The Host Cell Receptors for Measles Virus and Their Interaction with the Viral Hemagglutinin (H) Protein
Abstract
:1. Introduction
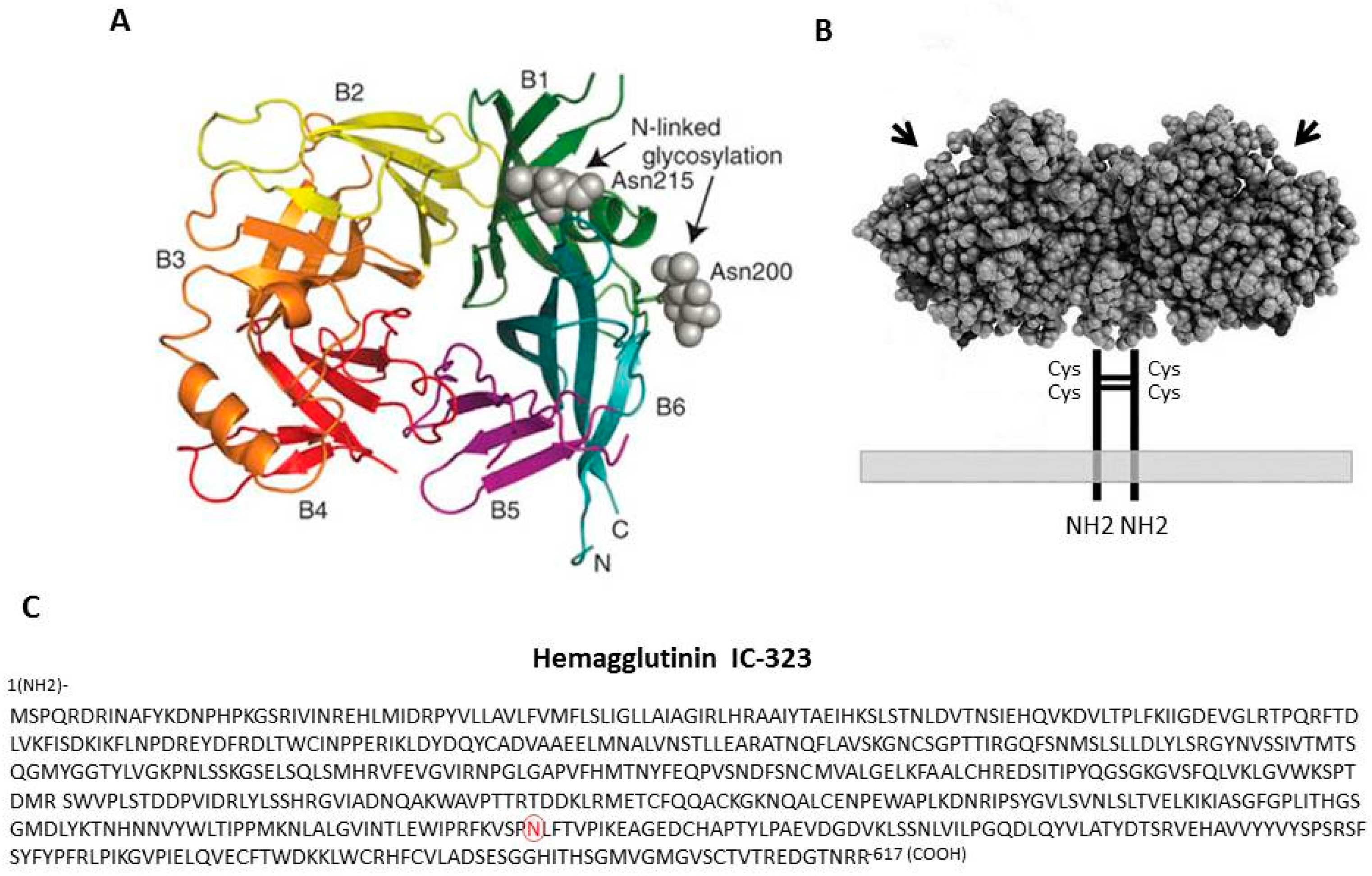
2. The Hemagglutinin Protein and Its Properties
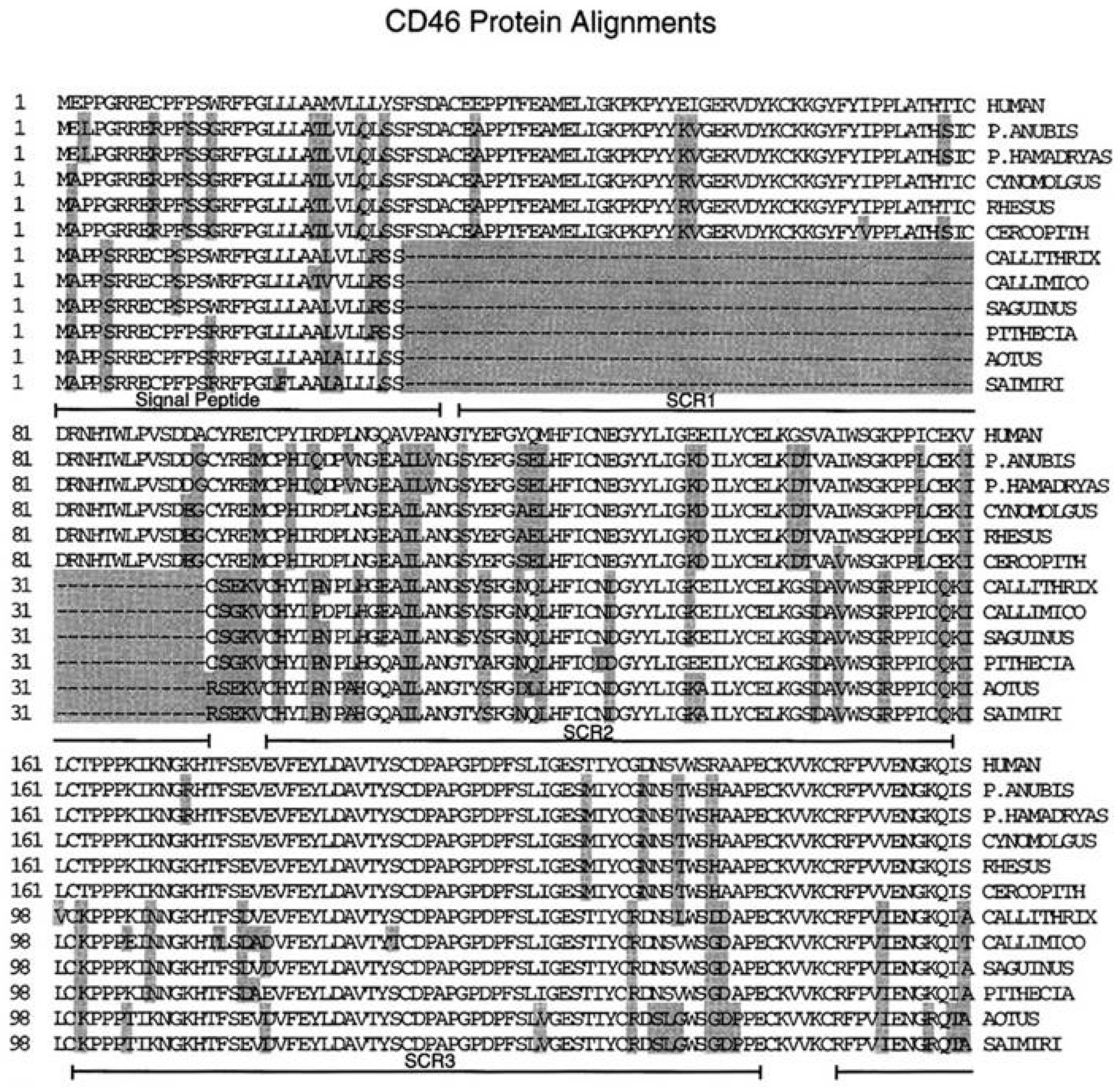
3. The Membrane Cofactor Protein CD46 Is a Cellular Receptor for the Hemagglutinin Proteins of Laboratory-Adapted and Vaccine Strains of Measles Virus
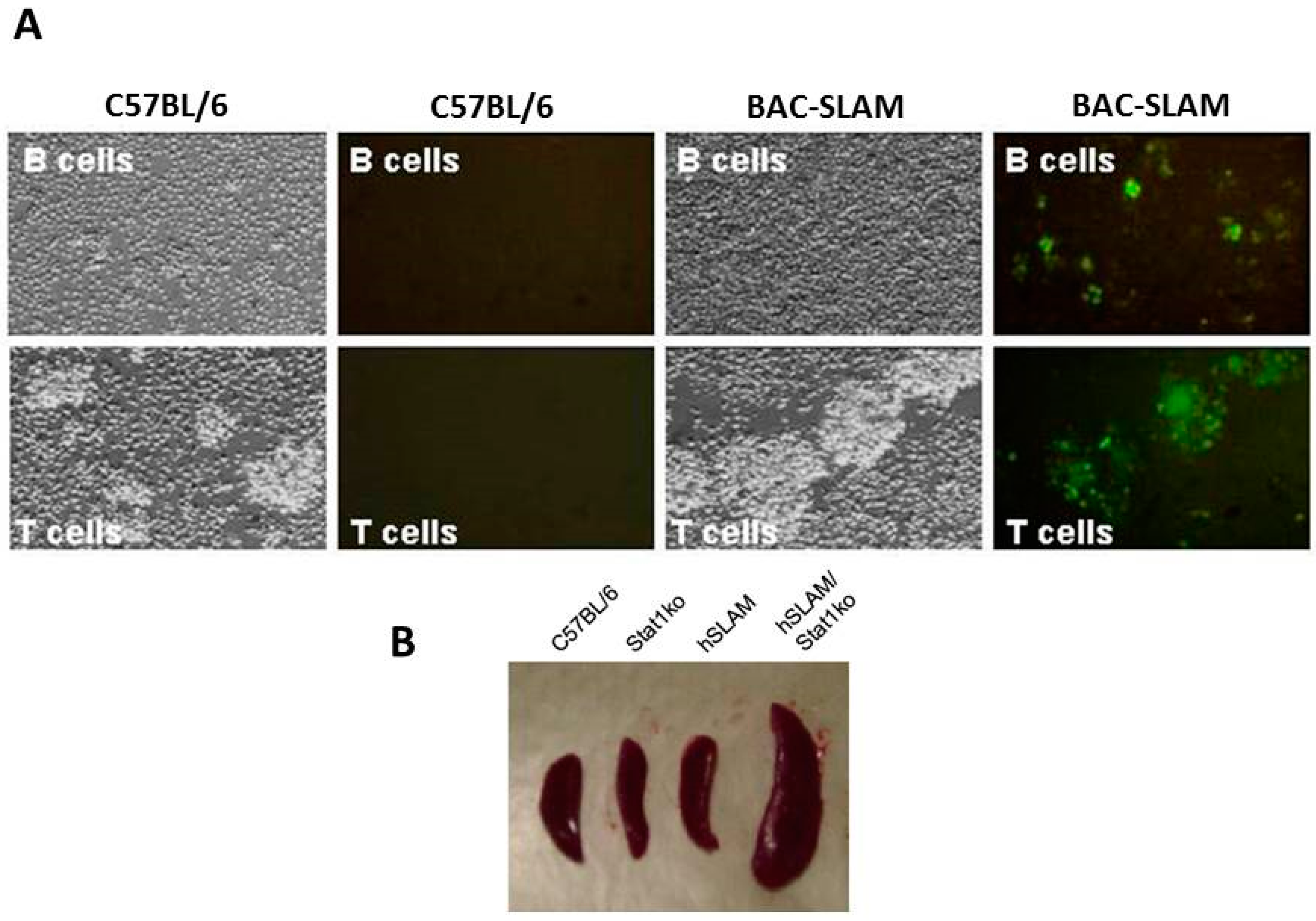
4. SLAMF1/SLAM/CD150 Is the Lymphocyte Cellular Receptor for the Hemagglutinin Proteins of Wild-Type and Vaccine Strains of Measles Virus
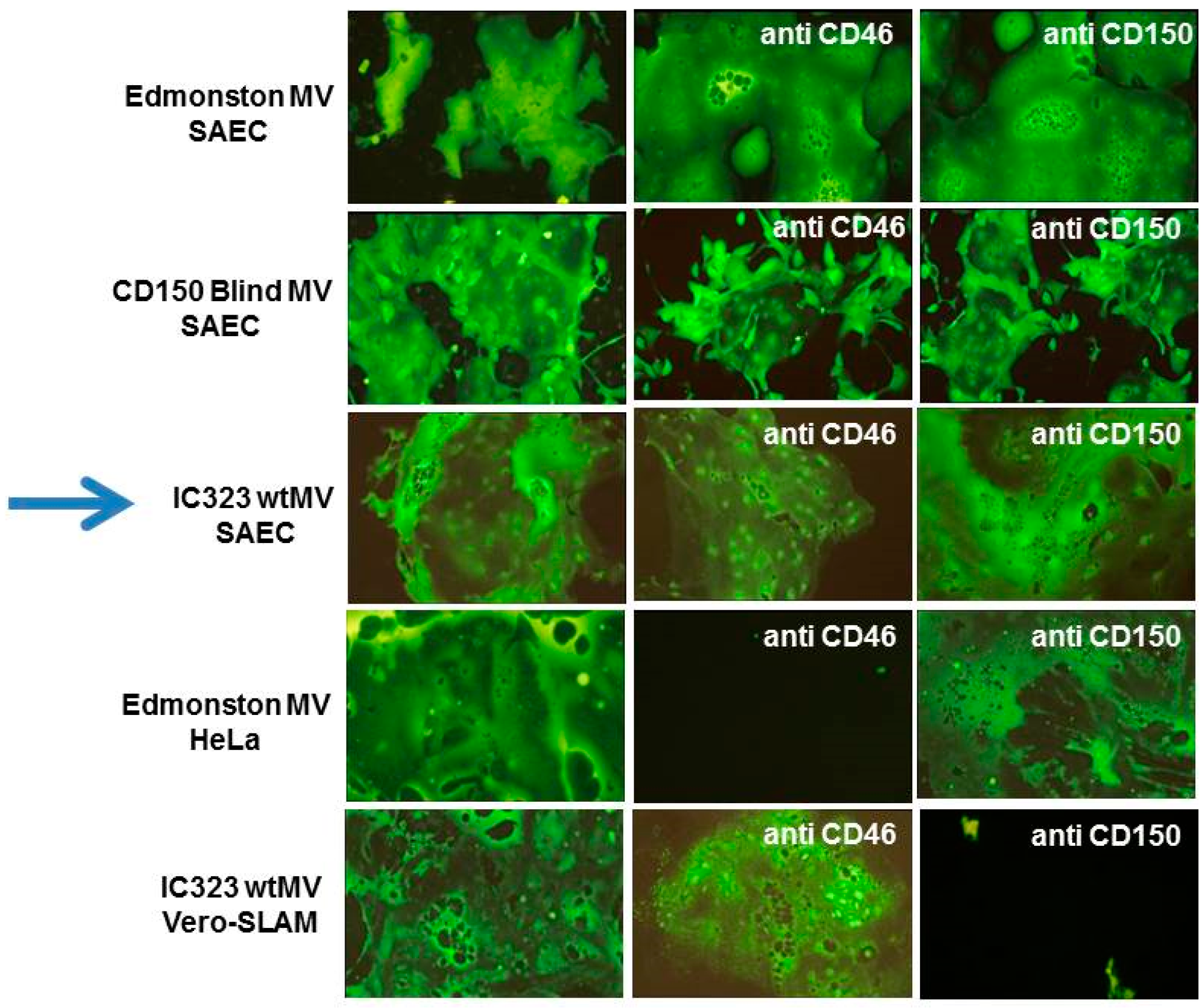
5. The Epithelial Cell Receptor for the Hemagglutinin Protein of Wild-Type Measles Virus
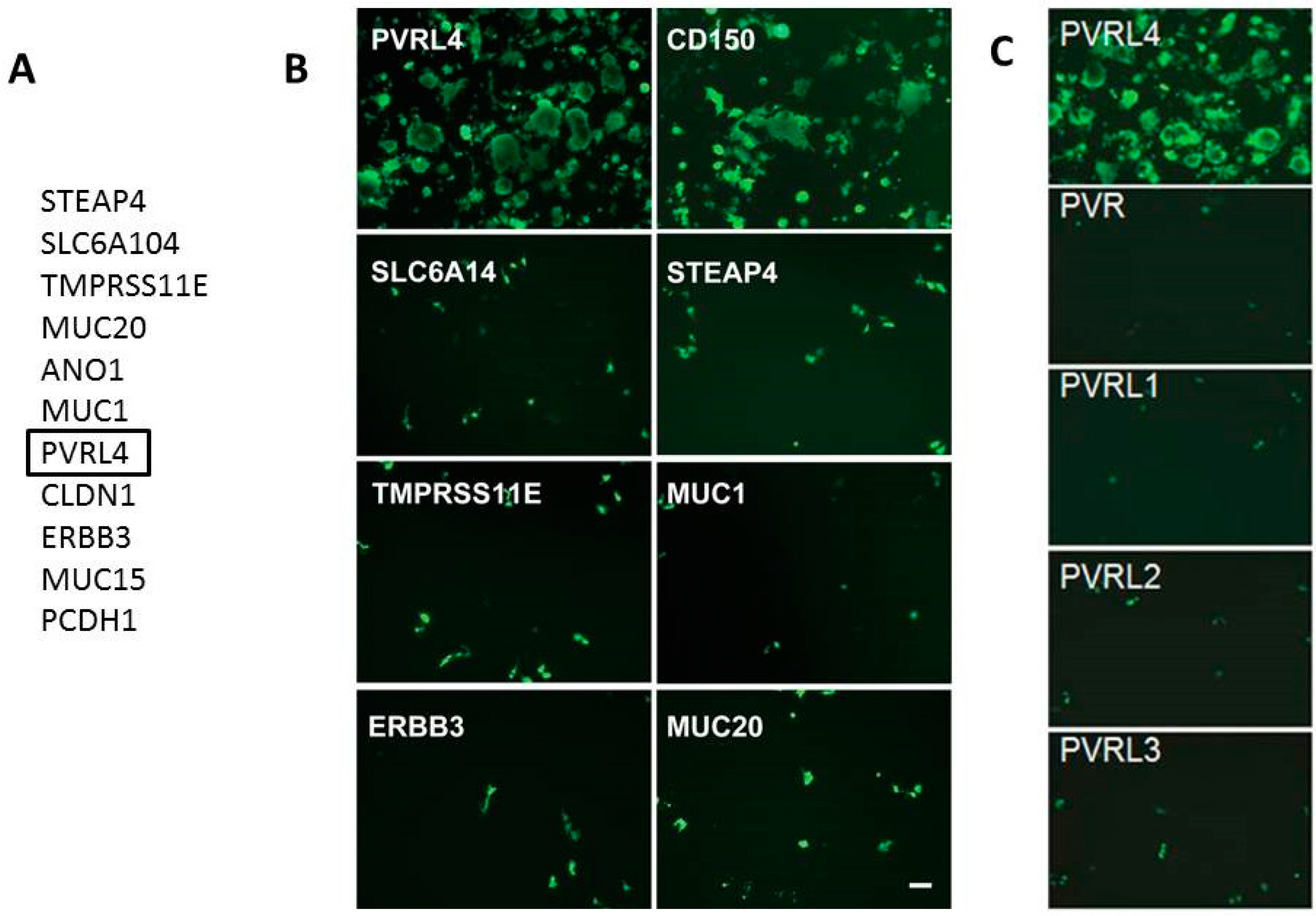
6. Identification of Nectin-4 (PVRL4) as the Epithelial Receptor for MeV
7. SLAMF1 and Nectin-4 Are Also the Receptors for Other Related Morbilliviruses
8. The Discovery of Nectin-4 Revealed a New Paradigm for Host Infection and Pathogenesis of MeV
9. The Receptors of Measles Virus Are Highly Expressed on Different Cancer Cells and Facilitate Viral Oncolytic Properties
10. Conclusions and Future Directions
Acknowledgments
Conflicts of Interest
References
- Griffin, D.E. Measles Virus. In Fields’ Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams and Wilkins: New York, NY, USA, 2001; pp. 1401–1442. [Google Scholar]
- Griffin, D.E. Measles Virus. In Fields’ Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams and Wilkins: New York, NY, USA, 2006. [Google Scholar]
- Griffin, D.E. Measles Virus. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott, Williams, & Wilkins: Philadelphia, PA, USA, 2013; Volume 1, pp. 1042–1069. [Google Scholar]
- Moss, W.J.; Griffin, D.E. Measles. Lancet 2012, 379, 153–164. [Google Scholar] [CrossRef]
- Billeter, M.A.; Naim, H.Y.; Udem, S.A. Reverse genetics of measles virus and resulting multivalent recombinant vaccines: Applications of recombinant measles viruses. Curr. Top. Microbiol. Immunol. 2009, 329, 129–162. [Google Scholar] [PubMed]
- Radecke, F.; Spielhofer, P.; Schneider, H.; Kaelin, K.; Huber, M.; Dotsch, C.; Christiansen, G.; Billeter, M.A. Rescue of measles viruses from cloned DNA. EMBO J. 1995, 14, 5773–5784. [Google Scholar] [PubMed]
- Duprex, W.P.; McQuaid, S.; Hangartner, L.; Billeter, M.A.; Rima, B.K. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J. Virol. 1999, 73, 9568–9575. [Google Scholar] [PubMed]
- Duprex, W.P.; McQuaid, S.; Roscic-Mrkic, B.; Cattaneo, R.; McCallister, C.; Rima, B.K. In vitro and in vivo infection of neural cells by a recombinant measles virus expressing enhanced green fluorescent protein. J. Virol. 2000, 74, 7972–7979. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, T.; Kajikawa, M.; Maita, N.; Takeda, M.; Kuroki, K.; Sasaki, K.; Kohda, D.; Yanagi, Y.; Maenaka, K. Crystal structure of measles virus hemagglutinin provides insight into effective vaccines. Proc. Natl. Acad. Sci. USA 2007, 104, 19535–19540. [Google Scholar] [CrossRef] [PubMed]
- Navaratnarajah, C.K.; Leonard, V.H.; Cattaneo, R. Measles virus glycoprotein complex assembly, receptor attachment, and cell entry. Curr. Top. Microbiol. Immunol. 2009, 329, 59–76. [Google Scholar] [PubMed]
- Navaratnarajah, C.K.; Negi, S.; Braun, W.; Cattaneo, R. Membrane fusion triggering: Three modules with different structure and function in the upper half of the measles virus attachment protein stalk. J. Biol. Chem. 2012, 287, 38543–38551. [Google Scholar] [CrossRef] [PubMed]
- Navaratnarajah, C.K.; Oezguen, N.; Rupp, L.; Kay, L.; Leonard, V.H.; Braun, W.; Cattaneo, R. The heads of the measles virus attachment protein move to transmit the fusion-triggering signal. Nat. Struct. Mol. Biol. 2011, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Plattet, P.; Plemper, R.K. Envelope protein dynamics in paramyxovirus entry. mBio 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, Y.; Takeda, M.; Ohno, S.; Hashiguchi, T. Measles virus receptors. Curr. Top. Microbiol. Immunol. 2009, 329, 13–30. [Google Scholar] [PubMed]
- Albrecht, P.; Lorenz, D.; Klutch, M.J. Encephalitogenicity of measles virus in marmosets. Infect. Immun. 1981, 34, 581–587. [Google Scholar] [PubMed]
- Albrecht, P.; Lorenz, D.; Klutch, M.J.; Vickers, J.H.; Ennis, F.A. Fatal measles infection in marmosets pathogenesis and prophylaxis. Infect. Immun. 1980, 27, 969–978. [Google Scholar] [PubMed]
- Blake, F.G.; Trask, J.D. Studies on Measles: I. Susceptibility of Monkeys to the Virus of Measles. J. Exp. Med. 1921, 33, 385–412. [Google Scholar] [CrossRef] [PubMed]
- Kobune, F.; Takahashi, H.; Terao, K.; Ohkawa, T.; Ami, Y.; Suzaki, Y.; Nagata, N.; Sakata, H.; Yamanouchi, K.; Kai, C. Nonhuman primate models of measles. Lab. Anim. Sci. 1996, 46, 315–320. [Google Scholar] [PubMed]
- McChesney, M.B.; Miller, C.J.; Rota, P.A.; Zhu, Y.D.; Antipa, L.; Lerche, N.W.; Ahmed, R.; Bellini, W.J. Experimental measles. I. Pathogenesis in the normal and the immunized host. Virology 1997, 233, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Van Binnendijk, R.S.; van der Heijden, R.W.; Osterhaus, A.D. Monkeys in measles research. Curr. Top. Microbiol. Immunol. 1995, 191, 135–148. [Google Scholar] [PubMed]
- Zhu, Y.D.; Heath, J.; Collins, J.; Greene, T.; Antipa, L.; Rota, P.; Bellini, W.; McChesney, M. Experimental measles. II. Infection and immunity in the rhesus macaque. Virology 1997, 233, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Burnstein, T.; Jensen, J.H.; Waksman, B.H. The Development of a Neurotropic Strain of Measles Virus in Hamsters and Mice. J. Infect. Dis. 1964, 114, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Liebert, U.G.; Finke, D. Measles virus infections in rodents. Curr. Top. Microbiol. Immunol. 1995, 191, 149–166. [Google Scholar] [PubMed]
- Liebert, U.G.; ter Meulen, V. Virological aspects of measles virus-induced encephalomyelitis in Lewis and BN rats. J. Gen. Virol. 1987, 68, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- McFarland, H.F. The effect of measles virus infection on T and B lymphocytes in the mouse. I. Suppression of helper cell activity. J. Immunol. 1974, 113, 1978–1983. [Google Scholar] [PubMed]
- Fraser, K.B.; Martin, S.J. Measles Virus and Its Biology; Academic Press: London, UK, 1978. [Google Scholar]
- Enders, J.F.; Peebles, T.C. Propagation in tissue cultures of cytopathogenic agents from patients with measles. Exp. Biol. Med. 1954, 86, 277–286. [Google Scholar] [CrossRef]
