Analytical Challenges and Regulatory Requirements for Nasal Drug Products in Europe and the U.S.
Abstract
:1. Introduction
| Test | Pressurized metered dose nasal sprays | Nasal powders | Single and multiple use nasal drops | Single and multiple use nasal sprays |
|---|---|---|---|---|
| Specifications for the drug product | ||||
| Pump/valve delivery | yes # | yes # | ||
| Delivered dose/content uniformity | yes | yes, for multiple use drops | yes, for multiple use sprays | |
| Dose content uniformity through container life | yes # | yes # | ||
| Content uniformity/uniformity of dosage units | no * | no * | yes, for single use drops * | yes, for single use sprays * |
| Mean delivered dose | yes * | yes * | yes, for multiple use drops * | yes, for multiple use sprays * |
| Spray pattern | yes # | yes # | ||
| Particle/droplet size distribution | yes | yes | no | yes |
| Particle size distribution of API | yes, for suspensions # | yes, for suspensions # | ||
| Microscopic evaluation | yes, for suspensions # | |||
| Particulate matter | yes # | yes # | ||
| Microbial limits | yes | yes | yes | yes |
| Preservative content | no * | no * | yes, if present * | yes, if present * |
| Preservatives and stabilizing excipients assay | yes # | |||
| Sterility | no * | no * | yes, if product is sterile * | yes, if product is sterile * |
| Net content/minimum fill | yes # | yes # | ||
| Number of actuations per container | yes * | yes * | yes, for multiple use sprays * | |
| Weight loss (stability) | yes # | |||
| Leachables (stability) | yes # | yes # | ||
| Osmolality | yes # | |||
| Viscosity | yes # | |||
| Appearance and color of content and container closure system | yes # | |||
| Water or moisture content | yes | yes | no | no |
| Dehydrated alcohol content | yes, if used as a cosolvent # | |||
| Leak rate | yes | no | no | no |
| Pressure testing | yes, if cosolvent or more than one propellant is used # | |||
| Test | Pressurized metered dose nasal sprays | Nasal powders | Single and multiple use nasal drops | Single and multiple use nasal sprays |
|---|---|---|---|---|
| Drug product characterization/development studies | ||||
