Genetic Assessment of African Swine Fever Isolates Involved in Outbreaks in the Democratic Republic of Congo between 2005 and 2012 Reveals Co-Circulation of p72 Genotypes I, IX and XIV, Including 19 Variants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Samples
2.2. African Swine Fever (ASFV) DNA by Polymerase Chain Reaction (PCR)
2.3. Generation of ASFV Sequence Data
2.4. Molecular Characterization of ASFV
2.5. Central Hypervariable Region (CVR) of B602L Gene
3. Results
3.1. Clinical Findings and African Swine Fever (ASF) Diagnosis
Field Identification and Description of Collecting Localities
3.2. Laboratory Diagnostics
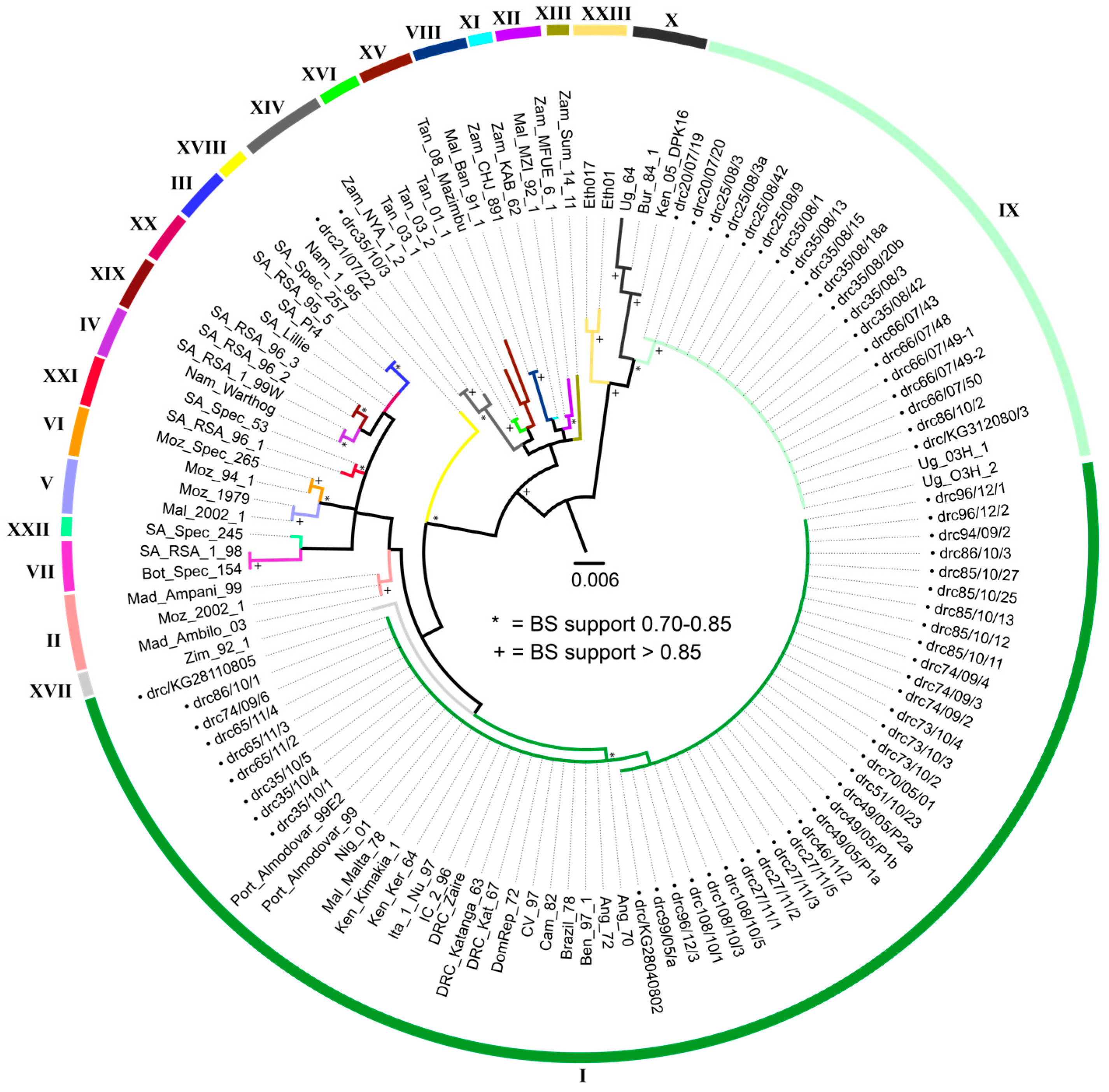
3.3. Molecular Characterization of ASFV
3.4. CVR of B602L Gene
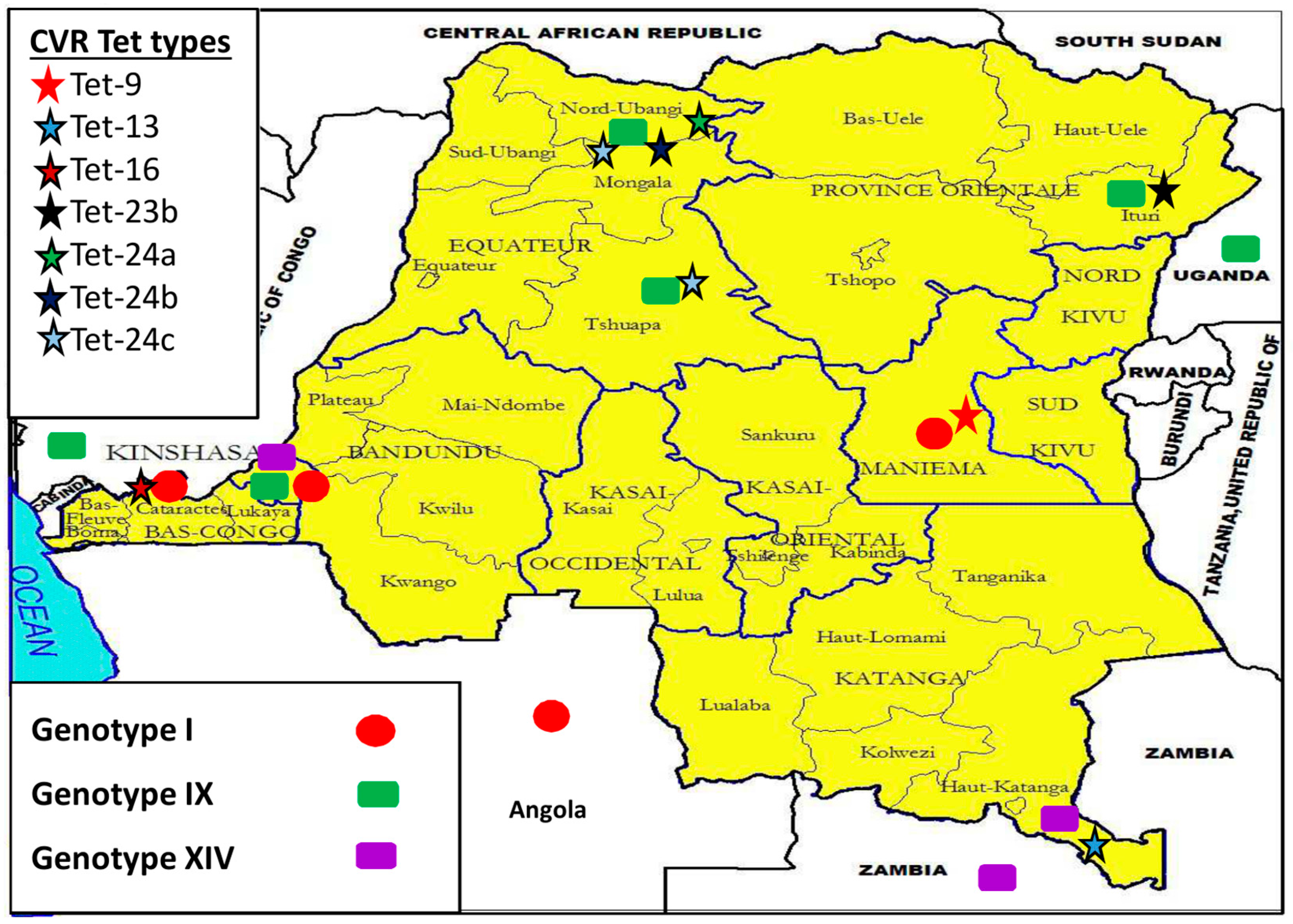
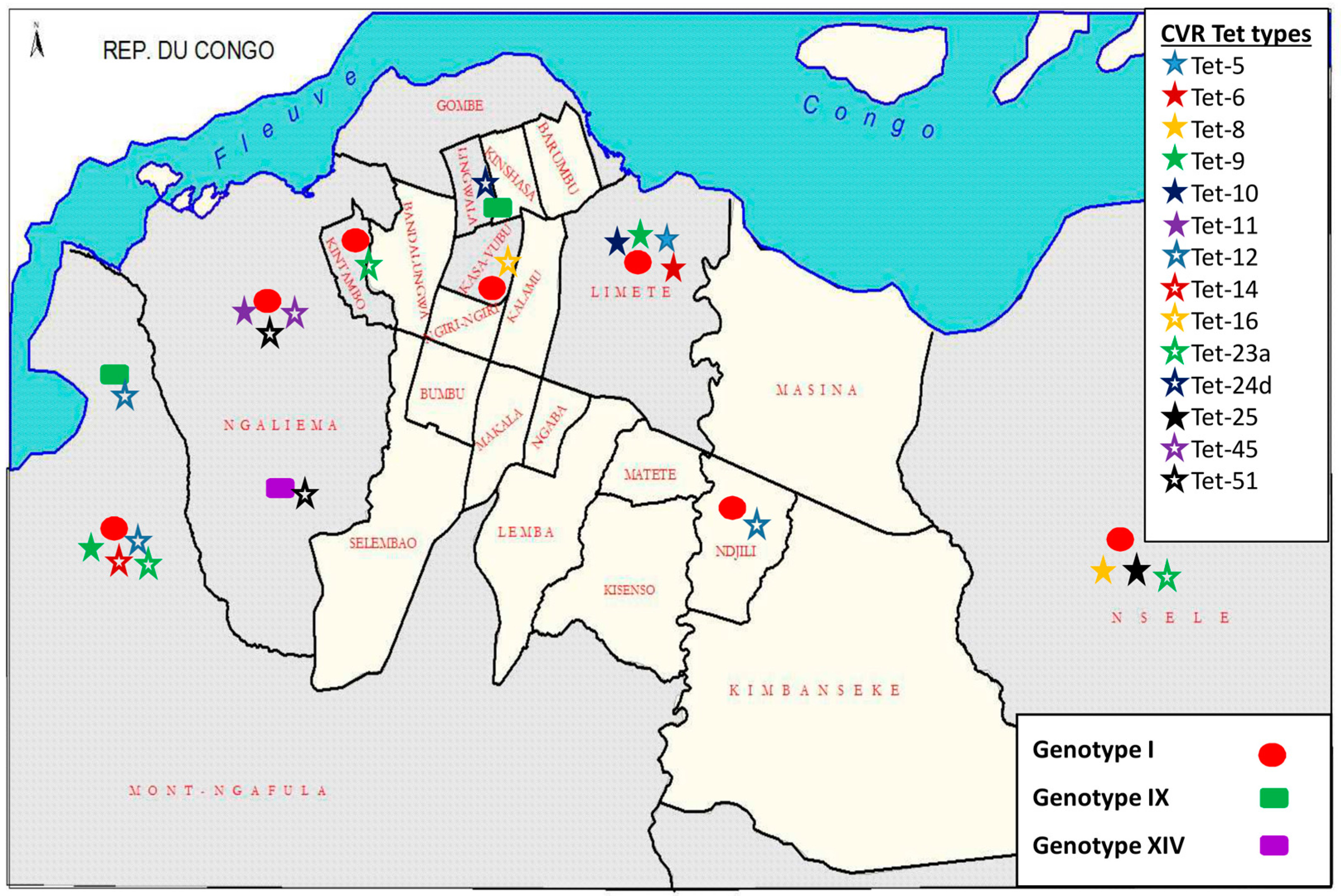
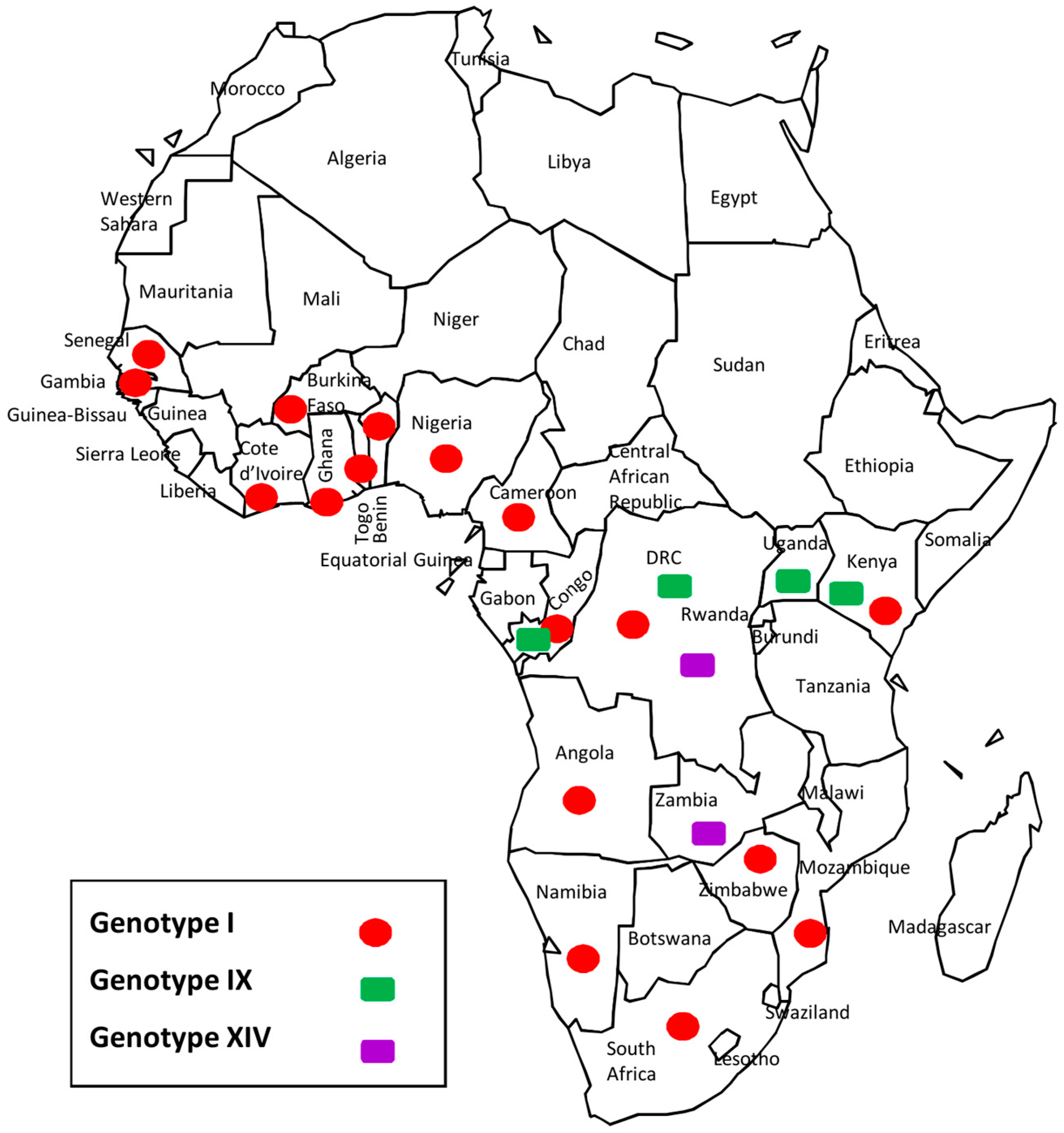
3.5. Democratic Republic of the Congo (DRC) ASFV Genotypes Geographical Distribution
4. Discussion
4.1. Molecular Characterization
4.2. Disease Ecology, Molecular Epidemiology and Case Investigations
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sánchez-Vizcaíno, J.M.; Mur, L.; Sánchez-Matamoros, A.; Martínez-López, B. African swine fever: New challenges and measures to prevent its spread. In Proceedings of the 82nd General Session World Assembly of Delegates of the World Organisation for Animal Health (OIE), Paris, France, 25–30 May 2014.
