Helicase Domain of West Nile Virus NS3 Protein Plays a Role in Inhibition of Type I Interferon Signalling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Generation of Parental and Chimeric Viruses by Circular Polymerase Extension Reaction (CPER)
2.3. Plaque Assay
2.4. Growth Kinetics
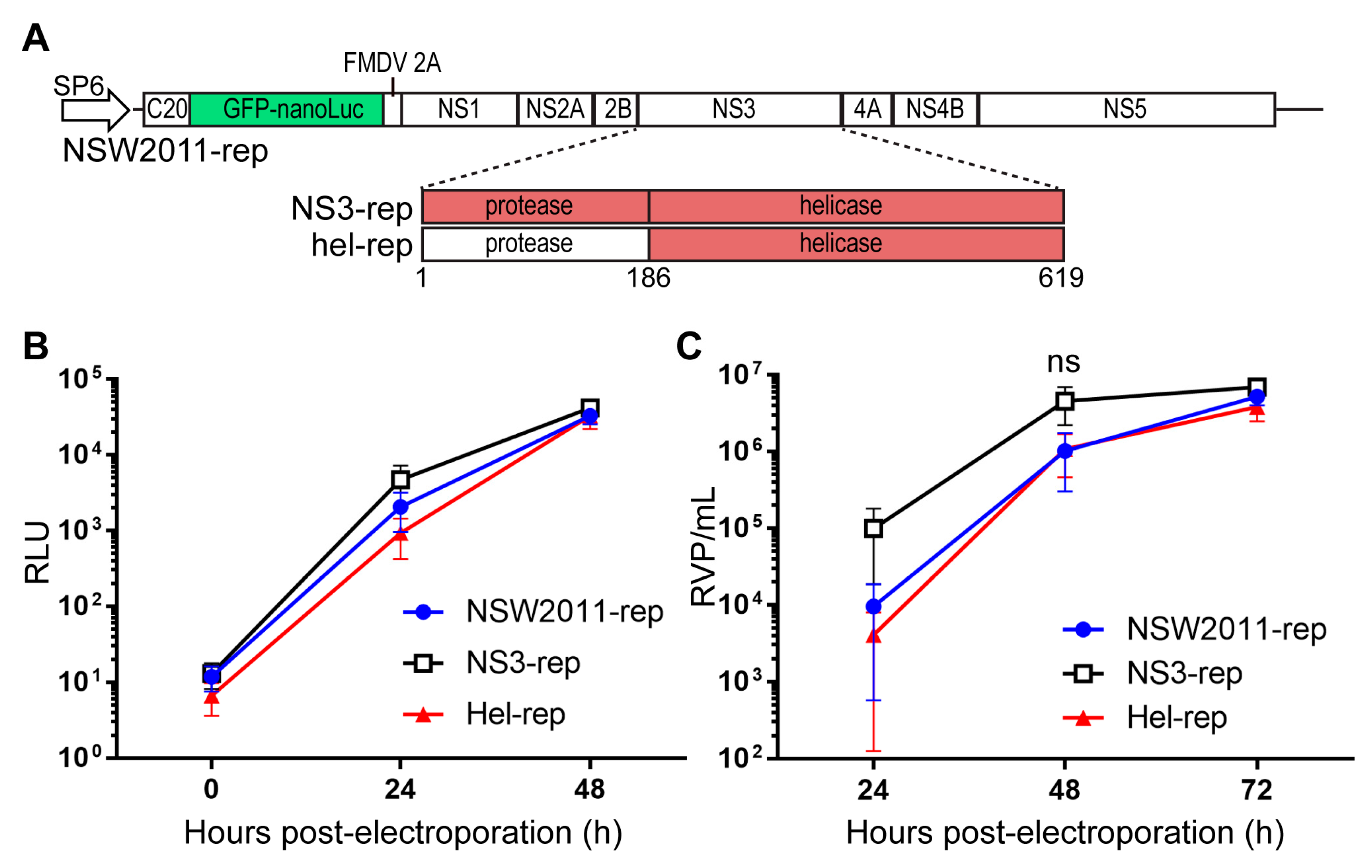
2.5. Construction of Replicons
2.6. RNA Replication and Virus-Like Particle (VLP) Production Assays
2.7. VLP Titration Assay
2.8. Flow Cytometry
2.9. Virulence in Mice
2.10. Statistical Analyses
3. Results
3.1. Helicase Domain of NY99 NS3 Protein Is Responsible for Enhanced Virus Replication in Type I IFN Response-Competent Mouse Cells
3.2. RNA Replication and Virus Assembly Is Not Enhanced by the Helicase Domain of NY99 NS3 Protein
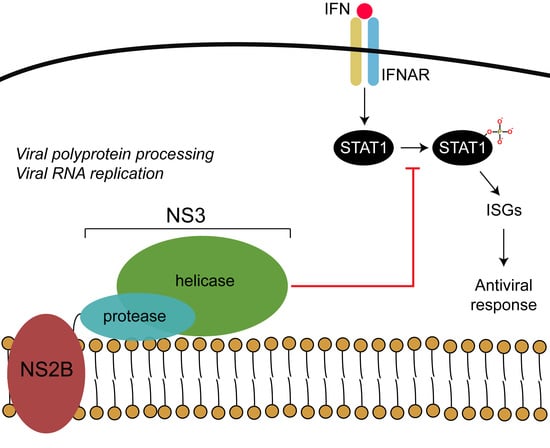
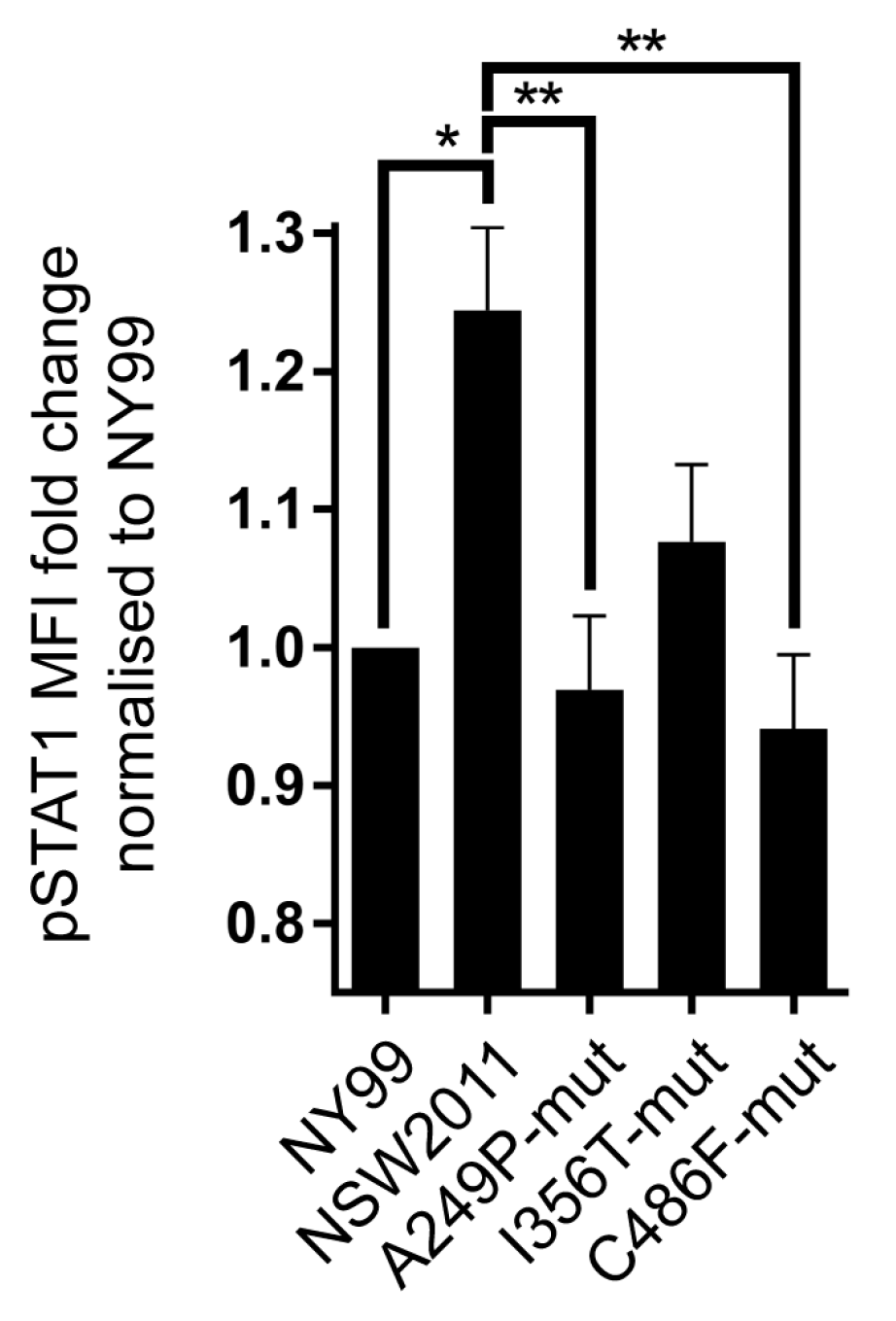
3.3. NS3-Helicase Domain of NY99 Inhibits Phosphorylation of STAT1
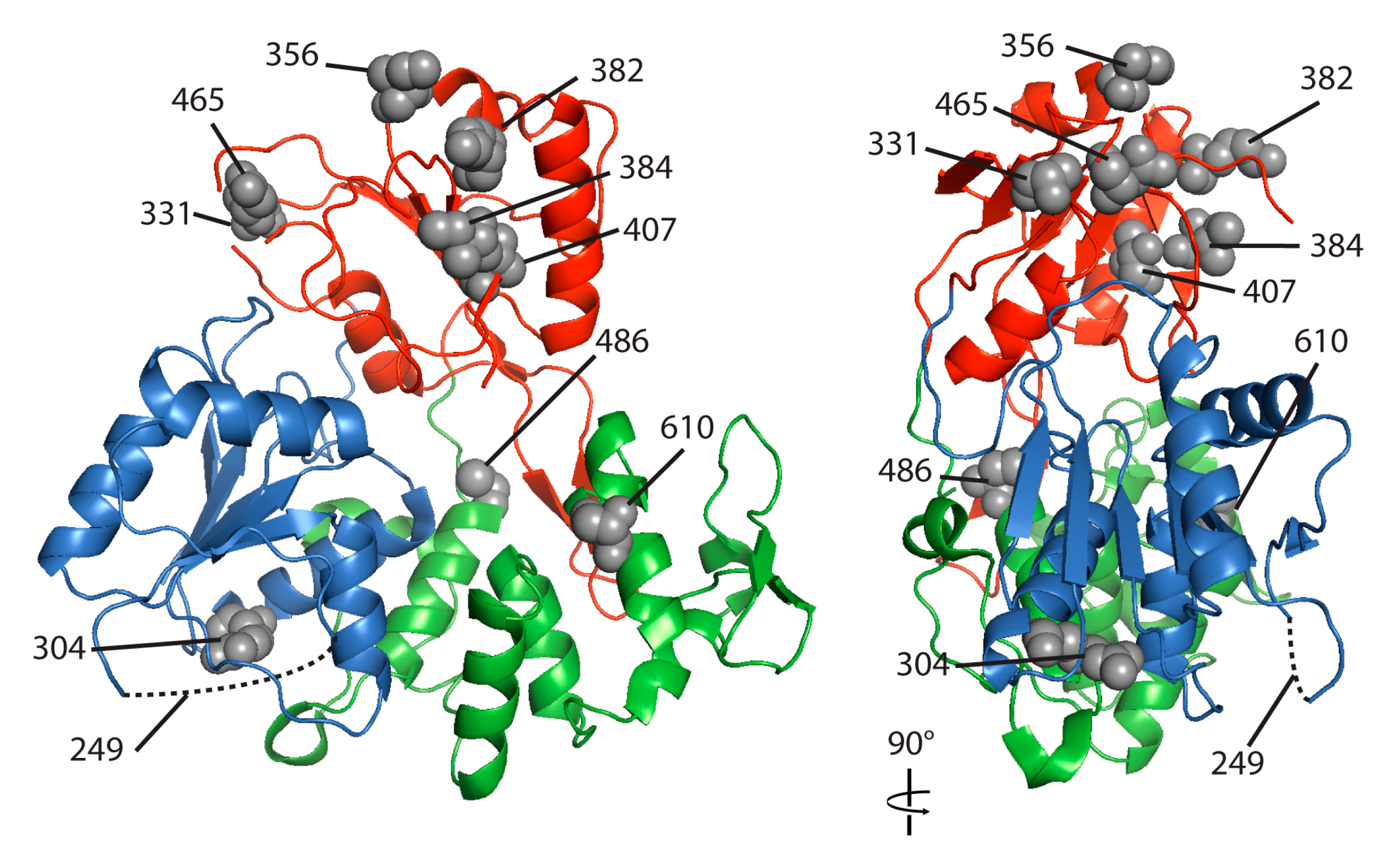
3.4. Proline at Position 249 and Phenylalanine at Position 486 in the Helicase Domain of NY99 NS3 Are Primarily Responsible for the Inhibition of STAT1 Phosphorylation
3.5. Helicase Domain of NY99 NS3 Does Not Significantly Enhance Virulence in Mice
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frost, M.J.; Zhang, J.; Edmonds, J.H.; Prow, N.A.; Gu, X.; Davis, R.; Hornitzky, C.; Arzey, K.E.; Finlaison, D.; Hick, P.; et al. Characterization of virulent west nile virus kunjin strain, australia, 2011. Emerg. Infect. Dis. 2012, 18, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Setoh, Y.X.; Prow, N.A.; Rawle, D.J.; Tan, C.S.; Edmonds, J.H.; Hall, R.A.; Khromykh, A.A. Systematic analysis of viral genes responsible for differential virulence between american and australian west nile virus strains. J. Gen. Virol. 2015, 96, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D.; Thiel, H.J.; Rice, C.M. Flaviviridae: The viruses and their replication. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott-Raven Publishers: Philadelphia, PA, USA, 2007; pp. 1101–1152. [Google Scholar]