| Physical characterization | yes, for suspensions * | yes * | yes, for suspensions * | yes, for suspensions * |
| Priming and repriming (in various orientations) | yes | no | no | yes |
| Plume geometry | yes # | yes # | ||
| Microscopic evaluation | yes, for suspensions # | |||
| Effect of resting time | yes # | |||
| Shaking requirements | yes, for suspensions | no | yes, for suspensions | yes, for suspensions |
| Minimum fill justification | yes * | yes * | yes * | yes * |
| Extractables/leachables | yes * | no * | yes * | yes * |
| Performance after temperature cycling | yes | no | no | yes |
| Effect of environmental moisture | yes * | yes * | no * | no * |
| Cleaning instructions | yes | yes | yes, for multiple use drops | yes, for multiple use sprays |
| Device robustness | yes | yes | yes | yes |
| Profiling of sprays near container exhaustion (tail off characteristics) | yes # | yes # | ||
| Delivered dose uniformity through container life | yes * | yes * | yes, for multiple use drops * | yes, for multiple use sprays * |
| Effect of storage on PSD | yes, for suspensions # | yes, for suspensions # | ||
| Particle/droplet size distribution | yes | yes | no | yes, for multiple use sprays |
| Preservative effectiveness (and sterility maintenance) | no | no | yes, if present | yes, if present |
| Photostability | yes, if drug is exposed to light # | yes, if drug is exposed to light # | yes, if drug is exposed to light # | yes, if drug is exposed to light # |
| Actuator/mouthpiece deposition | yes | yes | no | yes * |
| Determination of appropriate storage conditions | yes # | |||
| Stability of primary (unprotected) package | yes # | yes # | ||
| Delivery device development | yes | yes | yes | yes |
| Microbial challenge | yes # | |||
| Effect of dosing orientation | yes # | |||
| In vitro dose proportionality | yes, for suspensions in multiple strengths # | yes, for suspensions in multiple strengths # | ||
| Low temperature performance | yes * | no * | no * | no * |
2. Experimental Section
2.1. Materials
2.2. Model Formulations
2.3. Determination of Viscosity and Surface Tension
2.4. Determination of Droplet Size Distribution