- Montgomery, R.E. On a form of swine fever occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef]
- Costard, S.; Mur, L.; Lubroth, J.; Sanchez-Vizcaino, J.M.; Pfeiffer, D.U. Epidemiology of African swine fever virus. Virus Res. 2013, 173, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Detray, D.E. African swine fever. Adv. Vet. Sci. 1963, 8, 299–333. [Google Scholar] [PubMed]
- Sanchez-Vizcaino, J.M.; Mur, L.; Martinez-Lopez, B. African swine fever: An epidemiological update. Transbound. Emerg. Dis. 2012, 59 (Suppl. 1), 27–35. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, R.J.; Michaud, V.; Heath, L.; Hutchings, G.; Oura, C.; Vosloo, W.; Dwarka, R.; Onashvili, T.; Albina, E.; Dixon, L.K. African swine fever virus isolate, Georgia, 2007. Emerg. Infect Dis. 2008, 14, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Plowright, W.; Thomson, G.R.; Neser, J.A. African swine fever. Infect. Dis. Livest. Spec. Ref. S. Afr. 1994, 1, 568–599. [Google Scholar]
- Hess, W.R.; Endris, R.G.; Haslett, T.M.; Monahan, M.J.; McCoy, J.P. Potential arthropod vectors of African swine fever virus in North America and the Caribbean basin. Vet. Parasitol. 1987, 26, 145–155. [Google Scholar] [CrossRef]
- Lubisi, B.A.; Bastos, A.D.; Dwarka, R.M.; Vosloo, W. Molecular epidemiology of African swine fever in East Africa. Arch. Virol. 2005, 150, 2439–2452. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Abrams, C.C.; Bowick, G.; Goatley, L.C.; Kay-Jackson, P.C.; Chapman, D.; Liverani, E.; Nix, R.; Silk, R.; Zhang, F. African swine fever virus proteins involved in evading host defence systems. Vet. Immunol. Immunopathol. 2004, 100, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.D.; Penrith, M.L.; Cruciere, C.; Edrich, J.L.; Hutchings, G.; Roger, F.; Couacy-Hymann, E.; Thomson, R. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch. Virol. 2003, 148, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Phologane, S.B.; Bastos, A.D.; Penrith, M.L. Intra- and inter-genotypic size variation in the central variable region of the 9RL open reading frame of diverse African swine fever viruses. Virus Genes 2005, 31, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Mwaengo, D.M.; Macharia, J.M.; Arias, M.; Taracha, E.A.; Soler, A.; Okoth, E.; Martin, E.; Kasiti, J.; Bishop, R.P. Enhanced discrimination of African swine fever virus isolates through nucleotide sequencing of the p54, p72, and pB602L (CVR) genes. Virus Genes 2009, 38, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, J.E.; Gallardo, C.; Nieto-Pelegrín, E.; Rivera-Arroyo, B.; Degefa-Negi, T.; Arias, M.; Jenberie, S.; Mulisa, D.D.; Gizaw, D.; Gelaye, E.; et al. Identification of a New Genotype of African Swine Fever Virus in Domestic Pigs from Ethiopia. Transbound. Emerg. Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kouakou, K.V.; Michaud, V.; Biego, H.G.; Gnabro, H.P.; Kouakou, A.V.; Mossoun, A.M.; Awuni, J.A.; Minoungou, G.L.; Aplogan, G.L.; Awoumé, F.K.; et al. African and classical swine fever situation in Ivory-Coast and neighboring countries, 2008–2013. Acta Trop. 2017, 166, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Luka, P.D.; Achenbach, J.E.; Mwiine, F.N.; Lamien, C.E.; Shamaki, D.; Unger, H.; Erume, J. Genetic Characterization of Circulating African Swine Fever Viruses in Nigeria (2007–2015). Transbound. Emerg. Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Anchuelo, R.; Pelayo, V.; Poudevigne, F.; Leon, T.; Nzoussi, J.; Bishop, R.; Pérez, C.; Soler, A.; Nieto, R.; et al. African swine fever virus p72 genotype IX in domestic pigs, Congo, 2009. Emerg. Infect. Dis. 2011, 7, 1556–1558. [Google Scholar] [CrossRef] [PubMed]
- Atuhaire, D.K.; Afayoa, M.; Ochwo, S.; Mwesigwa, S.; Okuni, J.B.; Olaho-Mukani, W.; Ojok, L. Molecular characterization and phylogenetic study of African swine fever virus isolates from recent outbreaks in Uganda (2010–2013). Virol. J. 2013. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Ademun, A.R.; Nieto, R.; Nantima, N.; Arias, M.; Martín, E.; Pelayo, V.; Bishop, R.P. Genotyping of African swine fever virus (ASFV) isolates associated with disease outbreaks in Uganda in 2007. Afr. J. Biotechnol. 2011, 10, 3488–3497. [Google Scholar]