- Roby, J.A.; Setoh, Y.X.; Hall, R.A.; Khromykh, A.A. Post-translational regulation and modifications of flavivirus structural proteins. J. Gen. Virol. 2015, 96, 1551–1569. [Google Scholar] [CrossRef] [PubMed]
- Singleton, M.R.; Dillingham, M.S.; Wigley, D.B. Structure and mechanism of helicases and nucleic acid translocases. Annu. Rev. Biochem. 2007, 76, 23–50. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, E.; Milani, M.; Bollati, M.; Selisko, B.; Peyrane, F.; Pandini, V.; Sorrentino, G.; Canard, B.; Konarev, P.V.; Svergun, D.I.; et al. Crystal structure and activity of kunjin virus NS3 helicase; protease and helicase domain assembly in the full length NS3 protein. J. Mol. Biol. 2007, 372, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Patkar, C.G.; Kuhn, R.J. Yellow fever virus NS3 plays an essential role in virus assembly independent of its known enzymatic functions. J. Virol. 2008, 82, 3342–3352. [Google Scholar] [CrossRef] [PubMed]
- Pijlman, G.P.; Kondratieva, N.; Khromykh, A.A. Translation of the flavivirus kunjin NS3 gene in cis but not its rna sequence or secondary structure is essential for efficient rna packaging. J. Virol. 2006, 80, 11255–11264. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, S.; Maestre, A.M.; Pagni, S.; Patel, J.R.; Savage, T.; Gutman, D.; Maringer, K.; Bernal-Rubio, D.; Shabman, R.S.; Simon, V.; et al. Denv inhibits type i ifn production in infected cells by cleaving human sting. PLoS Pathog. 2012, 8, e1002934. [Google Scholar] [CrossRef] [PubMed]
- De Borba, L.; Strottmann, D.M.; de Noronha, L.; Mason, P.W.; Dos Santos, C.N. Synergistic interactions between the NS3(hel) and e proteins contribute to the virulence of dengue virus type 1. PLoS Negl. Trop. Dis. 2012, 6, e1624. [Google Scholar] [CrossRef] [PubMed]