2.5. Determination of Plume Geometry
2.6. Determination of Shot Weights/Validation of Pump Delivery
3. Results and Discussion
3.1. Droplet Size Distribution
3.1.1. Regulatory Aspects
3.1.2. Analytical Aspects
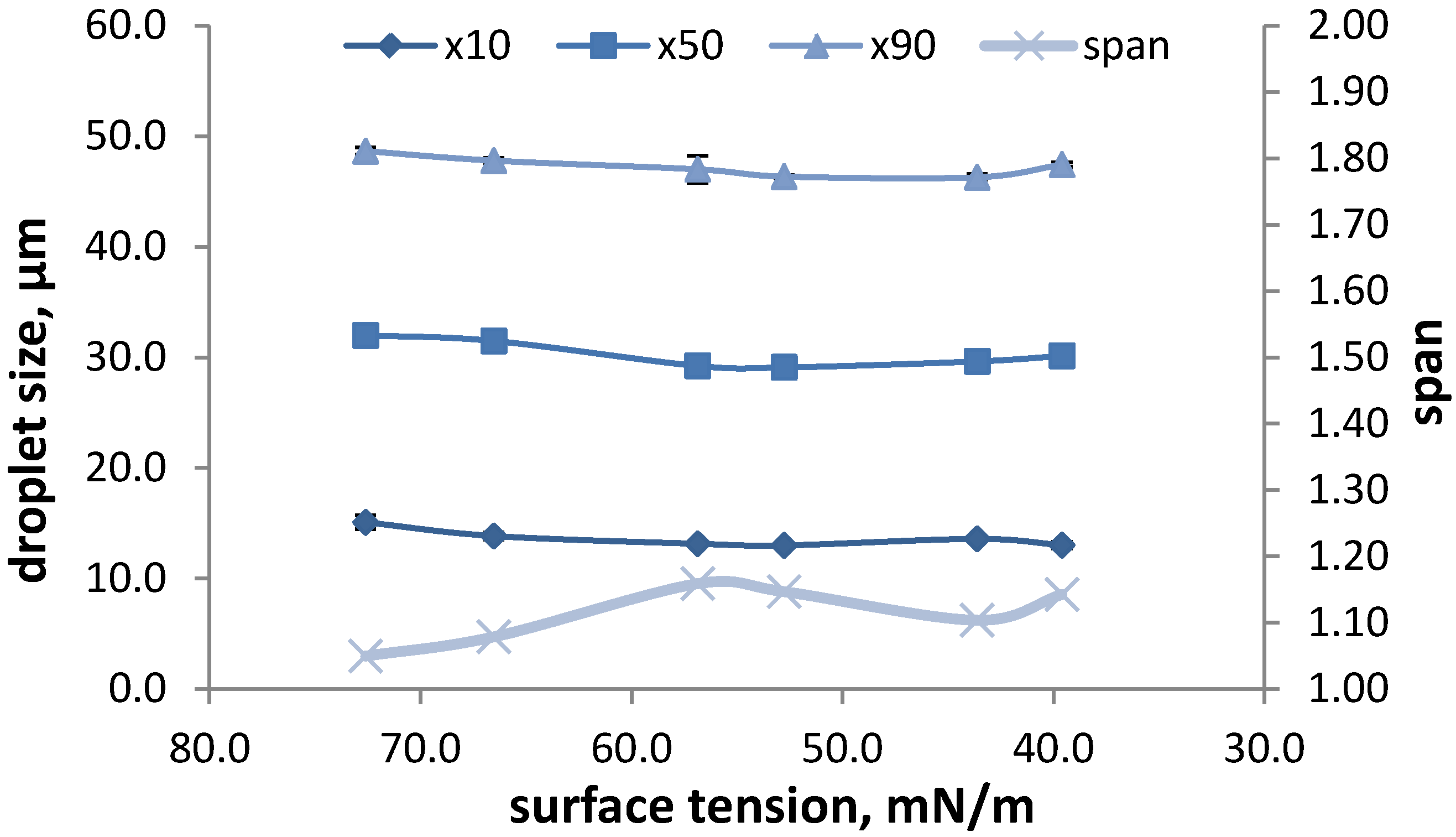
3.1.2.1. Formulation Dependent Variables
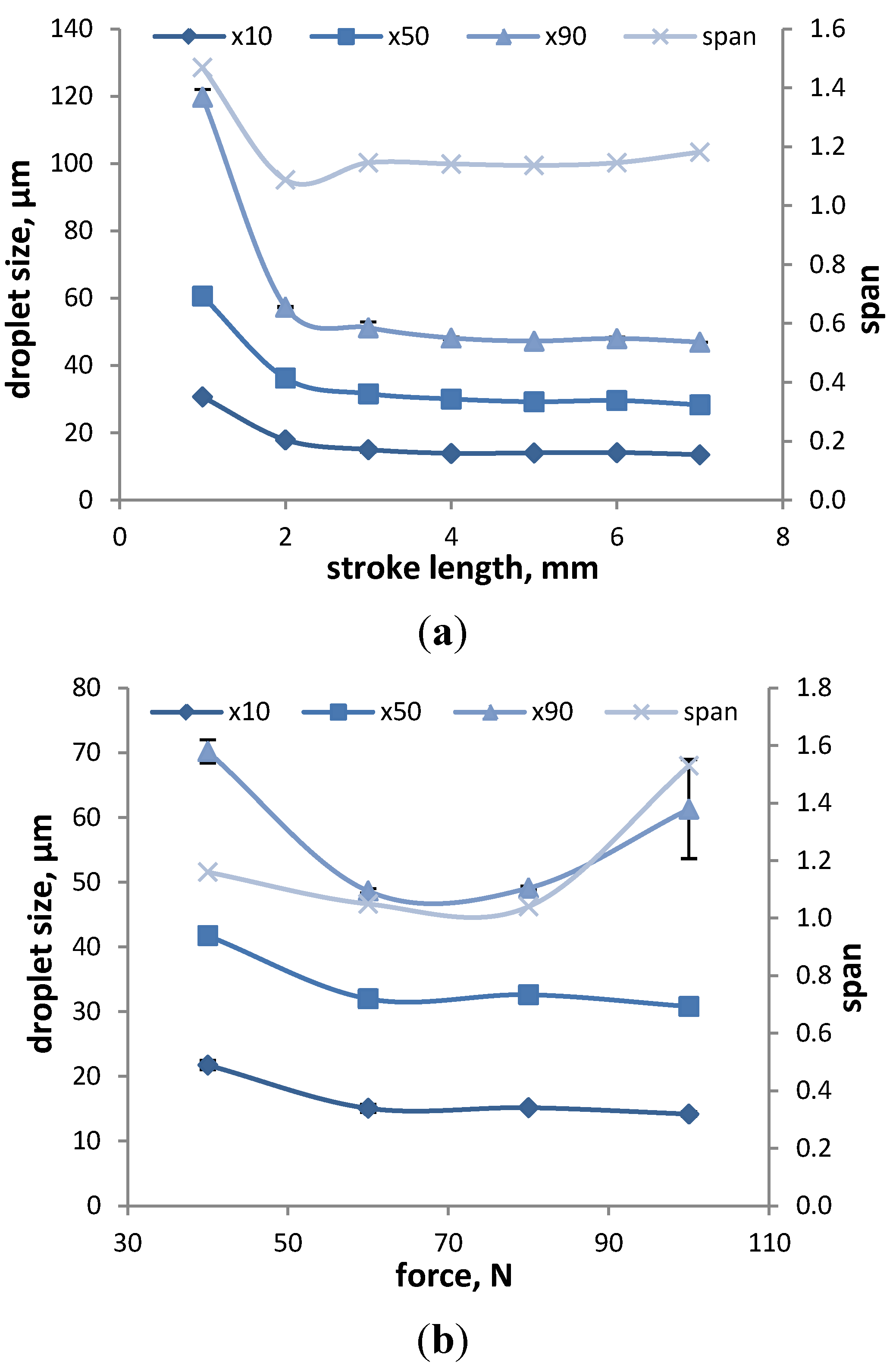
3.1.2.2. Device-Dependent Variables
3.1.2.3. Method- and Actuation-Dependent Variables

3.2. Plume Geometry and Spray Pattern
3.2.1. Regulatory Aspects
3.2.2. Analytical Aspects
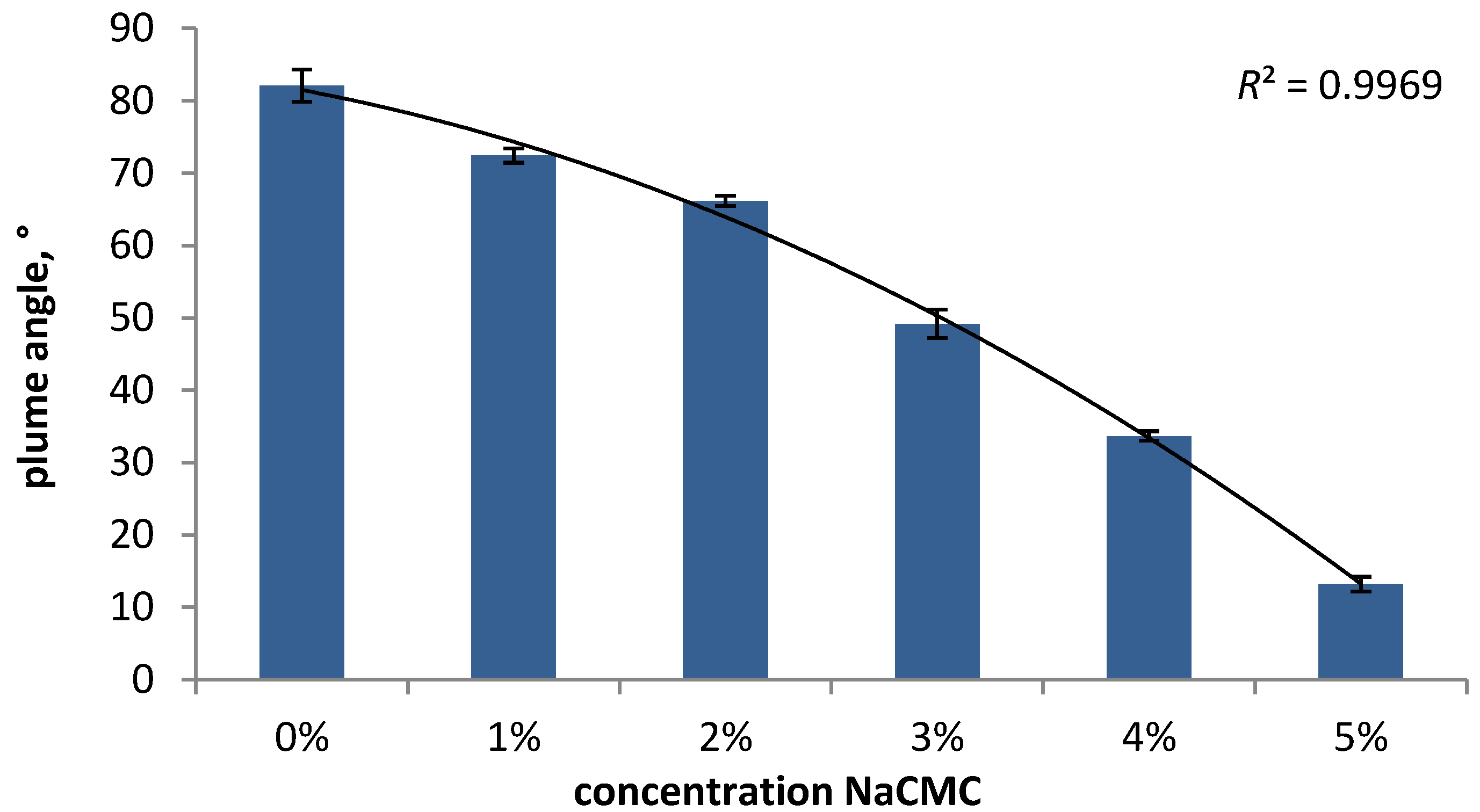
3.2.2.1. Formulation-Dependent Variables

3.2.2.2. Device-Dependent Variables
3.2.2.3. Method- and Actuation-Dependent Variables
3.3. Shot Weights/Pump Delivery
3.3.1. Regulatory Aspects
3.3.2. Analytical Aspects
3.3.2.1. Formulation-Dependent Variables
3.3.2.2. Device-Dependent Variables
3.3.2.3. Method- and Actuation-Dependent Variables

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Illum, L. Nasal drug delivery—Possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Jadhav, K.R.; Gambhire, M.N.; Shaikh, I.M.; Kadam, V.J.; Pisal, S.S. Nasal drug delivery system-factors affecting and applications. Curr. Drug Ther. 2007, 2, 27–38. [Google Scholar]
- Costantino, H.R.; Illum, L.; Brandt, G.; Johnson, P.H.; Quay, S.C. Intranasal delivery: Physicochemical and therapeutic aspects. Int. J. Pharm. 2007, 337, 1–24. [Google Scholar]
- Kulkarni, V.; Shaw, C. Formulation and characterization of nasal sprays. An examination of nasal spray formulation parameters and excipients and their influence on key in vitro tests. Inhalation 2012, 10–15. [Google Scholar]
- Guo, C.; Stine, K.J.; Kauffman, J.F.; Doub, W.H. Assessment of the influence factors on in vitro testing of nasal sprays using Box-Behnken experimental design. Eur. J. Pharm. Sci. 2008, 35, 417–426. [Google Scholar] [CrossRef]
- Behl, C.R.; Pimplaskar, H.K.; Sileno, A.P.; de Meireles, J.; Romeo, V.D. Effects of physicochemical properties and other factors on systemic nasal drug delivery. Adv. Drug Deliv. Rev. 1997, 29, 89–116. [Google Scholar]
- Suman, J. In vitro nasal spray characterization. Inhalation 2009, 15–19. [Google Scholar]