- Misinzo, G.; Magambo, J.; Masambu, J.; Yongolo, M.G.; Van, D.J.; Nauwynck, H.J. Genetic characterization of African swine fever viruses from a 2008 outbreak in Tanzania. Transbound. Emerg. Dis. 2011, 58, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Gogin, A.; Gerasimov, V.; Malogolovkin, A.; Kolbasov, D. African swine fever in the North Caucasus region and the Russian Federation in years 2007–2012. Virus Res. 2013, 173, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Rodríguez, A.; Iglesias, I.; Muñoz, M.J.; Jurado, C.; Sánchez-Vizcaíno, J.M.; de la Torre, A. Update on the Risk of Introduction of African Swine Fever by Wild Boar into Disease-Free European Union Countries. Transbound. Emerg. Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Fernández-Pinero, J.; Pelayo, V.; Gazaev, I.; Markowska-Daniel, I.; Pridotkas, G.; Nieto, R.; Fernández-Pacheco, P.; Bokhan, S.; Nevolko, O.; et al. Genetic variation among African swine fever genotype II viruses, eastern and central Europe. Emerg. Infect. Dis. 2014, 20, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- El-Sawalhy, A.; Soumaré, B.; Nouala, S.; Mukanda, B.; Wamwayi, H.; Ahmed, I.G. African Swine fever. In Pan African Animal Health Yearbook; Interafrican Bureau for Animal Resources, African Union: Nairobi, Kenya, 2011; pp. 16–17. [Google Scholar]
- King, D.P.; Reid, S.M.; Hutchings, G.H.; Grierson, S.S.; Wilkinson, P.J.; Dixon, L.K.; Bastos, A.D.; Drew, T.W. Development of a TaqMan PCR assay with internal amplification control for the detection of African swine fever virus. J. Virol. Methods 2003, 107, 53–61. [Google Scholar] [CrossRef]
- Nix, R.J.; Gallardo, C.; Hutchings, G.; Blanco, E.; Dixon, L.K. Molecular epidemiology of African swine fever virus studied by analysis of four variable genome regions. Arch. Virol. 2006, 151, 2475–2494. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Boshoff, C.I.; Bastos, A.D.; Gerber, L.J.; Vosloo, W. Genetic characterisation of African swine fever viruses from outbreaks in southern Africa (1973–1999). Vet. Microbiol. 2007, 121, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Lubisi, B.A.; Bastos, A.D.; Dwarka, R.M.; Vosloo, W. Intra-genotypic resolution of African swine fever viruses from an East African domestic pig cycle: A combined p72-CVR approach. Virus Genes 2007, 35, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Misinzo, G.; Kwavi, D.E.; Sikombe, C.D.; Makange, M.; Peter, E.; Muhairwa, A.P.; Madege, M.J. Molecular characterization of African swine fever virus from domestic pigs in northern Tanzania during an outbreak in 2013. Trop. Anim. Health Prod. 2014, 46, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Ekue, N.F.; Wilkinson, P.J. Comparison of genomes of African swine fever virus isolates from Cameroon, other African countries and Europe. Rev. Elev. Méd. Vét. Pays Trop. 2000, 53, 229–238. [Google Scholar]
- Mulumba-Mfumu, L.K.; Goatley, L.C.; Saegerman, C.; Takamatsu, H.H.; Dixon, L.K. Immunization of African Indigenous Pigs with Attenuated Genotype I African Swine Fever Virus OURT88/3 Induces Protection Against Challenge with Virulent Strains of Genotype I. Transbound. Emerg. Dis. 2016, 63, e323–e327. [Google Scholar] [CrossRef] [PubMed]
- Praet, N.; Kanobana, K.; Kabwe, C.; Maketa, V.; Lukanu, P.; Lutumba, P.; Polman, K.; Matondo, P.; Speybroeck, N.; Dorny, P. Taenia solium cysticercosis in the Democratic Republic of Congo: How does pork trade affect the transmission of the parasite. PLoS Negl. Trop. Dis. 2010, 4, e817. [Google Scholar] [CrossRef] [PubMed]
- Atuhaire, D.K.; Ochwo, S.; Afayoa, M.; Mwesigwa, S.; Mwiine, F.N.; Okuni, J.B.; Olaho-Mukani, W.; Ojak, L. Molecular characterization of African swine fever virus in apparently healthy domestic pigs in Uganda. Afr. J. Biotechnol. 2014, 13, 2491–2499. [Google Scholar]
- Giammarioli, M.; Gallardo, C.; Oggiano, A.; Iscaro, C.; Nieto, R.; Pellegrini, C.; Dei, G.S.; Arias, M.; De Mia, G.M. Genetic characterisation of African swine fever viruses from recent and historical outbreaks in Sardinia (1978–2009). Virus Genes 2011, 42, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Goller, K.V.; Malogolovkin, A.S.; Katorkin, S.; Kolbasov, D.; Titov, I.; Hoper, D.; Beer, M.; Keil, G.M.; Portugal, R.; Blome, S. Tandem repeat insertion in African swine fever virus, Russia, 2012. Emerg. Infect. Dis. 2015, 21, 731–732. [Google Scholar] [CrossRef] [PubMed]
