Key Determinants of Human α-Defensin 5 and 6 for Enhancement of HIV Infectivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. HIV Infection
2.3. HIV Attachment Assays
2.4. Late HIV Reverse Transcription Quantitative PCR
2.5. Statistical Analysis
3. Results
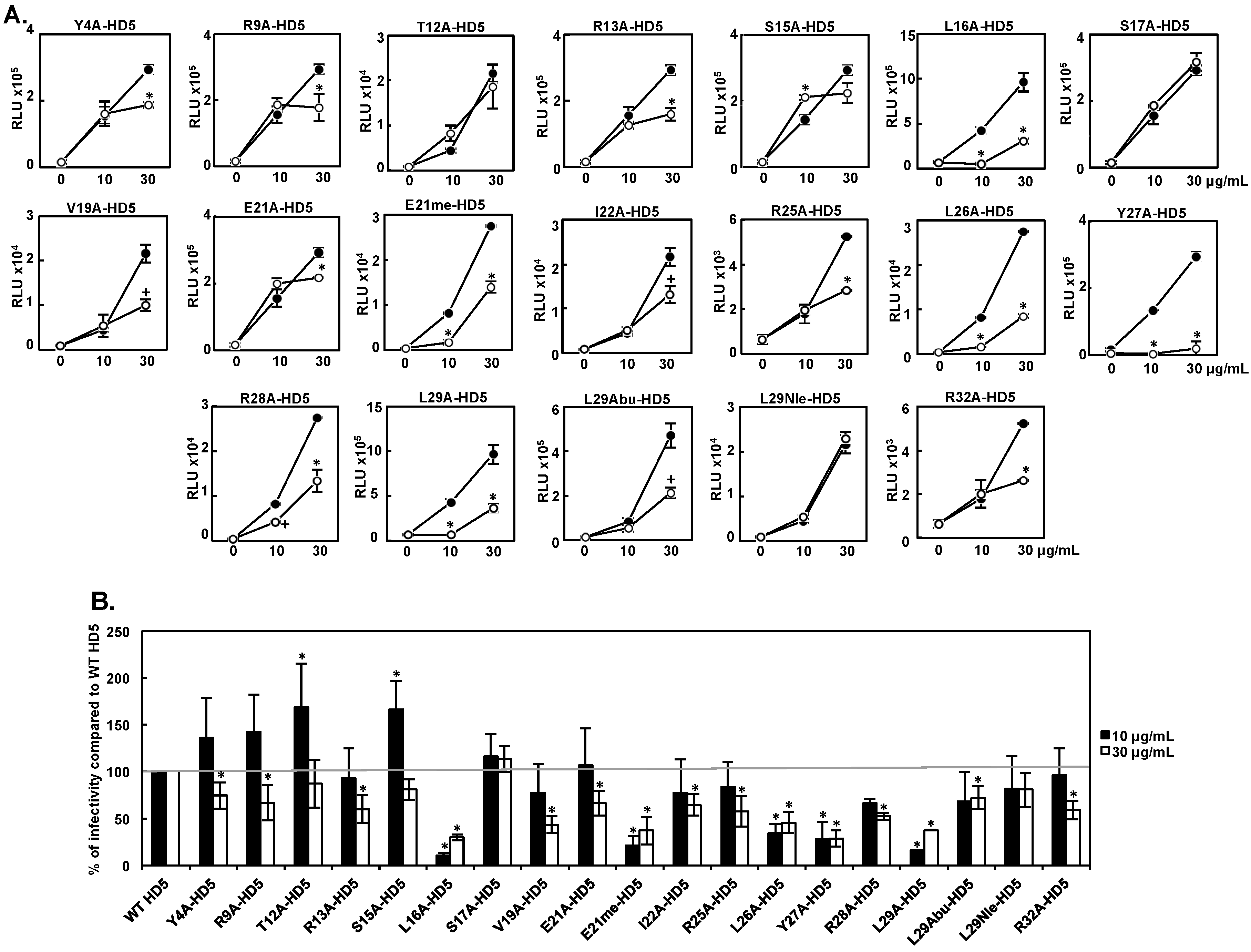
3.1. Effect of HD5 Mutants on HIV Infectivity
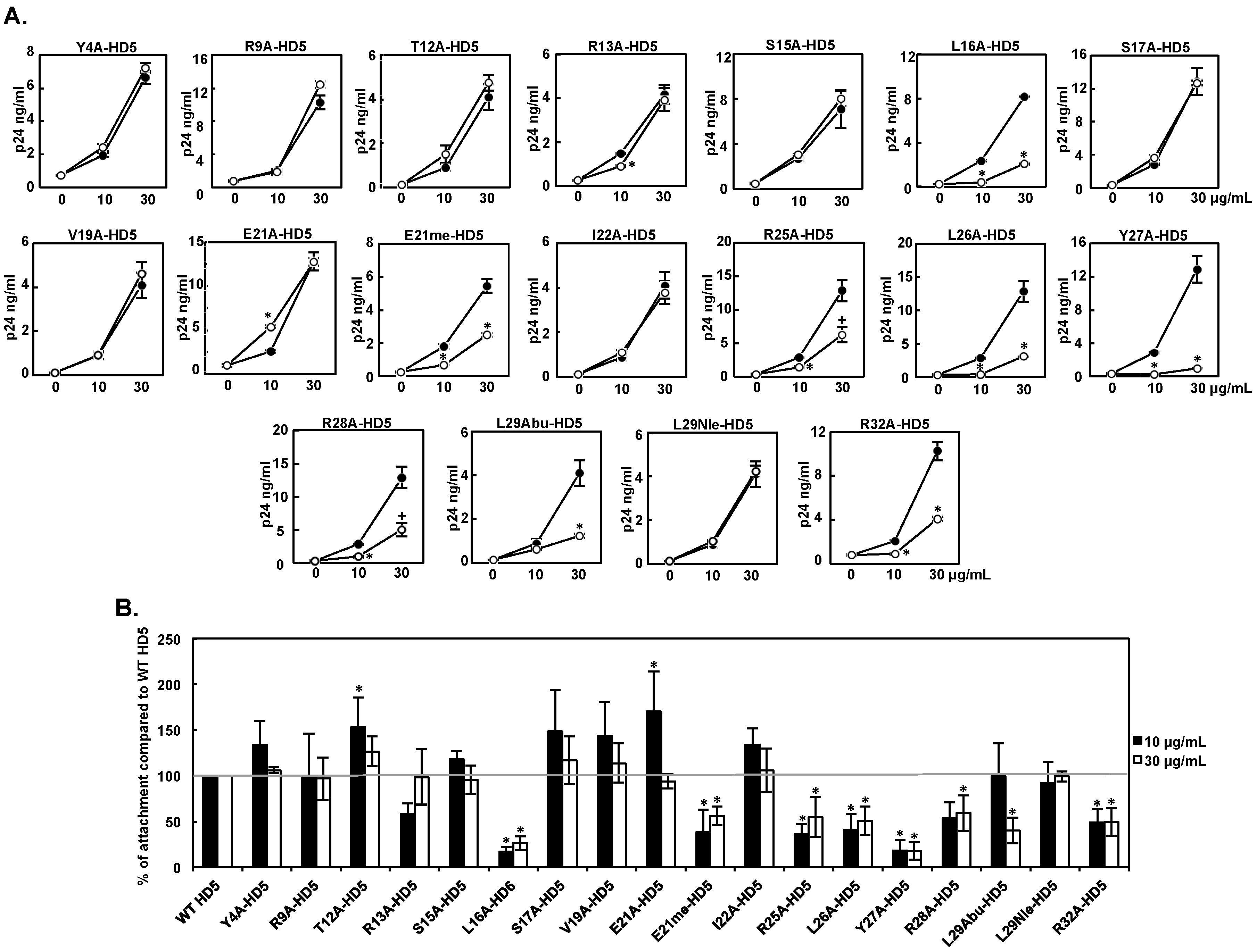
3.2. Effect of HD5 Mutants on HIV Attachment
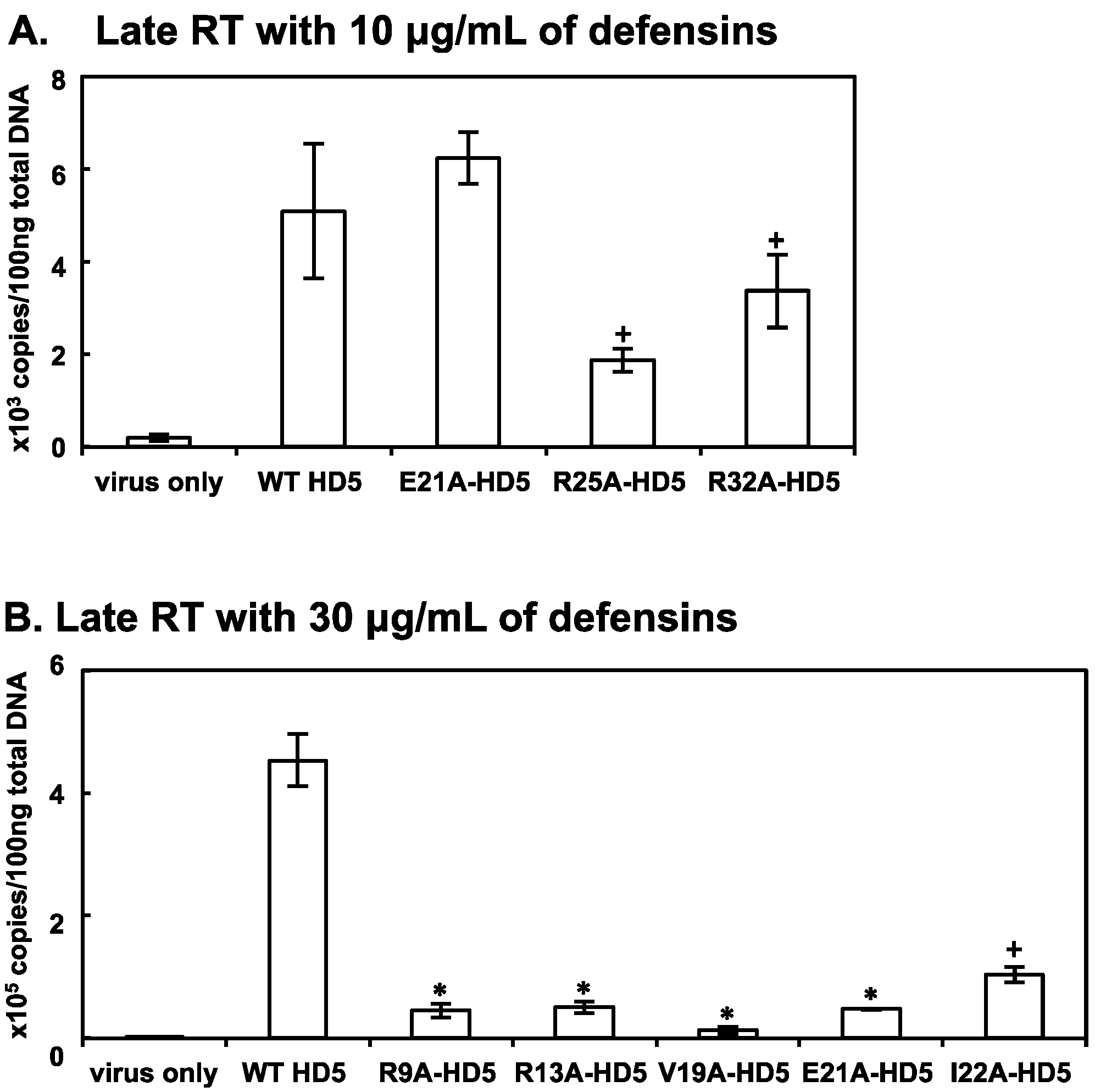
3.3. Some HD5 Mutants Affect HIV Reverse Transcription
3.4. Effect of HD6 Mutants on HIV Infection-Enhancing Activity
3.5. Effect of HD6 Mutants on HIV Attachment-Enhancing Activity
3.6. Effect of HD6 Mutations on Reverse Transcription
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ganz, T. The role of antimicrobial peptides in innate immunity. Integr. Comp. Biol. 2003, 43, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Klotman, M.E.; Chang, T.L. Defensins in innate antiviral immunity. Nat. Rev. Immunol. 2006, 6, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Lu, W. α-Defensins in human innate immunity. Immunol. Rev. 2012, 245, 84–112. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.R.; Liu, X.P.; Liao, Q.P. Human defensins and cytokines in vaginal lavage fluid of women with bacterial vaginosis. Int. J. Gynaecol. Obstet. 2008, 103, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Levinson, P.; Kaul, R.; Kimani, J.; Ngugi, E.; Moses, S.; MacDonald, K.S.; Broliden, H.; Hribod, T.; Kibera HIV Study Group. Levels of innate immune factors in genital fluids: Association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS 2009, 23, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.; Yang, H.; Yavagal, S.; Preza, G.C.; Murillo, O.; Lima, H.; Greene, S.; Mahoozi, L.; Klein-Patel, M.; Diamond, G.; et al. Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions. Infect. Immun. 2005, 73, 4823–4833. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Chu, H.; Shen, B.; Feathers, R.W.; Kays, R.J.; Lee, S.K.; Bevins, C.L. Paneth cell antimicrobial peptides: topographical distribution and quantification in human gastrointestinal tissues. FEBS Lett. 2006, 580, 5344–5350. [Google Scholar] [CrossRef] [PubMed]