- Silveira, G.F.; Strottmann, D.M.; de Borba, L.; Mansur, D.S.; Zanchin, N.I.; Bordignon, J.; dos Santos, C.N. Single point mutations in the helicase domain of the NS3 protein enhance dengue virus replicative capacity in human monocyte-derived dendritic cells and circumvent the type i interferon response. Clin. Exp. Immunol. 2016, 183, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Langevin, S.A.; Bowen, R.A.; Reisen, W.K.; Andrade, C.C.; Ramey, W.N.; Maharaj, P.D.; Anishchenko, M.; Kenney, J.L.; Duggal, N.K.; Romo, H.; et al. Host competence and helicase activity differences exhibited by west nile viral variants expressing NS3-249 amino acid polymorphisms. PLoS ONE 2014, 9, e100802. [Google Scholar] [CrossRef] [PubMed]
- Brault, A.C.; Huang, C.Y.; Langevin, S.A.; Kinney, R.M.; Bowen, R.A.; Ramey, W.N.; Panella, N.A.; Holmes, E.C.; Powers, A.M.; Miller, B.R. A single positively selected west nile viral mutation confers increased virogenesis in american crows. Nat. Genet. 2007, 39, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Audsley, M.; Edmonds, J.; Liu, W.; Mokhonov, V.; Mokhonova, E.; Melian, E.B.; Prow, N.; Hall, R.A.; Khromykh, A.A. Virulence determinants between new york 99 and kunjin strains of west nile virus. Virology 2011, 414, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, J.; van Grinsven, E.; Prow, N.; Bosco-Lauth, A.; Brault, A.C.; Bowen, R.A.; Hall, R.A.; Khromykh, A.A. A novel bacterium-free method for generation of flavivirus infectious DNA by circular polymerase extension reaction allows accurate recapitulation of viral heterogeneity. J. Virol. 2013, 87, 2367–2372. [Google Scholar] [CrossRef] [PubMed]
- Khromykh, A.A.; Westaway, E.G. Subgenomic replicons of the flavivirus kunjin: Construction and applications. J. Virol. 1997, 71, 1497–1505. [Google Scholar] [PubMed]