- Enders, J.F.; Katz, S.L.; Holloway, A. Development of attenuated measles-virus vaccines. A summary of recentinvestigation. Am. J. Dis. Child. 1962, 103, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Enders, J.F.; Katz, S.L.; Milovanovic, M.V.; Holloway, A. Studies on an attenuated measles-virus vaccine. I. Development and preparations of the vaccine: Technics for assay of effects of vaccination. N. Engl. J. Med. 1960, 263, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.L.; Enders, J.F.; Holloway, A. Studies on an attenuated measles-virus vaccine. II. Clinical, virologic and immunologic effects of vaccine in institutionalized children. N. Engl. J. Med. 1960, 263, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.L.; Kempe, C.H.; Black, F.L.; Lepow, M.L.; Krugman, S.; Haggerty, R.J.; Enders, J.F. Studies on an attenuated measles-virus vaccine. VIII. General summary and evaluation of the results of vaccine. N. Engl. J. Med. 1960, 263, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.L.; Milovanovic, M.V.; Enders, J.F. Propagation of measles virus in cultures of chick embryo cells. Soc. Exp. Biol. Med. 1958, 97, 23–29. [Google Scholar] [CrossRef]
- Demeio, J.L.; Gower, T.A. Hemagglutination by measles virus. Virology 1961, 13, 367–368. [Google Scholar] [CrossRef]
- Kohn, A. Haemadsorption by measles syncytia. Nature 1962, 193, 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Peries, J.R.; Chany, C. Studies on measles viral hemagglutination. Soc. Exp. Biol. Med. 1962, 110, 477–482. [Google Scholar] [CrossRef]
- Rosanoff, E.I. Hemagglutination and hemadsorption of measles virus. Soc. Exp. Biol. Med. 1961, 106, 563–567. [Google Scholar] [CrossRef]
- Alkhatib, G.; Briedis, D.J. The predicted primary structure of the measles virus hemagglutinin. Virology 1986, 150, 479–490. [Google Scholar] [CrossRef]
- Kimura, H.; Saitoh, M.; Kobayashi, M.; Ishii, H.; Saraya, T.; Kurai, D.; Tsukagoshi, H.; Shirabe, K.; Nishina, A.; Kozawa, K.; et al. Molecular evolution of haemagglutinin (H) gene in measles virus. Sci. Rep. 2015, 5, 11648. [Google Scholar] [CrossRef] [PubMed]
- Rota, J.S.; Hummel, K.B.; Rota, P.A.; Bellini, W.J. Genetic variability of the glycoprotein genes of current wild-type measles isolates. Virology 1992, 188, 135–142. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Zhu, Z.; Liu, C.; Mao, N.; Ji, Y.; Wang, H.; Jiang, X.; Li, C.; Tang, W.; et al. Genetic characterization of the hemagglutinin genes of wild-type measles virus circulating in china, 1993–2009. PLoS ONE 2013, 8, e73374. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.C.; Sarangi, F.; Iorio, C.; Sidhu, M.S.; Udem, S.A.; Dillehay, D.L.; Xu, W.; Rota, P.A.; Bellini, W.J.; Richardson, C.D. A single amino acid change in the hemagglutinin protein of measles virus determines its ability to bind CD46 and reveals another receptor on marmoset B cells. J. Virol. 1998, 72, 2905–2916. [Google Scholar] [PubMed]
- Lecouturier, V.; Fayolle, J.; Caballero, M.; Carabana, J.; Celma, M.L.; Fernandez-Munoz, R.; Wild, T.F.; Buckland, R. Identification of two amino acids in the hemagglutinin glycoprotein of measles virus (MV) that govern hemadsorption, HeLa cell fusion, and CD46 downregulation: Phenotypic markers that differentiate vaccine and wild-type MV strains. J. Virol. 1996, 70, 4200–4204. [Google Scholar] [PubMed]
- Colf, L.A.; Juo, Z.S.; Garcia, K.C. Structure of the measles virus hemagglutinin. Nat. Struct. Mol. Biol. 2007, 14, 1227–1228. [Google Scholar] [CrossRef] [PubMed]
- Brindley, M.A.; Plemper, R.K. Blue native PAGE and biomolecular complementation reveal a tetrameric or higher-order oligomer organization of the physiological measles virus attachment protein H. J. Virol. 2010, 84, 12174–12184. [Google Scholar] [CrossRef] [PubMed]
- Jardetzky, T.S.; Lamb, R.A. Activation of paramyxovirus membrane fusion and virus entry. Curr. Opin. Virol. 2014, 5, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Ader-Ebert, N.; Khosravi, M.; Herren, M.; Avila, M.; Alves, L.; Bringolf, F.; Orvell, C.; Langedijk, J.P.; Zurbriggen, A.; Plemper, R.K.; et al. Sequential conformational changes in the morbillivirus attachment protein initiate the membrane fusion process. PLoS Pathog. 2015, 11, e1004880. [Google Scholar] [CrossRef] [PubMed]
- Rasbach, A.; Abel, T.; Münch, R.C.; Boller, K.; Schneider-Schaulies, J.; Buchholz, C.J. The receptor attachment function of measles virus hemagglutinin can be replaced with an autonomous protein that binds Her2/neu while maintaining its fusion-helper function. J. Virol. 2013, 87, 6246–6256. [Google Scholar] [CrossRef] [PubMed]
- Dorig, R.E.; Marcil, A.; Chopra, A.; Richardson, C.D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 1993, 75, 295–305. [Google Scholar] [CrossRef]
- Dorig, R.E.; Marcil, A.; Richardson, C.D. CD46, a primate-specific receptor for measles virus. Trends Microbiol. 1994, 2, 312–318. [Google Scholar] [CrossRef]
- Naniche, D.; Wild, T.F.; Rabourdin-Combe, C.; Gerlier, D. A monoclonal antibody recognizes a human cell surface glycoprotein involved in measles virus binding. J. Gen. Virol. 1992, 73, 2617–2624. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A.N. The Leucocyte Antigen Factsbook, 2nd ed.; Academic Press: San Diego, CA, USA, 1997; pp. 248–250. [Google Scholar]
- Liszewski, M.K.; Atkinson, J.P. Membrane cofactor protein. Curr. Top. Microbiol. Immunol. 1992, 178, 45–60. [Google Scholar] [PubMed]
- Liszewski, M.K.; Post, T.W.; Atkinson, J.P. Membrane cofactor protein (MCP or CD46): Newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 1991, 9, 431–455. [Google Scholar] [CrossRef] [PubMed]
- Naniche, D.; Varior-Krishnan, G.; Cervoni, F.; Wild, T.F.; Rossi, B.; Rabourdin-Combe, C.; Gerlier, D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 1993, 67, 6025–6032. [Google Scholar] [PubMed]
- Buchholz, C.J.; Gerlier, D.; Hu, A.; Cathomen, T.; Liszewski, M.K.; Atkinson, J.P.; Cattaneo, R. Selective expression of a subset of measles virus receptor-competent CD46 isoforms in human brain. Virology 1996, 217, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, C.J.; Koller, D.; Devaux, P.; Mumenthaler, C.; Schneider-Schaulies, J.; Braun, W.; Gerlier, D.; Cattaneo, R. Mapping of the primary binding site of measles virus to its receptor CD46. J. Biol. Chem. 1997, 272, 22072–22079. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, C.J.; Schneider, U.; Devaux, P.; Gerlier, D.; Cattaneo, R. Cell entry by measles virus: Long hybrid receptors uncouple binding from membrane fusion. J. Virol. 1996, 70, 3716–3723. [Google Scholar] [PubMed]
- Hsu, E.C.; Dorig, R.E.; Sarangi, F.; Marcil, A.; Iorio, C.; Richardson, C.D. Artificial mutations and natural variations in the CD46 molecules from human and monkey cells define regions important for measles virus binding. J. Virol. 1997, 71, 6144–6154. [Google Scholar] [PubMed]