- Klotman, M.E.; Rapista, A.; Teleshova, N.; Micsenyi, A.; Jarvis, G.A.; Lu, W.; Porter, E.; Chang, T.L. Neisseria gonorrhoeae-induced human defensins 5 and 6 increase HIV infectivity: Role in enhanced transmission. J. Immunol. 2008, 180, 6176–6185. [Google Scholar] [CrossRef] [PubMed]
- Chairatana, P.; Nolan, E.M. Molecular basis for self-assembly of a human host-defense peptide that entraps bacterial pathogens. J. Am. Chem. Soc. 2014, 136, 13267–13276. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Pazgier, M.; Jung, G.; Nuccio, S.P.; Castillo, P.A.; de Jong, M.F.; Winter, M.J.; Winter, S.E.; Wehkamp, J.; Shen, B.; et al. Human α-defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science 2012, 337, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Ericksen, B.; Wu, X.; de Leeuw, E.; Zhao, L.; Pazgier, M.; Lu, W. Functional determinants of human enteric α-defensin HD5: Crucial role for hydrophobicity at dimer interface. J. Biol. Chem. 2012, 287, 21615–21627. [Google Scholar] [CrossRef] [PubMed]
- Tenge, V.R.; Gounder, A.P.; Wiens, M.E.; Lu, W.; Smith, J.G. Delineation of interfaces on human alpha-defensins critical for human adenovirus and human papillomavirus inhibition. PLoS Pathog. 2014, 10, e1004360. [Google Scholar] [CrossRef] [PubMed]
- Rapista, A.; Ding, J.; Benito, B.; Lo, Y.T.; Neiditch, M.B.; Lu, W.; Chang, T.L. Human defensins 5 and 6 enhance HIV-1 infectivity through promoting HIV attachment. Retrovirology 2011, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabuddhe, V.V.; Vermund, S.H. The future of HIV prevention: Control of sexually transmitted infections and circumcision