU/g dwt) | 5.3 ± 11.5 | 3 | 1.1 | 4.7 | 0 | 113.9 | 180 |
| oxidases (nU/g dwt) | 1.8 ± 3.5 | 0 | 0 | 2.1 | 0 | 16.3 | 180 |
| peroxidases (nU/g dwt) | 46.1 ± 29.5 | 41.0 | 24.4 | 56.5 | 4.3 | 161.1 | 180 |
| Variable | Median (Trampling = 0) | Median (Trampling = 1) | p-Value |
|---|---|---|---|
| PLFAtot (mg/kg dwt) | 4.67 | 4.41 | 0.928 |
| PLFAfun (mg/kg dwt) | 0 | 0.06 | 2.0 × 10−10 |
| PLFAbac (mg/kg dwt) | 0.58 | 1.52 | 6.5 × 10−4 |
| PLFAG+ (mg/kg dwt) | 0.2 | 0.54 | 7.6 × 10−7 |
| PLFAG− (mg/kg dwt) | 0.19 | 0.475 | 4.2 × 10−3 |
| PLFAAc (mg/kg dwt) | 0.08 | 0.31 | 2.02 × 10−5 |
| F/B | 0 | 0.065 | 2.86 × 10−5 |
| G+/G- | 0.71 | 1.185 | 5.3 × 10−5 |
| trans/cis | 0.12 | 0.14 | 0.485 |
| cy/pre | 0.54 | 1.365 | 9.3 × 10−7 |
| Dry weight | 0.95 | 0.96 | 0.418 |
| Phosphatases (U/g d.wt) | 0.029 | 0.017 | 0.0112 |
| β-glucosidases (U/g d.wt) | 0.004 | 0.0013 | 0.0112 |
| Proteases (U/g d.wt) | 0.0032 | 0.0031 | 0.933 |
| Oxidases (U/g d.wt) | 0 | 9.1 × 10−8 | 0.376 |
| Peroxidases (U/g d.wt) | 4.6 × 10−5 | 3.5 × 10−5 | 0.025 |
| Parameter | Attendance | PLFAtot |
|---|---|---|
| PLFAtot (mg/kg dwt) | 0.57 | - |
| PLFAfun (mg/kg dwt) | 0.60 | 0.38 |
| PLFAbac (mg/kg dwt) | 0.46 | 0.81 |
| PLFAG+ (mg/kg dwt) | 0.47 | 0.71 |
| PLFAG− (mg/kg dwt) | 0.28 | 0.74 |
| PLFAAc (mg/kg dwt) | 0.52 | 0.72 |
| F/B | 0.54 | 0.17 |
| G+/G− | 0.40 | −0.03 |
| Actinobacteria (%) | 0.34 | 0.16 |
| trans/cis | −0.46 | −0.40 |
| cy/pre | −0.13 | −0.20 |
| phosphatases (μU/g dwt) | −0.26 | −0.11 |
| glucosidases (μU/g dwt) | −0.18 | −0.07 |
| proteases (μU/g dwt) | 0.16 | 0.03 |
| oxidases (nU/g dwt) | 0.32 | 0.06 |
| peroxidases (nU/g dwt) | −0.68 | −0.33 |
| Dry weight | 0.75 | 0.75 |
| pH (CaCl2) | 0.60 | −0.12 |
| Extractable P (mg/kg dwt) | 0.76 | 0.32 |
| Extractable K (mg/kg dwt) | 0.83 | 0.23 |
| Extractable Ca (mg/kg dwt) | 0.83 | 0.75 |
| Extractable Mg (mg/kg dwt) | 0.38 | 0.00 |
| Cox (% dwt) | −0.38 | −0.39 |
| humus (% dwt) | −0.38 | −0.39 |
| Ntot (% dwt) | 0.47 | 0.10 |
| N-NH4 (mg/kg dwt) | 0.60 | −0.12 |
| N-NO3 (mg/kg dwt) | 0.83 | 0.23 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukla, J.; Holec, M.; Trögl, J.; Holcová, D.; Hofmanová, D.; Kuráň, P.; Popelka, J.; Pacina, J.; Kříženecká, S.; Usťak, S.; et al. Tourist Traffic Significantly Affects Microbial Communities of Sandstone Cave Sediments in the Protected Landscape Area “Labské Pískovce” (Czech Republic): Implications for Regulatory Measures. Sustainability 2018, 10, 396. https://doi.org/10.3390/su10020396
Kukla J, Holec M, Trögl J, Holcová D, Hofmanová D, Kuráň P, Popelka J, Pacina J, Kříženecká S, Usťak S, et al. Tourist Traffic Significantly Affects Microbial Communities of Sandstone Cave Sediments in the Protected Landscape Area “Labské Pískovce” (Czech Republic): Implications for Regulatory Measures. Sustainability. 2018; 10(2):396. https://doi.org/10.3390/su10020396
Chicago/Turabian StyleKukla, Jaroslav, Michal Holec, Josef Trögl, Diana Holcová, Dagmar Hofmanová, Pavel Kuráň, Jan Popelka, Jan Pacina, Sylvie Kříženecká, Sergej Usťak, and et al. 2018. "Tourist Traffic Significantly Affects Microbial Communities of Sandstone Cave Sediments in the Protected Landscape Area “Labské Pískovce” (Czech Republic): Implications for Regulatory Measures" Sustainability 10, no. 2: 396. https://doi.org/10.3390/su10020396
APA StyleKukla, J., Holec, M., Trögl, J., Holcová, D., Hofmanová, D., Kuráň, P., Popelka, J., Pacina, J., Kříženecká, S., Usťak, S., & Honzík, R. (2018). Tourist Traffic Significantly Affects Microbial Communities of Sandstone Cave Sediments in the Protected Landscape Area “Labské Pískovce” (Czech Republic): Implications for Regulatory Measures. Sustainability, 10(2), 396. https://doi.org/10.3390/su10020396