- Hsu, E.C.; Sabatinos, S.; Hoedemaeker, F.J.; Rose, D.R.; Richardson, C.D. Use of site-specific mutagenesis and monoclonal antibodies to map regions of CD46 that interact with measles virus H protein. Virology 1999, 258, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Seya, T.; Yanagi, Y.; Pesando, J.M.; Johnson, P.M.; Okabe, M.; Ueda, S.; Ariga, H.; Nagasawa, S. Diversity of sites for measles virus binding and for inactivation of complement C3b and C4b on membrane cofactor protein CD46. J. Biol. Chem. 1995, 270, 15148–15152. [Google Scholar] [CrossRef] [PubMed]
- Maisner, A.; Alvarez, J.; Liszewski, M.K.; Atkinson, D.J.; Atkinson, J.P.; Herrler, G. The N-glycan of the SCR 2 region is essential for membrane cofactor protein (CD46) to function as a measles virus receptor. J. Virol. 1996, 70, 4973–4977. [Google Scholar] [PubMed]
- Maisner, A.; Herrler, G. Membrane cofactor protein with different types of N-glycans can serve as measles virus receptor. Virology 1995, 210, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Manchester, M.; Gairin, J.E.; Patterson, J.B.; Alvarez, J.; Liszewski, M.K.; Eto, D.S.; Atkinson, J.P.; Oldstone, M.B. Measles virus recognizes its receptor, CD46, via two distinct binding domains within SCR1-2. Virology 1997, 233, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Manchester, M.; Liszewski, M.K.; Atkinson, J.P.; Oldstone, M.B. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc. Natl. Acad. Sci. USA 1994, 91, 2161–2165. [Google Scholar] [CrossRef] [PubMed]
- Mumenthaler, C.; Schneider, U.; Buchholz, C.J.; Koller, D.; Braun, W.; Cattaneo, R. A 3D model for the measles virus receptor CD46 based on homology modeling, Monte Carlo simulations, and hemagglutinin binding studies. Protein Sci. 1997, 6, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Devaux, P.; Loveland, B.; Christiansen, D.; Milland, J.; Gerlier, D. Interactions between the ectodomains of haemagglutinin and CD46 as a primary step in measles virus entry. J. Gen. Virol. 1996, 77, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.C.; Tannenbaum, P.L.; Abbott, D.H.; Atkinson, J.P. Cutting edge: Inhibiting measles virus infection but promoting reproduction: An explanation for splicing and tissue-specific expression of CD46. J. Immunol. 2002, 169, 5405–5409. [Google Scholar] [CrossRef] [PubMed]
- Kallstrom, H.; Blackmer Gill, D.; Albiger, B.; Liszewski, M.K.; Atkinson, J.P.; Jonsson, A.B. Attachment of Neisseria gonorrhoeae to the cellular pilus receptor CD46: Identification of domains important for bacterial adherence. Cell. Microbiol. 2001, 3, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Kennedy, P.E.; Locatelli, G.; Malnati, M.S.; Berger, E.A.; Lusso, P. CD46 is a cellular receptor for human herpesvirus 6. Cell 1999, 99, 817–827. [Google Scholar] [CrossRef]
- Gaggar, A.; Shayakhmetov, D.M.; Lieber, A. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 2003, 9, 1408–1412. [Google Scholar] [CrossRef] [PubMed]
- Segerman, A.; Atkinson, J.P.; Marttila, M.; Dennerquist, V.; Wadell, G.; Arnberg, N. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 2003, 77, 9183–9191. [Google Scholar] [CrossRef] [PubMed]
- Maurer, K.; Krey, T.; Moennig, V.; Thiel, H.J.; Rumenapf, T. CD46 is a cellular receptor for bovine viral diarrhea virus. J. Virol. 2004, 78, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Santiago, C.; Celma, M.L.; Stehle, T.; Casasnovas, J.M. Structure of the measles virus hemagglutinin bound to the CD46 receptor. Nat. Struct. Mol. Biol. 2010, 17, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Santiago, C.; Gutierrez-Rodriguez, A.; Tucker, P.A.; Stehle, T.; Casasnovas, J.M. Crystallization and preliminary crystallographic analysis of the measles virus hemagglutinin in complex with the CD46 receptor. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2010, 66, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Navaratnarajah, C.K.; Vongpunsawad, S.; Oezguen, N.; Stehle, T.; Braun, W.; Hashiguchi, T.; Maenaka, K.; Yanagi, Y.; Cattaneo, R. Dynamic interaction of the measles virus hemagglutinin with its receptor signaling lymphocytic activation molecule (SLAM, CD150). J. Biol. Chem. 2008, 283, 11763–11771. [Google Scholar] [CrossRef] [PubMed]
- Kemper, C.; Atkinson, J.P. Measles virus and CD46. Curr. Top. Microbiol. Immunol. 2009, 329, 31–57. [Google Scholar] [PubMed]
- Evlashev, A.; Moyse, E.; Valentin, H.; Azocar, O.; Trescol-Biemont, M.C.; Marie, J.C.; Rabourdin-Combe, C.; Horvat, B. Productive measles virus brain infection and apoptosis in CD46 transgenic mice. J. Virol. 2000, 74, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Horvat, B.; Rivailler, P.; Varior-Krishnan, G.; Cardoso, A.; Gerlier, D.; Rabourdin-Combe, C. Transgenic mice expressing human measles virus (MV) receptor CD46 provide cells exhibiting different permissivities to MV infections. J. Virol. 1996, 70, 6673–6681. [Google Scholar] [PubMed]
- Rall, G.F.; Manchester, M.; Daniels, L.R.; Callahan, E.M.; Belman, A.R.; Oldstone, M.B. A transgenic mouse model for measles virus infection of the brain. Proc. Natl. Acad. Sci. USA 1997, 94, 4659–4663. [Google Scholar] [CrossRef] [PubMed]
- Oldstone, M.B.; Lewicki, H.; Thomas, D.; Tishon, A.; Dales, S.; Patterson, J.; Manchester, M.; Homann, D.; Naniche, D.; Holz, A. Measles virus infection in a transgenic model: Virus-induced immunosuppression and central nervous system disease. Cell 1999, 98, 629–640. [Google Scholar] [CrossRef]
- Tishon, A.; Lewicki, H.; Andaya, A.; McGavern, D.; Martin, L.; Oldstone, M.B. CD4 T cell control primary measles virus infection of the CNS: Regulation is dependent on combined activity with either CD8 T cells or with B cells: CD4, CD8 or B cells alone are ineffective. Virology 2006, 347, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Mrkic, B.; Odermatt, B.; Klein, M.A.; Billeter, M.A.; Pavlovic, J.; Cattaneo, R. Lymphatic dissemination and comparative pathology of recombinant measles viruses in genetically modified mice. J. Virol. 2000, 74, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Bartz, R.; Firsching, R.; Rima, B.; ter Meulen, V.; Schneider-Schaulies, J. Differential receptor usage by measles virus strains. J. Gen. Virol. 1998, 79, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Buckland, R.; Wild, T.F. Is CD46 the cellular receptor for measles virus? Virus Res. 1997, 48, 1–9. [Google Scholar] [CrossRef]