interventions. Infect. Dis. Clin. N. Am. 2007, 21, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ericksen, B.; Tucker, K.; Lubkowski, J.; Lu, W. Synthesis and characterization of human alpha-defensins 4–6. J. Pept. Res. 2004, 64, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; de Leeuw, E.; Pazgier, M.; Li, J.; Lubkowski, J.; Lu, W. The conserved salt bridge in human alpha-defensin 5 is required for its precursor processing and proteolytic stability. J. Biol. Chem. 2008, 283, 21509–21518. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zeng, P.; Ericksen, B.; Wu, Z.; Lu, W.Y.; Lu, W. Effects of the terminal charges in human neutrophil alpha-defensin 2 on its bactericidal and membrane activity. Peptides 2005, 26, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Szyk, A.; Wu, Z.; Tucker, K.; Yang, D.; Lu, W.; Lubkowski, J. Crystal structures of human alpha-defensins HNP4, HD5, and HD6. Protein Sci. 2006, 15, 2749–2760. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Saksela, K.; Andino, R.; Baltimore, D. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J. Virol. 1994, 68, 654–660. [Google Scholar] [PubMed]
- Connor, R.I.; Sheridan, K.E.; Ceradini, D.; Choe, S.; Landau, N.R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 1997, 185, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Zack, J.A.; Arrigo, S.J.; Weitsman, S.R.; Go, A.S.; Haislip, A.; Chen, I.S. HIV-1 entry into quiescent primary lymphocytes: Molecular analysis reveals a labile, latent viral structure. Cell 1990, 61, 213–222. [Google Scholar] [CrossRef]
- Gounder, A.P.; Wiens, M.E.; Wilson, S.S.; Lu, W.; Smith, J.G. Critical determinants of human α-defensin 5 activity against non-enveloped viruses. J. Biol. Chem. 2012, 287, 24554–24562. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Jung, G.; Ruchala, P.; Andre, S.; Gabius, H.J.; Lu, W. Multivalent binding of carbohydrates by the human α-defensin, HD5. J. Immunol. 2009, 183, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K.O. The roles of antimicrobial peptides in innate host defense. Curr. Pharm. Des. 2009, 15, 2377–2392. [Google Scholar] [CrossRef] [PubMed]