- Irusta, P.M.; Borca, M.V.; Kutish, G.F.; Lu, Z.; Caler, E.; Carrillo, C.; Rock, D.L. Amino acid tandem repeats within a late viral gene define the central variable region of African swine fever virus. Virology 1996, 220, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.D.; Penrith, M.L.; Macome, F.; Pinto, F.; Thomson, G.R. Co-circulation of two genetically distinct viruses in an outbreak of African swine fever in Mozambique: No evidence for individual co-infection. Vet. Microbiol. 2004, 103, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Soler, A.; Nieto, R.; Cano, C.; Pelayo, V.; Sanchez, M.A.; Pridotkas, G.; Fernandez-Pinero, J.; Briones, V.; Arias, M. Experimental Infection of Domestic Pigs with African Swine Fever Virus Lithuania 2014 Genotype II Field Isolate. Transbound. Emerg. Dis. 2017, 64, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Central Veterinary Laboratory (CVL). Rapport Annuel D’Activites (Kinshasa/RDC); CVL: Kinshasa, Democratic Republic of Congo, 2010; pp. 1–52. [Google Scholar]
- Owolodun, O.A.; Bastos, A.D.; Antiabong, J.F.; Ogedengbe, M.E.; Ekong, P.S.; Yakubu, B. Molecular characterisation of African swine fever viruses from Nigeria (2003–2006) recovers multiple virus variants and reaffirms CVR epidemiological utility. Virus Genes 2010, 41, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P. Evolution of repeated DNA sequences by unequal crossover. Science 1976, 191, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Blasco, R.; de la Vega, I.; Almazan, F.; Aguero, M.; Vinuela, E. Genetic variation of African swine fever virus: Variable regions near the ends of the viral DNA. Virology 1989, 173, 251–257. [Google Scholar] [CrossRef]
- Garcia-Barreno, B.; Sanz, A.; Nogal, M.L.; Vinuela, E.; Enjuanes, L. Monoclonal antibodies of African swine fever virus: Antigenic differences among field virus isolates and viruses passaged in cell culture. J. Virol. 1986, 58, 385–392. [Google Scholar] [PubMed]

| Virus | Outbreak Date | Location | GPS | Ecological Profile | Tissues | Farming System | Genotype |
|---|---|---|---|---|---|---|---|
| drc49/05/p1a | May 2005 | Limete | 4°18 S/15°22 E | City | Spl | Commercial | I |
| drc49/05p1b | May 2005 | Limete | 4°18 S/15°22 E | City | Ln | Commercial | I |
| drc49/05/P2a | May 2005 | Limete | 4°18 S/15°22 E | City | Spl | Commercial | I |
| drc70/05/1 | 2005 | Limete | 4°18 S/15°22 E | City | Spl | Commercial | I |
| drc75/05/1 | 2005 | Maniema | 2°93 S/25°86 E | City | Spl | Commercial | I |
| drc99/05/a | 2005 | Ngafula | 4°21 S/15°14 E | Peri-urban | Spl | Commercial | I |
| drcKG28110805 | Nov 2008 | Ngaliema | 4°21 S/15°21 E | City | Spl | Backyard | I |
| drcKG28040802 | Apr 2008 | Kasavubu | 4°20 S/15°20 E | City | Spl | Backyard | I |
| drc74/09/2 | 2009 | Nsele | 4°24 S/15°30 E | Peri-urban | Kd | Commercial | I |
| drc74/09/3 | 2009 | Nsele | 4°24 S/15°30 E | Peri-urban | Lg | Commercial | I |
| drc74/09/4 | 2009 | Nsele | 4°24 S/15°30 E | Peri-urban | Lv | Commercial | I |
| drc74/09/6 | 2009 | Nsele | 4°24 S/15°30 E | Peri-urban | Hrt | Commercial | I |
| drc94/09/2 | 2009 | Kintambo | 4°20 S/15°18 E | City | Ln | Commercial | I |
| drc35/10/1 | 2010 | Ngaliema | 4°21 S/15°05 E | City | Spl | Backyard | I |
| drc35/10/5 | 2010 | Ngaliema | 4°21 S/15°05 E | City | Ln | Backyard | I |
| drc35/10/4 | 2010 | Ngaliema | 4°21 S/15°05 E | City | Ln | Backyard | I |
| drc51/10/23 | 2010 | Ndjili | 4°24 S/15°21 E | City | Spl | Commercial | I |
| drc73/10/2 | 2010 | Ngaliema | 4°21 S/15°05 E | City | Kn | Backyard | I |
| drc73/10/3 | 2010 | Ngaliema | 4°21 S/15°05 E | City | Lv | Backyard | I |
| drc73/10/4 | 2010 | Ngaliema | 4°21 S/15°05 E | City | Ln | Backyard | I |
| drc85/10/13 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Spl | Commercial | I |
| drc85/10/12 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Hrt | Commercial | I |
| drc85/10/11 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Lg | Commercial | I |
| drc85/10/27 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Hrt | Commercial | I |
| drc85/10/25 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Lv | Commercial | I |
| drc86/10/1 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Kd | Commercial | I |
| drc86/10/3 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Lv | Commercial | I |
| drc108/10/3 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Lv | Commercial | I |