- Harvey, T.J.; Liu, W.J.; Wang, X.J.; Linedale, R.; Jacobs, M.; Davidson, A.; Le, T.T.; Anraku, I.; Suhrbier, A.; Shi, P.Y.; et al. Tetracycline-inducible packaging cell line for production of flavivirus replicon particles. J. Virol. 2004, 78, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.C.; Lobigs, M.; Lee, E.; Howard, M.J.; Clark, K.; Blitvich, B.J.; Hall, R.A. In situ reactions of monoclonal antibodies with a viable mutant of murray valley encephalitis virus reveal an absence of dimeric ns1 protein. J. Gen. Virol. 2007, 88, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Setoh, Y.X.; Prow, N.A.; Peng, N.; Hugo, L.E.; Devine, G.; Hazlewood, J.E.; Suhrbier, A.; Khromykh, A.A. De novo generation and characterization of new zika virus isolate using sequence data from a microcephaly case. mSphere 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Amarilla, A.A.; Setoh, Y.X.; Periasamy, P.; Peng, N.Y.; Pali, G.; Figueiredo, L.T.; Khromykh, A.A.; Aquino, V.H. Chimeric viruses between rocio and west nile: The role for rocio prm-e proteins in virulence and inhibition of interferon-alpha/beta signaling. Sci. Rep. 2017, 7, 44642. [Google Scholar] [CrossRef] [PubMed]
- Laurent-Rolle, M.; Boer, E.F.; Lubick, K.J.; Wolfinbarger, J.B.; Carmody, A.B.; Rockx, B.; Liu, W.; Ashour, J.; Shupert, W.L.; Holbrook, M.R.; et al. The NS5 protein of the virulent west nile virus NY99 strain is a potent antagonist of type i interferon-mediated JAK-STAT signaling. J. Virol. 2010, 84, 3503–3515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazear, H.M.; Lancaster, A.; Wilkins, C.; Suthar, M.S.; Huang, A.; Vick, S.C.; Clepper, L.; Thackray, L.; Brassil, M.M.; Virgin, H.W.; et al. IRF-3, IRF-5 and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS Pathog. 2013, 9, e1003118. [Google Scholar] [CrossRef]