- Hsu, E.C.; Iorio, C.; Sarangi, F.; Khine, A.A.; Richardson, C.D. CDw150(SLAM) is a receptor for a lymphotropic strain of measles virus and may account for the immunosuppressive properties of this virus. Virology 2001, 279, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Kobune, F.; Sakata, H.; Sugiura, A. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J. Virol. 1990, 64, 700–705. [Google Scholar] [PubMed]
- Schneider-Schaulies, J.; Dunster, L.M.; Kobune, F.; Rima, B.; ter Meulen, V. Differential downregulation of CD46 by measles virus strains. J. Virol. 1995, 69, 7257–7259. [Google Scholar] [PubMed]
- Shibahara, K.; Hotta, H.; Katayama, Y.; Homma, M. Increased binding activity of measles virus to monkey red blood cells after long-term passage in Vero cell cultures. J. Gen. Virol. 1994, 75, 3511–3516. [Google Scholar] [CrossRef] [PubMed]
- Tatsuo, H.; Ono, N.; Tanaka, K.; Yanagi, Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature 2000, 406, 893–897. [Google Scholar] [PubMed]
- Ono, N.; Tatsuo, H.; Hidaka, Y.; Aoki, T.; Minagawa, H.; Yanagi, Y. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J. Virol. 2001, 75, 4399–4401. [Google Scholar] [CrossRef] [PubMed]
- Vongpunsawad, S.; Oezgun, N.; Braun, W.; Cattaneo, R. Selectively receptor-blind measles viruses: Identification of residues necessary for SLAM- or CD46-induced fusion and their localization on a new hemagglutinin structural model. J. Virol. 2004, 78, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Cocks, B.G.; Chang, C.C.; Carballido, J.M.; Yssel, H.; de Vries, J.E.; Aversa, G. A novel receptor involved in T-cell activation. Nature 1995, 376, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Sidorenko, S.P.; Clark, E.A. The dual-function CD150 receptor subfamily: The viral attraction. Nat. Immunol. 2003, 4, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Romero, X.; Sintes, J.; Engel, P. Role of SLAM family receptors and specific adapter SAP in innate-like lymphocytes. Crit. Rev. Immunol. 2014, 34, 263–299. [Google Scholar] [CrossRef] [PubMed]
- van Driel, B.J.; Liao, G.; Engel, P.; Terhorst, C. Responses to Microbial Challenges by SLAMF Receptors. Front. Immunol. 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Veillette, A.; Dong, Z.; Latour, S. Consequence of the SLAM-SAP signaling pathway in innate-like and conventional lymphocytes. Immunity 2007, 27, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Coffey, A.J.; Brooksbank, R.A.; Brandau, O.; Oohashi, T.; Howell, G.R.; Bye, J.M.; Cahn, A.P.; Durham, J.; Heath, P.; Wray, P.; et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat. Genet. 1998, 20, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.E.; Harkin, D.P.; Levitz, S.; Krainer, M.; Kolquist, K.A.; Genovese, C.; Bernard, A.; Ferguson, M.; Zuo, L.; Snyder, E.; et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc. Natl. Acad. Sci. USA 1998, 95, 13765–13770. [Google Scholar] [CrossRef] [PubMed]
- Sayos, J.; Wu, C.; Morra, M.; Wang, N.; Zhang, X.; Allen, D.; van Schaik, S.; Notarangelo, L.; Geha, R.; Roncarolo, M.G.; et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature 1998, 395, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.S.; Deenick, E.K. The role of SAP and SLAM family molecules in the humoral immune response. Ann. N. Y. Acad. Sci. 2011, 1217, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Avota, E.; Gulbins, E.; Schneider-Schaulies, S. DC-SIGN mediated sphingomyelinase-activation and ceramide generation is essential for enhancement of viral uptake in dendritic cells. PLoS Pathog. 2011, 7, e1001290. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, T.; Ose, T.; Kubota, M.; Maita, N.; Kamishikiryo, J.; Maenaka, K.; Yanagi, Y. Structure of the measles virus hemagglutinin bound to its cellular receptor SLAM. Nat. Struct. Mol. Biol. 2011, 18, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Hahm, B.; Arbour, N.; Naniche, D.; Homann, D.; Manchester, M.; Oldstone, M.B. Measles virus infects and suppresses proliferation of T lymphocytes from transgenic mice bearing human signaling lymphocytic activation molecule. J. Virol. 2003, 77, 3505–3515. [Google Scholar] [CrossRef] [PubMed]
- Hahm, B.; Arbour, N.; Oldstone, M.B. Measles virus interacts with human SLAM receptor on dendritic cells to cause immunosuppression. Virology 2004, 323, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Hahm, B.; Trifilo, M.J.; Zuniga, E.I.; Oldstone, M.B. Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity 2005, 22, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Hahm, B.; Cho, J.H.; Oldstone, M.B. Measles virus-dendritic cell interaction via SLAM inhibits innate immunity: Selective signaling through TLR4 but not other TLRs mediates suppression of IL-12 synthesis. Virology 2007, 358, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Sellin, C.I.; Horvat, B. Current animal models: Transgenic animal models for the study of measles pathogenesis. Curr. Top. Microbiol. Immunol. 2009, 330, 111–127. [Google Scholar] [PubMed]
- Welstead, G.G.; Iorio, C.; Draker, R.; Bayani, J.; Squire, J.; Vongpunsawad, S.; Cattaneo, R.; Richardson, C.D. Measles virus replication in lymphatic cells and organs of CD150 (SLAM) transgenic mice. Proc. Natl. Acad. Sci. USA 2005, 102, 16415–16420. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.; Frenzke, M.; Leonard, V.H.; Welstead, G.G.; Richardson, C.D.; Cattaneo, R. Measles virus infection of alveolar macrophages and dendritic cells precedes spread to lymphatic organs in transgenic mice expressing human signaling lymphocytic activation molecule (SLAM, CD150). J. Virol. 2010, 84, 3033–3042. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Ono, N.; Seki, F.; Takeda, M.; Kura, S.; Tsuzuki, T.; Yanagi, Y. Measles virus infection of SLAM (CD150) knockin mice reproduces tropism and immunosuppression in human infection. J. Virol. 2007, 81, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Koga, R.; Ohno, S.; Ikegame, S.; Yanagi, Y. Measles virus-induced immunosuppression in SLAM knock-in mice. J. Virol. 2010, 84, 5360–5367. [Google Scholar] [CrossRef] [PubMed]
- Niewiesk, S. Current animal models: Cotton rat animal model. Curr. Top. Microbiol. Immunol. 2009, 330, 89–110. [Google Scholar] [PubMed]
- Carsillo, T.; Huey, D.; Levinsky, A.; Obojes, K.; Schneider-Schaulies, J.; Niewiesk, S. Cotton rat (Sigmodon hispidus) signaling lymphocyte activation molecule (CD150) is an entry receptor for measles virus. PLoS ONE 2014, 9, e110120. [Google Scholar] [CrossRef] [PubMed]
- De Swart, R.L.; Ludlow, M.; de Witte, L.; Yanagi, Y.; van Amerongen, G.; McQuaid, S.; Yuksel, S.; Geijtenbeek, T.B.; Duprex, W.P.; Osterhaus, A.D. Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog. 2007, 3, e178. [Google Scholar] [CrossRef] [PubMed]