- U.S. Food and Drug Administration. Guidance for industry: Nasal spray and inhalation solution, suspension, and spray drug products—Chemistry, manufacturing, and controls documentation. In Fed. Regist.; 2002; pp. 1–49. [Google Scholar]
- U.S. Food and Drug Administration. Draft guidance for industry: Bioavailability and bioequivalence studies for nasal aerosols and nasal sprays for local action. In Fed. Regist.; 2003; pp. 1–37. [Google Scholar]
- U.S. Food and Drug Administration. Draft guidance for industry: Metered dose inhaler (MDI) and dry powder inhaler drug products—Chemistry, manufacturing, and controls documentation. In Fed. Regist.; 1998; pp. 1–66. [Google Scholar]
- European Medicines Agency. Guideline on the Pharmaceutical Quality of Inhalation and Nasal Products; European Medicines Agency: London, UK, 2006; pp. 1–27. [Google Scholar]
- Cheng, Y.S.; Holmes, T.D.; Gao, J.; Guilmette, R.A.; Li, S.; Surakitbanharn, Y.; Rowlings, C. Characterization of nasal spray pumps and deposition pattern in a replica of the human nasal airway. J. Aerosol. Med. 2001, 14, 267–280. [Google Scholar] [CrossRef]
- Dayal, P.; Pillay, V.; Babu, R.J.; Singh, M. Box-Behnken experimental design in the development of a nasal drug delivery system of model drug hydroxyurea: Characterization of viscosity, in vitro drug release, droplet size, and dynamic surface tension. AAPS Pharm. Sci. Tech. 2005, 6, E573–E585. [Google Scholar] [CrossRef]
- Dayal, P.; Shaik, M.S.; Singh, M. Evaluation of different parameters that affect droplet-size distribution from nasal sprays using the Malvern Spraytec®. J. Pharm. Sci. 2004, 93, 1725–1742. [Google Scholar] [CrossRef]
- Suman, J.D.; Laube, B.L.; Lin, T.-C.; Brouet, G.; Dalby, R. Validity of in vitro tests on aqueous spray pumps as surrogates for nasal deposition. Pharm. Res. 2002, 19, 1–6. [Google Scholar]
- Suman, J.D.; Laube, B.; Dalby, R. Comparison of nasal deposition and clearance of aerosol generated by a nebulizer and an aqueous spray pump. Pharm. Res. 1999, 16, 1648–1652. [Google Scholar] [CrossRef]