- Suthar, M.S.; Ma, D.Y.; Thomas, S.; Lund, J.M.; Zhang, N.; Daffis, S.; Rudensky, A.Y.; Bevan, M.J.; Clark, E.A.; Kaja, M.K.; et al. IPS-1 is essential for the control of west nile virus infection and immunity. PLoS Pathog. 2010, 6, e1000757. [Google Scholar] [CrossRef] [PubMed]
- Caruthers, J.M.; McKay, D.B. Helicase structure and mechanism. Curr. Opin. Struct. Biol. 2002, 12, 123–133. [Google Scholar] [CrossRef]
- Wu, J.; Bera, A.K.; Kuhn, R.J.; Smith, J.L. Structure of the flavivirus helicase: Implications for catalytic activity, protein interactions and proteolytic processing. J. Virol. 2005, 79, 10268–10277. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.D.; Styer, L.M.; Ebel, G.D. A global perspective on the epidemiology of west nile virus. Annu. Rev. Entomol. 2008, 53, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Prow, N.A. The changing epidemiology of kunjin virus in australia. Int. J. Environ. Res. Public Health 2013, 10, 6255–6272. [Google Scholar] [CrossRef] [PubMed]
- Roche, S.E.; Wicks, R.; Garner, M.G.; East, I.J.; Paskin, R.; Moloney, B.J.; Carr, M.; Kirkland, P. Descriptive overview of the 2011 epidemic of arboviral disease in horses in australia. Aust. Vet. J. 2013, 91, 5–13. [Google Scholar] [CrossRef] [PubMed]



| Amplicon Name | Primer Name | Primer Sequence (5′–3′) |
|---|---|---|
| UTRlinker | flaviUTRlinker_F | GTGGTGCGAGAACACAGGA |
| flaviUTRlinker_R | CAGCTCACACAGGCGAACTACT | |
| 5′UTR | 5′UTR_F | AGTAGTTCGCCTGTGTGAGCTG |
| 5′UTR_R | GGCCCTCCTGGTTTCTTAGAC | |
| C-prM-E | CprME_F | GTCTAAGAAACCAGGAGGGCC |
| CprME_R | GGAAAGAAAGAGCAGAACTCCTCCAAC | |
| NS1-2A-2B | NS1_F | GTTGGAGGAGTTCTGCTCTTTCTTTCC |
| NS2B_R | CTCCTCTCTTTGTGTATTGGAGAGTTATC | |
| NS3-4A-4B | NS3_F | GATAACTCTCCAATACACAAAGAGAGGAG |
| NS4B_R | CGTCCTTTTGCCCCACCTC | |
| NS5-3′UTR | NS5_F | GAGGTGGGGCAAAAGGACG |
| 3′UTR_R | TCCTGTGTTCTCGCACCAC | |
| NS1 | NS1_F | GTTGGAGGAGTTCTGCTCTTTCTTTCC |