- Leonard, V.H.; Sinn, P.L.; Hodge, G.; Miest, T.; Devaux, P.; Oezguen, N.; Braun, W.; McCray, P.B., Jr.; McChesney, M.B.; Cattaneo, R. Measles virus blind to its epithelial cell receptor remains virulent in rhesus monkeys but cannot cross the airway epithelium and is not shed. J. Clin. Investig. 2008, 118, 2448–2458. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, M.; Rennick, L.J.; Sarlang, S.; Skibinski, G.; McQuaid, S.; Moore, T.; de Swart, R.L.; Duprex, W.P. Wild-type measles virus infection of primary epithelial cells occurs via the basolateral surface without syncytium formation or release of infectious virus. J. Gen. Virol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Lemon, K.; de Vries, R.D.; Measman, A.W.; McQuaid, S.; van Amerongen, G.; Yuksel, S.; Ludlow, M.; Rennick, L.J.; Kuken, T.; Rima, B.K.; et al. Early target cells of measles virus after aerosol infection of non-human primates. PLoS Pathog. 2010, 7, e1001263. [Google Scholar] [CrossRef] [PubMed]
- Craighead, J.E. Pathology and pathogenesis of human viral disease. In Rubeola (Measles); Craighead, J.E., Ed.; Elsevier Inc.: Philadelphia, PA, USA, 2000; pp. 397–410. [Google Scholar]
- Lightwood, R.; Nolan, R. Epithelial giant cells in measles as an acid in diagnosis. J. Pediatr. 1970, 77, 59–64. [Google Scholar] [CrossRef]
- Llanes-Rodas, R.; Liu, C. Rapid diagnosis of measles from urinary sediments stained with fluorescent antibody. N. Engl. J. Med. 1966, 275, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Moench, T.R.; Griffin, D.E.; Obriecht, C.R.; Vaisberg, A.J.; Johnson, R.T. Acute measles in patients with and without neurological involvement: Distribution of measles virus antigen and RNA. J. Infect. Dis. 1988, 158, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Nii, S.; Kamahora, J.; Mori, Y.; Takahashi, M.; Nishimura, S.; Okuno, Y. Experimental Pathology of Measles in Monkeys. Biken J. 1964, 6, 271–297. [Google Scholar] [PubMed]
- Sakaguchi, M.; Yoshikawa, Y.; Yamanouchi, K.; Sata, T.; Nagashima, K.; Takeda, K. Growth of measles virus in epithelial and lymphoid tissues of cynomolgus monkeys. Microbiol. Immunol. 1986, 30, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Miyajima, N.; Nagata, N.; Takeda, M.; Tashiro, M. Wild-type measles virus induces large syncytium formation in primary human small airway epithelial cells by a SLAM(CD150)-independent mechanism. Virus Res. 2003, 94, 11–16. [Google Scholar] [CrossRef]
- De Swart, R.L. The pathogenesis of measles revisited. Pediatr. Infect. Dis. J. 2008, 27 (Suppl. 10), S84–S88. [Google Scholar] [CrossRef] [PubMed]
- Becroft, D.M.; Osborne, D.R. The lungs in fatal measles infection in childhood: Pathological, radiological and immunological correlations. Histopathology 1980, 4, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, A.; Deutsch, M.; Ageletopoulou, J.; Delladetsima, J.K.; Marinos, E.; Kapranos, N.; Dourakis, S.P. A fatal case of postinfantile giant cell hepatitis in a patient with chronic lymphocytic leukaemia. Eur. J. Gastroenterol. Hepatol. 2003, 15, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.; Eto, H.; Morshed, S.A.; Itakura, H. Giant cell pneumonia: Light microscopy, immunohistochemical, and ultrastructural study of an autopsy case. Ultrastruct. Pathol. 1996, 20, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Harboldt, S.L.; Dugan, J.M.; Tronic, B.S. Cytologic diagnosis of measles pneumonia in a bronchoalveolar lavage specimen. A case report. Acta Cytol. 1994, 38, 403–406. [Google Scholar] [PubMed]
- Richardson, C.D. Towards identification and characterization of a new receptor for measles virus on airway epithelial cells. 13th International Negative Strand Virus Meeting (Salamanca, Spain), 2006. [Google Scholar]
- Takeda, M.; Tahara, M.; Hashiguchi, T.; Sato, T.A.; Jinnouchi, F.; Ueki, S.; Ohno, S.; Yanagi, Y. A human lung carcinoma cell line supports efficient measles virus growth and syncytium formation via a SLAM- and CD46-independent mechanism. J. Virol. 2007, 81, 12091–12096. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.; Bondre, D.; Noyce, R.; Ha, M.; Sisson, G. Wild type measles virus infects polarized adenocarcinoma cell lines using a unique receptor on the cellular apical surface. 14th International Negative Strand Virus Meeting (Bruges, Belgium), 2010. [Google Scholar]
- Noyce, R.S.; Bondre, D.G.; Ha, M.N.; Lin, L.T.; Sisson, G.; Tsao, M.S.; Richardson, C.D. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011, 7, e1002240. [Google Scholar] [CrossRef] [PubMed]
- Sinn, P.L.; Williams, G.; Vongpunsawad, S.; Cattaneo, R.; McCray, P.B., Jr. Measles virus preferentially transduces the basolateral surface of well-differentiated human airway epithelia. J. Virol. 2002, 76, 2403–2409. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Takeda, M.; Shirogane, Y.; Hashiguchi, T.; Ohno, S.; Yanagi, Y. Measles virus infects both polarized epithelial and immune cells by using distinctive receptor-binding sites on its hemagglutinin. J. Virol. 2008, 82, 4630–4637. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Ono, N.; Tatsuo, H.; Minagawa, H.; Takeda, M.; Takeuchi, K.; Yanagi, Y. SLAM (CD150)-independent measles virus entry as revealed by recombinant virus expressing green fluorescent protein. J. Virol. 2002, 76, 6743–6749. [Google Scholar] [CrossRef] [PubMed]
- Shirogane, Y.; Takeda, M.; Tahara, M.; Ikegame, S.; Nakamura, T.; Yanagi, Y. Epithelial-mesenchymal transition abolishes the susceptibility of polarized epithelial cell lines to measles virus. J. Biol. Chem. 2010, 285, 20882–20890. [Google Scholar] [CrossRef] [PubMed]
- Muhlebach, M.D.; Mateo, M.; Sinn, P.L.; Prufer, S.; Uhlig, K.M.; Leonard, V.H.; Navaratnarajah, C.K.; Frenzke, M.; Wong, X.X.; Sawatsky, B.; et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 2011, 480, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Reymond, N.; Fabre, S.; Lecocq, E.; Adelaide, J.; Dubreuil, P.; Lopez, M. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J. Biol. Chem. 2001, 276, 43205–43215. [Google Scholar] [CrossRef] [PubMed]
- Derycke, M.S.; Pambuccian, S.E.; Gilks, C.B.; Kalloger, S.E.; Ghidouche, A.; Lopez, M.; Bliss, R.L.; Geller, M.A.; Argenta, P.A.; Harrington, K.M.; et al. Nectin 4 overexpression in ovarian cancer tissues and serum: Potential role as a serum biomarker. Am. J. Clin. Pathol. 2010, 134, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.; Ishikawa, N.; Nishino, R.; Masuda, K.; Yasui, W.; Inai, K.; Nishimura, H.; Ito, H.; Nakayama, H.; Miyagi, Y.; et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009, 69, 6694–6703. [Google Scholar] [CrossRef] [PubMed]