- Guo, C.; Doub, W.H. The influence of actuation parameters on in vitro testing of nasal spray products. J. Pharm. Sci. 2006, 95, 2029–2040. [Google Scholar]
- Harris, A.S.; Svensson, E.; Wagner, Z.G.; Lethagen, S.; Nilsson, I.M. Effect of viscosity on particle size, deposition, and clearance of nasal delivery systems containing desmopressin. J. Pharm. Sci. 1988, 77, 405–408. [Google Scholar]
- Copley, M.; Kippax, P. From actuation to deposition: Particle sizing techniques for characterizing nasal drug delivery systems. Inhalation 2012, 12–16. [Google Scholar]
- Sangolkar, S.; Adhao, V.; Mundhe, D.; Sawarkar, H. Particle size determination of nasal drug delivery system: A review. Int. J. Pharm. Sci. Rev. Res. 2012, 17, 66–73. [Google Scholar]
- Farina, D.J. Regulatory aspects of nasal and pulmonary spray drug products. In Handbook of Non-Invasive Drug Delivery Systems, 1st ed.; Kulkarni, V.S., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 247–290. [Google Scholar]
- Pennington, J.; Pandey, P.; Tat, H.; Willson, J.; Donovan, B. Spray pattern and droplet size analyses for high-shear viscosity determination of aqueous suspension corticosteroid nasal sprays. Drug Dev. Ind. Pharm. 2008, 34, 923–929. [Google Scholar] [CrossRef]
- Bommer, R. Drug Delivery. Nasal Route. In Encyclopedia of Pharmaceutical Technology; Swabrick, J., Ed.; Informa Healthcare Inc.: New York, NY, USA, 2007; Volume 3, pp. 1201–1208. [Google Scholar]
- Suman, J.D.; Laube, B.L.; Dalby, R. Validity of in vitro tests on aqueous spray pumps as surrogates for nasal deposition, absorption, and biologic response. J. Aerosol. Med. 2006, 19, 510–521. [Google Scholar] [CrossRef]
- Farina, D.J. Nasal spray actuation. Advancing the science of in vitro testing and laboratory data management for nasal sprays. Drug Deliv. Tech. 2004, 4, 46–51. [Google Scholar]
- Eck, C.; McGrath, T.F.; Perlwitz, A.G. Droplet size distribution in a solution nasal spray. In Respiratory Drug Delivery VII; Byron, P.R., Dalby, R.N., Farr, S.J., Peart, J., Eds.; Serentec Press: Raleigh, NC, USA, 2000; pp. 475–478. [Google Scholar]