| NS1_R | GGCCAAGAACACGACCAGAAG | |
| NS2A | NS2A_F | CTTCTGGTCGTGTTCTTGGCC |
| NS2A_R | CAGCTGTCATCACTTCAGTTGC | |
| NS2B | NS2B_F | GCAACTGAAGTGATGACAGCTG |
| NS2B_R | CTCCTCTCTTTGTGTATTGGAGAGTTATC | |
| NS3 | NS3_F | GATAACTCTCCAATACACAAAGAGAGGAG |
| NS3_R | CCTGAGGCGAAGTCTTTGAA | |
| NS4A | NS4A_F | TTCAAAGACTTCGCCTCAGG |
| NS4A_R | CCCATCTCATTGGCTGCCAC | |
| NS4B | NS4B_F | GTGGCAGCCAATGAGATGGG |
| NS4B_R | CGTCCTTTTGCCCCACCTC | |
| Helicase chimera | Hel_F | CGGATTCGAACCTGAGATGTTGAGG |
| NS3_R | CCTGAGGCGAAGTCTTTGAA | |
| Protease chimera | NS3_F | GATAACTCTCCAATACACAAAGAGAGGAG |
| Pro_R | CCTCAACATCTCAGGTTCGAATCCG |
| Domain | Amino Acid Position | Domain | KUNV | NSW2011 | NY99 |
|---|---|---|---|---|---|
| Protease | 110 | Q | Q | R | |
| 175 | V | V | I | ||
| Helicase | 249 | 1 | A | A | P |
| 304 | 1 | R | R | K | |
| 331 | 2 | A | A | S | |
| 356 | 2 | I | I | T | |
| 382 | 2 | K | R | K | |
| 384 | 2 | I | I | V | |
| 407 | 2 | V | V | I | |
| 465 | 2 | N | S | N | |
| 486 | 3 | C | C | F | |
| 610 | 3 | S | S | A |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setoh, Y.X.; Periasamy, P.; Peng, N.Y.G.; Amarilla, A.A.; Slonchak, A.; Khromykh, A.A. Helicase Domain of West Nile Virus NS3 Protein Plays a Role in Inhibition of Type I Interferon Signalling. Viruses 2017, 9, 326. https://doi.org/10.3390/v9110326
Setoh YX, Periasamy P, Peng NYG, Amarilla AA, Slonchak A, Khromykh AA. Helicase Domain of West Nile Virus NS3 Protein Plays a Role in Inhibition of Type I Interferon Signalling. Viruses. 2017; 9(11):326. https://doi.org/10.3390/v9110326
Chicago/Turabian StyleSetoh, Yin Xiang, Parthiban Periasamy, Nias Yong Gao Peng, Alberto A. Amarilla, Andrii Slonchak, and Alexander A. Khromykh. 2017. "Helicase Domain of West Nile Virus NS3 Protein Plays a Role in Inhibition of Type I Interferon Signalling" Viruses 9, no. 11: 326. https://doi.org/10.3390/v9110326