- Fabre-Lafay, S.; Garrido-Urbani, S.; Reymond, N.; Goncalves, A.; Dubreuil, P.; Lopez, M. Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-alpha-converting enzyme (TACE)/ADAM-17. J. Biol. Chem. 2005, 280, 19543–19550. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Takeichi, M. Adherens junction: Molecular architecture and regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a002899. [Google Scholar] [CrossRef] [PubMed]
- Fabre-Lafay, S.; Monville, F.; Garrido-Urbani, S.; Berruyer-Pouyet, C.; Ginestier, C.; Reymond, N.; Finetti, P.; Sauvan, R.; Adelaide, J.; Geneix, J.; et al. Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer 2007, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Pallasch, C.; Elia, A.E.; Braun, C.J.; Westbrook, T.F.; Hemann, M.; Elledge, S.J. A role for PVRL4-driven cell-cell interactions in tumorigenesis. eLife 2013, 2, e00358. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, C.L.; Wimmer, E.; Racaniello, V.R. Cellular receptor for poliovirus: Molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell 1989, 56, 855–865. [Google Scholar] [CrossRef]
- Geraghty, R.J.; Krummenacher, C.; Cohen, G.H.; Eisenberg, R.J.; Spear, P.G. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 1998, 280, 1618–1620. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Cocchi, F.; Menotti, L.; Avitabile, E.; Dubreuil, P.; Campadelli-Fiume, G. Nectin2alpha (PRR2alpha or HveB) and nectin2delta are low-efficiency mediators for entry of herpes simplex virus mutants carrying the Leu25Pro substitution in glycoprotein D. J. Virol. 2000, 74, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.M.; Lin, E.; Susmarski, N.; Yoon, M.; Zago, A.; Ware, C.F.; Pfeffer, K.; Miyoshi, J.; Takai, Y.; Spear, P.G. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell. Host Microbe 2007, 2, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Adusumilli, P.S.; Eisenberg, D.P.; Darr, E.; Ghossein, R.A.; Li, S.; Liu, S.; Singh, B.; Shah, J.P.; Fong, Y.; et al. Nectin-1 expression by squamous cell carcinoma is a predictor of herpes oncolytic sensitivity. Mol. Ther. 2007, 15, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, G.; Qi, J.; Li, Y.; He, Y.; Xu, X.; Shi, J.; Zhang, C.W.; Yan, J.; Gao, G.F. Structure of measles virus hemagglutinin bound to its epithelial receptor nectin-4. Nat. Struct. Mol. Biol. 2013, 20, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Mateo, M.; Navaratnarajah, C.K.; Syed, S.; Cattaneo, R. The measles virus hemagglutinin beta-propeller head β4-β5 hydrophobic groove governs functional interactions with nectin-4 and CD46 but not those with the signaling lymphocytic activation molecule. J. Virol. 2013, 87, 9208–9216. [Google Scholar] [CrossRef] [PubMed]
- Mateo, M.; Navaratnarajah, C.K.; Willenbring, R.C.; Maroun, J.W.; Iankov, I.; Lopez, M.; Sinn, P.L.; Cattaneo, R. Different roles of the three loops forming the adhesive interface of nectin-4 in measles virus binding and cell entry, nectin-4 homodimerization, and heterodimerization with nectin-1. J. Virol. 2014, 88, 14161–14171. [Google Scholar] [CrossRef] [PubMed]
- Seki, F.; Someya, K.; Komase, K.; Takeda, M. A chicken homologue of nectin-4 functions as a measles virus receptor. Vaccine 2016, 34, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Adombi, C.M.; Lelenta, M.; Lamien, C.E.; Shamaki, D.; Koffi, Y.M.; Traore, A.; Silber, R.; Couacy-Hymann, E.; Bodjo, S.C.; Djaman, J.A.; et al. Monkey CV1 cell line expressing the sheep-goat SLAM protein: A highly sensitive cell line for the isolation of peste des petits ruminants virus from pathological specimens. J. Virol. Methods 2011, 173, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Tatsuo, H.; Ono, N.; Yanagi, Y. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J. Virol. 2001, 75, 5842–5850. [Google Scholar] [CrossRef] [PubMed]
- Tatsuo, H.; Yanagi, Y. The morbillivirus receptor SLAM (CD150). Microbiol. Immunol. 2002, 46, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Bieringer, M.; Han, J.W.; Kendl, S.; Khosravi, M.; Plattet, P.; Schneider-Schaulies, J. Experimental adaptation of wild-type canine distemper virus (CDV) to the human entry receptor CD150. PLoS ONE 2013, 8, e57488. [Google Scholar] [CrossRef] [PubMed]
- Sattler, U.; Khosravi, M.; Avila, M.; Pilo, P.; Langedijk, J.P.; Ader-Ebert, N.; Alves, L.A.; Plattet, P.; Origgi, F.C. Identification of amino acid substitutions with compensational effects in the attachment protein of canine distemper virus. J. Virol. 2014, 88, 8057–8064. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Suzuki, J.; Noguchi, K.; Terada, Y.; Shimoda, H.; Mizuno, T.; Maeda, K. Function of feline signaling lymphocyte activation molecule as a receptor of canine distemper virus. J. Vet. Med. Sci. Jpn. Soc. Vet. Sci. 2013, 75, 1085–1089. [Google Scholar] [CrossRef]
- Ohishi, K.; Suzuki, R.; Maeda, T.; Tsuda, M.; Abe, E.; Yoshida, T.; Endo, Y.; Okamura, M.; Nagamine, T.; Yamamoto, H.; et al. Recent host range expansion of canine distemper virus and variation in its receptor, the signaling lymphocyte activation molecule, in carnivores. J. Wildl. Dis. 2014, 50, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Nagata, N.; Ami, Y.; Seki, F.; Suzaki, Y.; Iwata-Yoshikawa, N.; Suzuki, T.; Fukushi, S.; Mizutani, T.; Yoshikawa, T.; et al. Lethal canine distemper virus outbreak in cynomolgus monkeys in Japan in 2008. J. Virol. 2013, 87, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Yoshikawa, T.; Seki, F.; Fukushi, S.; Tahara, M.; Nagata, N.; Ami, Y.; Mizutani, T.; Kurane, I.; Yamaguchi, R.; et al. Canine distemper virus associated with a lethal outbreak in monkeys can readily adapt to use human receptors. J. Virol. 2013, 87, 7170–7175. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, A.; Ye, H.; Shi, Y.; Hu, Z.; Zeng, L. Natural infection with canine distemper virus in hand-feeding Rhesus monkeys in China. Vet. Microbiol. 2010, 141, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Noyce, R.S.; Delpeut, S.; Richardson, C.D. Dog nectin-4 is an epithelial cell receptor for canine distemper virus that facilitates virus entry and syncytia formation. Virology 2013, 436, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Pratakpiriya, W.; Seki, F.; Otsuki, N.; Sakai, K.; Fukuhara, H.; Katamoto, H.; Hirai, T.; Maenaka, K.; Techangamsuwan, S.; Lan, N.T.; et al. Nectin4 is an epithelial cell receptor for canine distemper virus and involved in neurovirulence. J. Virol. 2012, 86, 10207–10210. [Google Scholar] [CrossRef] [PubMed]