- Aumiller, W.; Aydin, M.; Shelton, C.; Cummings, H.; Steele, A.; Evans, C. Time correlation of plume geometry and laser light scattering. In Respiratory Drug Delivery VIII; Dalby, R.N., Farr, S.J., Eds.; Interpharm Press: Buffalo Grove, IL, USA, 2002; pp. 497–499. [Google Scholar]
- Kippax, P.; Suman, J.D.; Williams, G. Enhancing the in vitro assessment of nasal sprays. Available online: http://www.pharmtech.com/pharmtech/Formulation/Enhancing-the-iin-vitroi-assessment-of-nasal-spray/ArticleStandard/Article/detail/525670 (accessed on 24 November 2013).
- Doughty, D.; Vibbert, C.; Kewalramani, A.; Bollinger, M.; Dalby, R. Automated actuation of nasal spray products: Determination and comparison of adult and pediatric settings. Drug Dev. Ind. Pharm. 2011, 37, 359–366. [Google Scholar] [CrossRef]
- Guo, Y.; Suman, J.; Dalby, R. Investigation of the effect of formulation variables on in vitro performance of nasal sprays. In Respiratory Drug Delivery IX; Byron, P.R., Dalby, R.N., Peart, J., Suman, J.D., Farr, S.J., Eds.; Davies Healthcare International Publishing: River Grove, IL, USA, 2004; pp. 785–787. [Google Scholar]
- Djupesland, P. Nasal drug delivery devices: Characteristics and performance in a clinical perspective—A review. Drug Deliv. Transl. Res. 2013, 3, 42–62. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Trows, S.; Wuchner, K.; Spycher, R.; Steckel, H. Analytical Challenges and Regulatory Requirements for Nasal Drug Products in Europe and the U.S. Pharmaceutics 2014, 6, 195-219. https://doi.org/10.3390/pharmaceutics6020195
Trows S, Wuchner K, Spycher R, Steckel H. Analytical Challenges and Regulatory Requirements for Nasal Drug Products in Europe and the U.S. Pharmaceutics. 2014; 6(2):195-219. https://doi.org/10.3390/pharmaceutics6020195
Chicago/Turabian StyleTrows, Sabrina, Klaus Wuchner, Rene Spycher, and Hartwig Steckel. 2014. "Analytical Challenges and Regulatory Requirements for Nasal Drug Products in Europe and the U.S." Pharmaceutics 6, no. 2: 195-219. https://doi.org/10.3390/pharmaceutics6020195
APA StyleTrows, S., Wuchner, K., Spycher, R., & Steckel, H. (2014). Analytical Challenges and Regulatory Requirements for Nasal Drug Products in Europe and the U.S. Pharmaceutics, 6(2), 195-219. https://doi.org/10.3390/pharmaceutics6020195