- Chairatana, P.; Chiang, I.L.; Nolan, E.M. Human α-defensin 6 self-assembly prevents adhesion and suppresses virulence traits of Candida albicans. Biochemistry 2017, 56, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Herrera, R.; Morris, M.; Rosbe, K.; Feng, Z.; Weinberg, A.; Tugizov, S. Human beta-defensins 2 and -3 cointernalize with human immunodeficiency virus via heparan sulfate and reduce infectivity of intracellular virions in tonsil epithelial cells. Virol. Proteoglycans 2016, 487, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Hazrati, E.; Galen, B.; Lu, W.; Wang, W.; Ouyang, Y.; Keller, M.J.; Lehrer, R.I.; Herold, B.C. Human alpha- and beta-defensins block multiple steps in herpes simplex virus infection. J. Immunol. 2006, 177, 8658–8666. [Google Scholar] [CrossRef] [PubMed]

| Defensin Mutants | Property |
|---|---|
| Y4A-HD5 | hydrophobicity |
| R9A-HD5 | cationicity |
| T12A-HD5 | NA |
| R13A-HD5 | cationicity |
| S15A-HD5 | NA |
| L16A-HD5 | hydrophobicity |
| S17A-HD5 | NA |
| V19A-HD5 | hydrophobicity |
| E21A-HD5 | anionicity |
| E21me-HD5 | dimerization |
| I22A-HD5 | hydrophobicity |
| R25A-HD5 | cationicity |
| L26A-HD5 | hydrophobicity |
| Y27A-HD5 | hydrophobicity |
| R28A-HD5 | cationicity |
| L29A-HD5 | hydrophobicity |
| L29Abu-HD5 | hydrophobicity |
| L29Nle-HD5 | hydrophobicity |
| R32A-HD5 | cationicity |
| Ac-HD6 | NA |
| HD6-NH2 | NA |
| F2A-HD6 | self-association/hydrophobicity |
| H5N/H27N-HD6 | polarity |
| H5A/H27A-HD6 | polarity |
| H27A-HD6 | self-association |
| H27W-HD6 | self-association |
| F29A-HD6 | self-association/hydrophobicity |
| Attachment 10 μg/mL | Infectivity 10 μg/mL | Attachment 30 μg/mL | Infectivity 30μg/mL | |
|---|---|---|---|---|