- Sawatsky, B.; Wong, X.X.; Hinkelmann, S.; Cattaneo, R.; von Messling, V. Canine distemper virus epithelial cell infection is required for clinical disease but not for immunosuppression. J. Virol. 2012, 86, 3658–3666. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.; Juleff, N.; Heaton, M.P.; Kalbfleisch, T.; Kijas, J.; Bailey, D. Characterization of ovine Nectin-4, a novel peste des petits ruminants virus receptor. J. Virol. 2013, 87, 4756–4761. [Google Scholar] [CrossRef] [PubMed]
- Fakri, F.; Elarkam, A.; Daouam, S.; Tadlaoui, K.; Fassi-Fihri, O.; Richardson, C.D.; Elharrak, M. VeroNectin-4 is a highly sensitive cell line that can be used for the isolation and titration of Peste des Petits Ruminants virus. J. Virol. Methods 2016, 228, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Melia, M.M.; Earle, J.P.; Abdullah, H.; Reaney, K.; Tangy, F.; Cosby, S.L. Use of SLAM and PVRL4 and identification of pro-HB-EGF as cell entry receptors for wild type phocine distemper virus. PLoS ONE 2014, 9, e106281. [Google Scholar] [CrossRef] [PubMed]
- Delpeut, S.; Noyce, R.S.; Richardson, C.D. The Tumor-Associated Marker, PVRL4 (Nectin-4), Is the Epithelial Receptor for Morbilliviruses. Viruses 2014, 6, 2268–2286. [Google Scholar] [CrossRef] [PubMed]
- Avota, E.; Gassert, E.; Schneider-Schaulies, S. Measles virus-induced immunosuppression: From effectors to mechanisms. Med. Microbiol. Immunol. 2010, 199, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Frenzke, M.; Sawatsky, B.; Wong, X.X.; Delpeut, S.; Mateo, M.; Cattaneo, R.; von Messling, V. Nectin-4-dependent measles virus spread to the cynomolgus monkey tracheal epithelium: Role of infected immune cells infiltrating the lamina propria. J. Virol. 2013, 87, 2526–2534. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, M.; Lemon, K.; de Vries, R.D.; McQuaid, S.; Millar, E.L.; van Amerongen, G.; Yuksel, S.; Verburgh, R.J.; Osterhaus, A.D.; de Swart, R.L.; et al. Measles virus infection of epithelial cells in the macaque upper respiratory tract is mediated by subepithelial immune cells. J. Virol. 2013, 87, 4033–4042. [Google Scholar] [CrossRef] [PubMed]
- Dingli, D.; Peng, K.W.; Harvey, M.E.; Greipp, P.R.; O’Connor, M.K.; Cattaneo, R.; Morris, J.C.; Russell, S.J. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood 2004, 103, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.W.; Ahmann, G.J.; Pham, L.; Greipp, P.R.; Cattaneo, R.; Russell, S.J. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood 2001, 98, 2002–2007. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.W.; Donovan, K.A.; Schneider, U.; Cattaneo, R.; Lust, J.A.; Russell, S.J. Oncolytic measles viruses displaying a single-chain antibody against CD38, a myeloma cell marker. Blood 2003, 101, 2557–2562. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.J. RNA viruses as virotherapy agents. Cancer Gene Ther. 2002, 9, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.J.; Peng, K.W. Measles virus for cancer therapy. Curr Top. Microbiol. Immunol. 2009, 330, 213–241. [Google Scholar] [PubMed]
- Bluming, A.Z.; Ziegler, J.L. Regression of Burkitt’s lymphoma in association with measles infection. Lancet 1971, 2, 105–106. [Google Scholar] [CrossRef]
- Mota, H.C. Infantile Hodgkin’s disease: Remission after measles. Br. Med. J. 1973, 2, 421. [Google Scholar] [CrossRef] [PubMed]
- Taqi, A.M.; Abdurrahman, M.B.; Yakubu, A.M.; Fleming, A.F. Regression of Hodgkin’s disease after measles. Lancet 1981, 1, 1112. [Google Scholar] [CrossRef]
- Ziegler, J.L. Spontaneous remission in Burkitt’s lymphoma. Natl. Cancer Inst. Monogr. 1976, 44, 61–65. [Google Scholar] [PubMed]
- Zygiert, Z. Hodgkin’s disease: Remissions after measles. Lancet 1971, 1, 593. [Google Scholar] [CrossRef]
- Blechacz, B.; Splinter, P.L.; Greiner, S.; Myers, R.; Peng, K.W.; Federspiel, M.J.; Russell, S.J.; LaRusso, N.F. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology 2006, 44, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Grote, D.; Russell, S.J.; Cornu, T.I.; Cattaneo, R.; Vile, R.; Poland, G.A.; Fielding, A.K. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood 2001, 97, 3746–3754. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Bangen, J.M.; Bayer, W.; Wildner, O. Synergy between expression of fusogenic membrane proteins, chemotherapy and facultative virotherapy in colorectal cancer. Gene Ther. 2006, 13, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.J.; Erlichman, C.; Ingle, J.N.; Rosales, G.A.; Allen, C.; Greiner, S.M.; Harvey, M.E.; Zollman, P.J.; Russell, S.J.; Galanis, E. A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer. Breast Cancer Res. Treat. 2006, 99, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.; Greiner, S.; Harvey, M.; Soeffker, D.; Frenzke, M.; Abraham, K.; Shaw, A.; Rozenblatt, S.; Federspiel, M.J.; Russell, S.J.; et al. Oncolytic activities of approved mumps and measles vaccines for therapy of ovarian cancer. Cancer Gene Ther. 2005, 12, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.W.; TenEyck, C.J.; Galanis, E.; Kalli, K.R.; Hartmann, L.C.; Russell, S.J. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002, 62, 4656–4662. [Google Scholar] [PubMed]
- Phuong, L.K.; Allen, C.; Peng, K.W.; Giannini, C.; Greiner, S.; TenEyck, C.J.; Mishra, P.K.; Macura, S.I.; Russell, S.J.; Galanis, E.C. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003, 63, 2462–2469. [Google Scholar] [PubMed]
- Heinzerling, L.; Kunzi, V.; Oberholzer, P.A.; Kundig, T.; Naim, H.; Dummer, R. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood 2005, 106, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Vongpunsawad, S.; Nakamura, T.; James, C.D.; Schroeder, M.; Cattaneo, R.; Giannini, C.; Krempski, J.; Peng, K.W.; Goble, J.M.; et al. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006, 66, 11840–11850. [Google Scholar] [CrossRef] [PubMed]
- Bucheit, A.D.; Kumar, S.; Grote, D.M.; Lin, Y.; von Messling, V.; Cattaneo, R.B.; Fielding, A.K. An oncolytic measles virus engineered to enter cells through the CD20 antigen. Mol. Ther. 2003, 7, 62–72. [Google Scholar] [CrossRef]