| drc108/10/5 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Spl | Commercial | I |
| drc27/11/1 | 2011 | Ngafula | 4°21 S/15°14 E | Peri-urban | Spl | Commercial | I |
| drc27/11/2 | 2011 | Ngafula | 4°21 S/15°14 E | Peri-urban | Ln | Commercial | I |
| drc27/11/3 | 2011 | Ngafula | 4°21 S/15°14 E | Peri-urban | Stm | Commercial | I |
| drc27/11/5 | 2011 | Ngafula | 4°21 S/15°14 E | Peri-urban | Lv | Commercial | I |
| drc65/11/2 | 2011 | Nsele | 4°24 S/15°30 E | Peri-urban | Kd | Commercial | I |
| drc65/11/3 | 2011 | Nsele | 4°24 S/15°30 E | Peri-urban | Spl | Commercial | I |
| drc65/11/4 | 2011 | Nsele | 4°24 S/15°30 E | Peri-urban | Lg | Commercial | I |
| drc96/12/1 | 2012 | Mayanda | 5°12 S/15°14 E | Rural | Lg | Village | I |
| drc96/12/2 | 2012 | Mayanda | 5°12 S/15°14 E | Rural | Spl | Village | I |
| drc96/12/3 | 2012 | Mayanda | 5°12 S/15°14 E | Rural | Hrt | Village | I |
| drc108/10/1 | Dec 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Lg | Commercial | I |
| drc46/11/2 | Jun 2011 | Kinshasa | 4°20 S/15°18 E | City | Hrt | Backyard | I |
| drc20/07/19 | Apr 2007 | Mahagi * | 2° S/31° E | Rift Valley | Kd | Backyard | IX |
| drc20/07/20 | Apr 2007 | Mahagi * | 2° S/31° E | Rift Valley | Ln | Backyard | IX |
| drc25/08/3a | Mar 2008 | Boende | 0°15 S/21°01 E | Forest | Kd | Free range | IX |
| drc25/08/3 | Mar 2008 | Boende | 0°15 S/21°01 E | Forest | Spl | Free range | IX |
| drc25/08/42 | Mar 2008 | Boende | 0°15 S/21°01 E | Forest | Kd | Free range | IX |
| drc25/08/9 | Mar 2008 | Boende | 0°15 S/21°01 E | Forest | Spl | Free range | IX |
| drc35/08/1 | Apr 2008 | Boende | 0°15 S/21°01 E | Forest | Spl | Free range | IX |
| drc35/08/13 | Apr 2008 | Boende | 0°15 S/21°01 E | Forest | Spl | Free range | IX |
| drc35/08/P42 | Apr 2008 | Boende | 0°15 S/21°01 E | Forest | Spl | Free range | IX |
| drc35/08/15 | Apr 2008 | Boende | 0°15 S/21°01 E | Forest | Spl | Free range | IX |
| drc35/08/18 | Apr 2008 | Boende | 0°15 S/21°01 E | Forest | Kd | Free range | IX |
| drc35/08/20 | Apr 2008 | Boende | 0°15 S/21°01 E | Forest | Spl | Free range | IX |
| drc35/08/3 | Apr 2008 | Boende | 0°15 S/21°01 E | Forest | Spl | Free range | IX |
| drc66/07/43 | Nov 2007 | Yakoma | 4° S/22° E | Forest | Lg | Free range | IX |
| drc66/07/48 | Nov 2007 | Yakoma | 4° S/22° E | Forest | Spl | Free range | IX |
| drc66/07/491 | Nov 2007 | Yakoma | 4° S/22° E | Forest | Spl | Free range | IX |
| drc66/07/492 | Nov 2007 | Yakoma | 4° S/22° E | Forest | Ln | Free range | IX |
| drc66/07/50 | Nov 2007 | Yakoma | 4° S/22° E | Forest | Spl | Free range | IX |
| drcKG31208/3 | Dec 2008 | Lingwala | 4°20 S/15°19 E | City | Spl | Backyard | IX |
| drc35/10/3 | Apr 2010 | Ngaliema | 4°21 S/15°05 E | City | Kd | Backyard | XIV |
| drc21/07/22 | 2007 | Kipushi † | 12° S/28° E | City | Spl | Backyard | XIV |
| Strain | Location | Year | Genotype | Tetrameric Repeats | TRS |
|---|---|---|---|---|---|
| drc35/10/1 | Ngaliema * | 2010 | I | AAAAAAAAAAAAAAAAAAAAAAAAAAAAABNAB NBTDBNAAAAAAAAAAAF | 51 |
| drcKG28110805 | Ngaliema * | 2010 | I | AAAAAAAAABNABNBTABNAAAAAAAAAAAAAAAAA AAAAAAAAF | 45 |
| drc65/11/4 | Nsele * | 2011 | I | AAAAAABNABNBTDBNAAAAAAAAF | 25 |
| drc74/09/2 | Nsele * | 2009 | I | AAAAAAAAAAAAAAAAAAAAAAF | 23a |
| drc74/09/3 | Nsele * | 2009 | I | AAAAAAAAAAAAAAAAAAAAAAF | 23a |
| drc74/09/4 | Nsele * | 2009 | I | AAAAAAAAAAAAAAAAAAAAAAF | 23a |
| drc74/09/6 | Nsele * | 2009 | I | AAAAAAAAAAAAAAAAAAAAAAF | 23a |
| drc94/09/2 | Kintambo * | 2009 | I | AAAAAAAAAAAAAAAAAAAAAAF | 23a |
| drc27/11/3 | Ngafula * | 2011 | I | AAAAAAAAAAAAAAAAAAAAAAF | 23a |
| drc27/11/5 | Ngafula * | 2011 | I | AAAAAAAAAAAAAAAAAAAAAAF | 23a |
| drcKG28040802 | Kasavubu * | 2008 | I | AAAAAAAAAAAAAAAF | 16 |
| drc96/12/1 | Mayanda | 2012 | I | AAAAAAAAAAAAAAAF | 16 |
| drc96/12/2 | Mayanda | 2012 | I | AAAAAAAAAAAAAAAF | 16 |
| drc96/12/3 | Mayanda | 2012 | I | AAAAAAAAAAAAAAAF | 16 |
| drc99/05a | Ngafula * | 2005 | I | AAAAAAAAAAAAAF | 14 |
| drc51/10/23 | Ndjili * | 2010 | I | AAAAAAAAAAAF | 12 |
| drc85/10/13 | Ngafula * | 2010 | I | AAAAAAAAAAAF | 12 |
| drc85/10/27 | Ngafula * | 2010 | I | AAAAAAAAAAAF | 12 |
| drc86/10/3 | Ngafula * | 2010 | I | AAAAAAAAAAAF | 12 |
| drc86/10/1 | Ngafula * | 2010 | I | AAAAAAAAAAAF | 12 |
| drc108/10/1 | Ngafula * | 2010 | I | AAAAAAAAAAAF | 12 |
| drc108/10/3 | Ngafula * | 2010 | I | AAAAAAAAAAAF | 12 |