| WT HD5 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Y4A-HD5 | 134 ± 26 | 136 ± 42 | 106 ± 3 | 75 ± 14 |
| R9A-HD5 | 100 ± 46 | 142 ± 40 | 97 ± 23 | 67 ± 19 |
| T12A-HD5 | 153 ± 32 | 169 ± 47 | 127 ± 16 | 87 ± 25 |
| R13A-HD5 | 59 ± 11 | 92 ± 32 | 98 ± 30 | 60 ± 15 |
| S15A-HD5 | 118± 9 | 166 ± 30 | 95 ± 16 | 81 ± 11 |
| L16A-HD5 | 17 ± 5 | 10 ± 3 | 27 ± 7 | 30 ± 3 |
| S17A-HD5 | 148 ± 46 | 116 ± 24 | 117 ± 26 | 113 ± 14 |
| V19A-HD5 | 144 ± 37 | 77 ± 31 | 114 ± 21 | 43 ± 9 |
| E21A-HD5 | 171 ± 44 | 107 ± 40 | 94 ± 8 | 66 ± 13 |
| E21me-HD5 | 38 ± 25 | 21 ± 10 | 56 ± 10 | 37 ± 15 |
| I22A-HD5 | 134 ± 18 | 77 ± 36 | 106 ± 24 | 64 ± 11 |
| R25A-HD5 | 36 ± 11 | 83 ± 27 | 55 ± 22 | 58 ± 16 |
| L26A-HD5 | 41 ± 18 | 35 ± 10 | 51 ± 16 | 46 ± 11 |
| Y27A-HD5 | 19 ± 11 | 28 ± 18 | 18 ± 9 | 29 ± 9 |
| R28A-HD5 | 54 ± 17 | 66 ± 5 | 59 ± 19 | 52 ± 3 |
| L29A-HD5 | NA | 16 ± 0 | NA | 38 ± 0 |
| L29Abu-HD5 | 99 ± 36 | 68 ± 32 | 40 ± 14 | 72 ± 12 |
| L29Nle-HD5 | 92 ± 23 | 82 ± 35 | 99 ± 6 | 81 ± 18 |
| R32A-HD5 | 49 ± 15 | 96 ± 29 | 50 ± 15 | 59 ± 10 |
 : Comparable activity for HIV attachment and infectivity at both concentrations to WT HD5;
: Comparable activity for HIV attachment and infectivity at both concentrations to WT HD5;  : Comparable activity for attachment or infectivity at a specific concentration to WT HD5;
: Comparable activity for attachment or infectivity at a specific concentration to WT HD5;  : Reduced activity for HIV attachment and infectivity at a specific concentration compared to WT HD5;
: Reduced activity for HIV attachment and infectivity at a specific concentration compared to WT HD5;  : Reduced activity, but not consistent profiles of attachment and infectivity, compared to WT HD5 (p < 0.05);
: Reduced activity, but not consistent profiles of attachment and infectivity, compared to WT HD5 (p < 0.05);  : Enhanced activity when compared to WT HD5 (p < 0.05).