- Hasegawa, K.; Nakamura, T.; Harvey, M.; Ikeda, Y.; Oberg, A.; Figini, M.; Canevari, S.; Hartmann, L.C.; Peng, K.W. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 6170–6178. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Peng, K.W.; Harvey, M.; Greiner, S.; Lorimer, I.A.; James, C.D.; Russell, S.J. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat. Biotechnol. 2005, 23, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Peng, K.W.; Vongpunsawad, S.; Harvey, M.; Mizuguchi, H.; Hayakawa, T.; Cattaneo, R.; Russell, S.J. Antibody-targeted cell fusion. Nat. Biotechnol. 2004, 22, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Paraskevakou, G.; Allen, C.; Nakamura, T.; Zollman, P.; James, C.D.; Peng, K.W.; Schroeder, M.; Russell, S.J.; Galanis, E. Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Mol. Ther. 2007, 15, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Ungerechts, G.; Springfeld, C.; Frenzke, M.E.; Lampe, J.; Johnston, P.B.; Parker, W.B.; Sorscher, E.J.; Cattaneo, R. Lymphoma chemovirotherapy: CD20-targeted and convertase-armed measles virus can synergize with fludarabine. Cancer Res. 2007, 67, 10939–10947. [Google Scholar] [CrossRef] [PubMed]
- Dingli, D.; Offord, C.; Myers, R.; Peng, K.W.; Carr, T.W.; Josic, K.; Russell, S.J.; Bajzer, Z. Dynamics of multiple myeloma tumor therapy with a recombinant measles virus. Cancer Gene Ther. 2009, 16, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Hartmann, L.C.; Cliby, W.A.; Long, H.J.; Peethambaram, P.P.; Barrette, B.A.; Kaur, J.S.; Haluska, P.J., Jr.; Aderca, I.; Zollman, P.J.; et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010, 70, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Penheiter, A.R.; Wegman, T.R.; Classic, K.L.; Dingli, D.; Bender, C.E.; Russell, S.J.; Carlson, S.K. Sodium iodide symporter (NIS)-mediated radiovirotherapy for pancreatic cancer. AJR Am. J. Roentgenol. 2010, 195, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Studebaker, A.W.; Kreofsky, C.R.; Pierson, C.R.; Russell, S.J.; Galanis, E.; Raffel, C. Treatment of medulloblastoma with a modified measles virus. Neuro Oncol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Amagai, Y.; Fujiyuki, T.; Yoneda, M.; Shoji, K.; Furukawa, Y.; Sato, H.; Kai, C. Oncolytic Activity of a Recombinant Measles Virus, Blind to Signaling Lymphocyte Activation Molecule, Against Colorectal Cancer Cells. Sci. Rep. 2016, 6, 24572. [Google Scholar] [CrossRef] [PubMed]
- Fujiyuki, T.; Yoneda, M.; Amagai, Y.; Obayashi, K.; Ikeda, F.; Shoji, K.; Murakami, Y.; Sato, H.; Kai, C. A measles virus selectively blind to signaling lymphocytic activation molecule shows anti-tumor activity against lung cancer cells. Oncotarget 2015, 6, 24895–24903. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Yoneda, M.; Kuraishi, T.; Hattori, S.; Inoue, Y.; Sato, H.; Kai, C. Measles virus selectively blind to signaling lymphocyte activation molecule as a novel oncolytic virus for breast cancer treatment. Gene Ther. 2013, 20, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Shoji, K.; Yoneda, M.; Fujiyuki, T.; Amagai, Y.; Tanaka, A.; Matsuda, A.; Ogihara, K.; Naya, Y.; Ikeda, F.; Matsuda, H.; et al. Development of new therapy for canine mammary cancer with recombinant measles virus. Mol. Ther. Oncolytics 2016, 3, 15022. [Google Scholar] [CrossRef] [PubMed]
- Suter, S.E.; Chein, M.B.; von Messling, V.; Yip, B.; Cattaneo, R.; Vernau, W.; Madewell, B.R.; London, C.A. In vitro canine distemper virus infection of canine lymphoid cells: A prelude to oncolytic therapy for lymphoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Karp, C.L. Measles: Immunosuppression, interleukin-12, and complement receptors. Immunol. Rev. 1999, 168, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Fara, A.F.; Dasgupta, P.; Kemper, C. CD46: The ‘multitasker’ of complement proteins. Int. J. Biochem. Cell Biol. 2013, 45, 2808–2820. [Google Scholar] [CrossRef] [PubMed]
- Romanets-Korbut, O.; Kovalevska, L.M.; Seya, T.; Sidorenko, S.P.; Horvat, B. Measles virus hemagglutinin triggers intracellular signaling in CD150-expressing dendritic cells and inhibits immune response. Cell. Mol. Immunol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Goncalves Carneiro, D.; Bailey, D. Measles virus is endocytosed and induces macropinocytosis in SLAM positive cells. 15th International Negative Strand Virus Meeting (Siena, Italy), 2015. [Google Scholar]
- Beaty, S.M.; Lee, B. Constraints on the Genetic and Antigenic Variability of Measles Virus. Viruses 2016, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Bankamp, B.; Takeda, M.; Zhang, Y.; Xu, W.; Rota, P.A. Genetic characterization of measles vaccine strains. J. Infect. Dis. 2011, 204 (Suppl. 1), S533–S548. [Google Scholar] [CrossRef] [PubMed]
- Fulton, B.O.; Sachs, D.; Beaty, S.M.; Won, S.T.; Lee, B.; Palese, P.; Heaton, N.S. Mutational Analysis of Measles Virus Suggests Constraints on Antigenic Variation of the Glycoproteins. Cell. Rep. 2015, 11, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Manchester, M.; Eto, D.S.; Valsamakis, A.; Liton, P.B.; Fernandez-Munoz, R.; Rota, P.A.; Bellini, W.J.; Forthal, D.N.; Oldstone, M.B. Clinical isolates of measles virus use CD46 as a cellular receptor. J. Virol. 2000, 74, 3967–3974. [Google Scholar] [CrossRef] [PubMed]
- Bonami, F.; Rudd, P.A.; von Messling, V. Disease duration determines canine distemper virus neurovirulence. J. Virol. 2007, 81, 12066–12070. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Khosravi, M.; Avila, M.; Ader-Ebert, N.; Bringolf, F.; Zurbriggen, A.; Vandevelde, M.; Plattet, P. SLAM- and nectin-4-independent noncytolytic spread of canine distemper virus in astrocytes. J. Virol. 2015, 89, 5724–5733. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.M.; Patterson, C.E.; Gales, T.L.; D’Orazio, J.L.; Vaughn, M.M.; Rall, G.F. Measles virus spread between neurons requires cell contact but not CD46 expression, syncytium formation, or extracellular virus production. J. Virol. 2000, 74, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Makhortova, N.R.; Askovich, P.; Patterson, C.E.; Gechman, L.A.; Gerard, N.P.; Rall, G.F. Neurokinin-1 enables measles virus trans-synaptic spread in neurons. Virology 2007, 362, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Brindley, M.A.; Suter, R.; Schestak, I.; Kiss, G.; Wright, E.R.; Plemper, R.K. A stabilized headless measles virus attachment protein stalk efficiently triggers membrane fusion. J. Virol. 2013, 87, 11693–11703. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.-T.; Richardson, C.D. The Host Cell Receptors for Measles Virus and Their Interaction with the Viral Hemagglutinin (H) Protein. Viruses 2016, 8, 250. https://doi.org/10.3390/v8090250
Lin L-T, Richardson CD. The Host Cell Receptors for Measles Virus and Their Interaction with the Viral Hemagglutinin (H) Protein. Viruses. 2016; 8(9):250. https://doi.org/10.3390/v8090250
Chicago/Turabian StyleLin, Liang-Tzung, and Christopher D. Richardson. 2016. "The Host Cell Receptors for Measles Virus and Their Interaction with the Viral Hemagglutinin (H) Protein" Viruses 8, no. 9: 250. https://doi.org/10.3390/v8090250