| drc108/10/5 | Ngafula * | 2010 | I | AAAAAAAAAAAF | 12 |
| drc85/10/12 | Ngafula * | 2010 | I | AAAAAAAAAAF | 11 |
| drc73/10/2 | Ngaliema * | 2010 | I | AAAAAAAAAAF | 11 |
| drc73/10/3 | Ngaliema * | 2010 | I | AAAAAAAAAAF | 11 |
| drc73/10/4 | Ngaliema * | 2010 | I | AAAAAAAAAAF | 11 |
| drc49/05/P2a | Limete * | 2005 | I | AAAAAAAAAF | 10 |
| drc85/10/25 | Ngafula * | 2010 | I | AAAAAAAAF | 9 |
| drc75/05/1 | Maniema | 2005 | I | AAAAAAAAF | 9 |
| drc49/05/p1b | Limete * | 2005 | I | AAAAAAAAF | 9 |
| drc65/11/3 | Nsele * | 2011 | I | AAAAAAAF | 8 |
| drc49/05/p1a | Limete * | 2005 | I | AAAAAF | 6 |
| drc70/05/1 | Limete * | 2005 | I | AAAAF | 5 |
| Con09/Ni16 | Congo 1 | 2009 | I | AAAAAAAAAF | 10 |
| Kat67 | DRC(Zaire) 2 | 1967 | I | AAAAAAAABNABTDBNAAAAAAA | 23 |
| Nig13_KAF_14 | Nigeria 3 | 2014 | I | ABNABNAAAAACBNAFA | 17 |
| drc66/07/491 | Yakoma | 2007 | IX | AAABBAABBNABBAABBNABNABA | 24a |
| drc66/07/43 | Yakoma | 2007 | IX | AAABNABBBNABBAABBNABNABA | 24b |
| drc66/07/50 | Yakoma | 2007 | IX | AAABNABBBNABBAABBNABNABA | 24b |
| drc66/07/492 | Yakoma | 2007 | IX | AAABNABBBNABBAABBNABNABA | 24b |
| drc66/07/48 | Yakoma | 2007 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc35/08/p42 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc35/08/18 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc35/08/13 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc35/08/3 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc35/08/20 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc35/08/1 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc35/08/15 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc35/08/3a | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc25/08/3 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc25/08/9 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc25/08/42 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drcKG31208/3 | Lingwala * | 2008 | IX | AAAABNABBNABBAAABNABNABA | 24d |
| drc20/07/19 | Mahagi | 2007 | IX | AAABNABBNABBAABBNABNABA | 23b |
| drc20/07/20 | Mahagi | 2007 | IX | AAABNABBNABBAABBNABNABA | 23b |
| drc86/10/2 | Ngafula * | 2010 | IX | AAAAAAAAAAAF | 12 |
| UG03H.1 | Uganda 4 | 2003 | IX | AAABNABBNABBAABBNABNABA | 23b |
| Ken06.B1 | Kenya 5 | 2006 | IX | AAABNABBNABBAABBNABNABA | 23b |
| drc35/10/3 | Ngaliema * | 2010 | XIV | AAAAAAAAAAAAAAAAAAAAAAAAAAAAABNABNBTDBN AAAAAAAAAAAF | 51 |
| drc21/07/p22 | Kipushi | 2007 | XIV | AVVOVAVVNBVOV | 13 |
| ETH/3 | Ethiopia 6 | 2011 | XXIII | ABNAAAAACBNABTDBNAFA | 20 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulumba–Mfumu, L.K.; Achenbach, J.E.; Mauldin, M.R.; Dixon, L.K.; Tshilenge, C.G.; Thiry, E.; Moreno, N.; Blanco, E.; Saegerman, C.; Lamien, C.E.; et al. Genetic Assessment of African Swine Fever Isolates Involved in Outbreaks in the Democratic Republic of Congo between 2005 and 2012 Reveals Co-Circulation of p72 Genotypes I, IX and XIV, Including 19 Variants. Viruses 2017, 9, 31. https://doi.org/10.3390/v9020031
Mulumba–Mfumu LK, Achenbach JE, Mauldin MR, Dixon LK, Tshilenge CG, Thiry E, Moreno N, Blanco E, Saegerman C, Lamien CE, et al. Genetic Assessment of African Swine Fever Isolates Involved in Outbreaks in the Democratic Republic of Congo between 2005 and 2012 Reveals Co-Circulation of p72 Genotypes I, IX and XIV, Including 19 Variants. Viruses. 2017; 9(2):31. https://doi.org/10.3390/v9020031
Chicago/Turabian StyleMulumba–Mfumu, Leopold K., Jenna E. Achenbach, Matthew R. Mauldin, Linda K. Dixon, Curé Georges Tshilenge, Etienne Thiry, Noelia Moreno, Esther Blanco, Claude Saegerman, Charles E. Lamien, and et al. 2017. "Genetic Assessment of African Swine Fever Isolates Involved in Outbreaks in the Democratic Republic of Congo between 2005 and 2012 Reveals Co-Circulation of p72 Genotypes I, IX and XIV, Including 19 Variants" Viruses 9, no. 2: 31. https://doi.org/10.3390/v9020031
APA StyleMulumba–Mfumu, L. K., Achenbach, J. E., Mauldin, M. R., Dixon, L. K., Tshilenge, C. G., Thiry, E., Moreno, N., Blanco, E., Saegerman, C., Lamien, C. E., & Diallo, A. (2017). Genetic Assessment of African Swine Fever Isolates Involved in Outbreaks in the Democratic Republic of Congo between 2005 and 2012 Reveals Co-Circulation of p72 Genotypes I, IX and XIV, Including 19 Variants. Viruses, 9(2), 31. https://doi.org/10.3390/v9020031