: Enhanced activity when compared to WT HD5 (p < 0.05).| Attachment 10 μg/mL | Infectivity 10 μg/mL | Attachment 30 μg/mL | Infectivity 30 μg/mL | |
|---|---|---|---|---|
| WT HD6 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Ac-HD6 | 57 ± 14 | 58 ± 24 | 87 ± 19 | 175 ± 43 |
| HD6-NH2 | 99 ± 14 | 28 ± 17 | 65 ± 12 | 205 ± 55 |
| F2A-HD6 | 17 ± 6 | 1 ± 1 | 4 ± 3 | 1 ± 0 |
| H5N/H27N-HD6 | 102 ± 21 | 65 ± 16 | 69 ± 9 | 60 ± 24 |
| H5A/H27A-HD6 | 43 ± 7 | 30 ± 16 | 68 ± 10 | 63 ± 24 |
| H27A-HD6 | 46 ± 16 | 39 ± 12 | 41 ± 1 | 108 ± 29 |
| H27W-HD6 | 17 ± 6 | 4 ± 3 | 6 ± 1 | 3 ± 2 |
| F29A-HD6 | 18 ± 7 | 2 ± 0 | 3 ± 2 | 2 ± 1 |
 : Comparable activity for attachment or infectivity at a specific concentration to WT HD6;
: Comparable activity for attachment or infectivity at a specific concentration to WT HD6;  : Reduced activity for HIV attachment and infectivity at a specific concentration compared to WT HD6;
: Reduced activity for HIV attachment and infectivity at a specific concentration compared to WT HD6;  : Reduced activity, but not consistent profiles of attachment and infectivity, compared to WT HD6 (p < 0.05);
: Reduced activity, but not consistent profiles of attachment and infectivity, compared to WT HD6 (p < 0.05);  : Enhanced activity when compared to WT HD6 (p < 0.05).
: Enhanced activity when compared to WT HD6 (p < 0.05).© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valere, K.; Lu, W.; Chang, T.L. Key Determinants of Human α-Defensin 5 and 6 for Enhancement of HIV Infectivity. Viruses 2017, 9, 244. https://doi.org/10.3390/v9090244
Valere K, Lu W, Chang TL. Key Determinants of Human α-Defensin 5 and 6 for Enhancement of HIV Infectivity. Viruses. 2017; 9(9):244. https://doi.org/10.3390/v9090244
Chicago/Turabian StyleValere, Kimyata, Wuyuan Lu, and Theresa L. Chang. 2017. "Key Determinants of Human α-Defensin 5 and 6 for Enhancement of HIV Infectivity" Viruses 9, no. 9: 244. https://doi.org/10.3390/v9090244





