A Linear Surface Epitope in a Proline-Rich Region of ORF3 Product of Genotype 1 Hepatitis E Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Transfection
2.2. Plasmids
2.3. Expression and Purification of VP13
2.4. Immunization and Monoclonal Antibody Preparation
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Western Blotting
2.7. Immunofluorescence Assay (IFA)
2.8. Statistical Analysis
3. Results
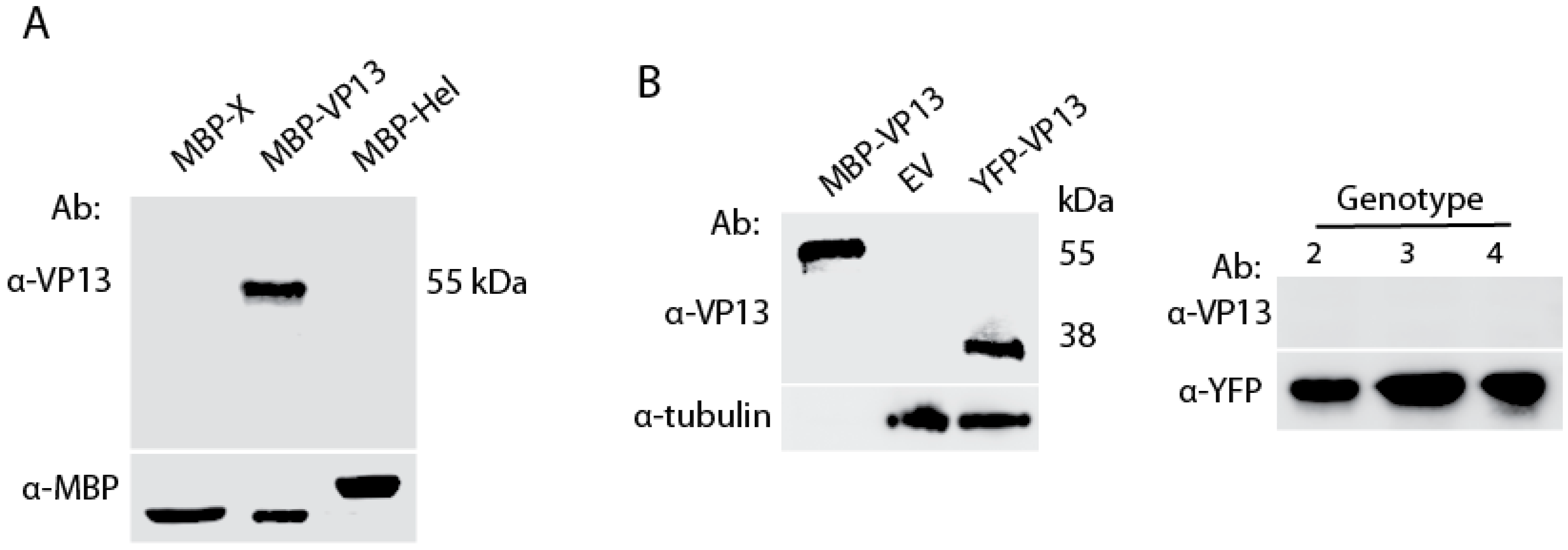
3.1. A VP13 Mab only Reacts with VP13 of Genotype 1 HEV
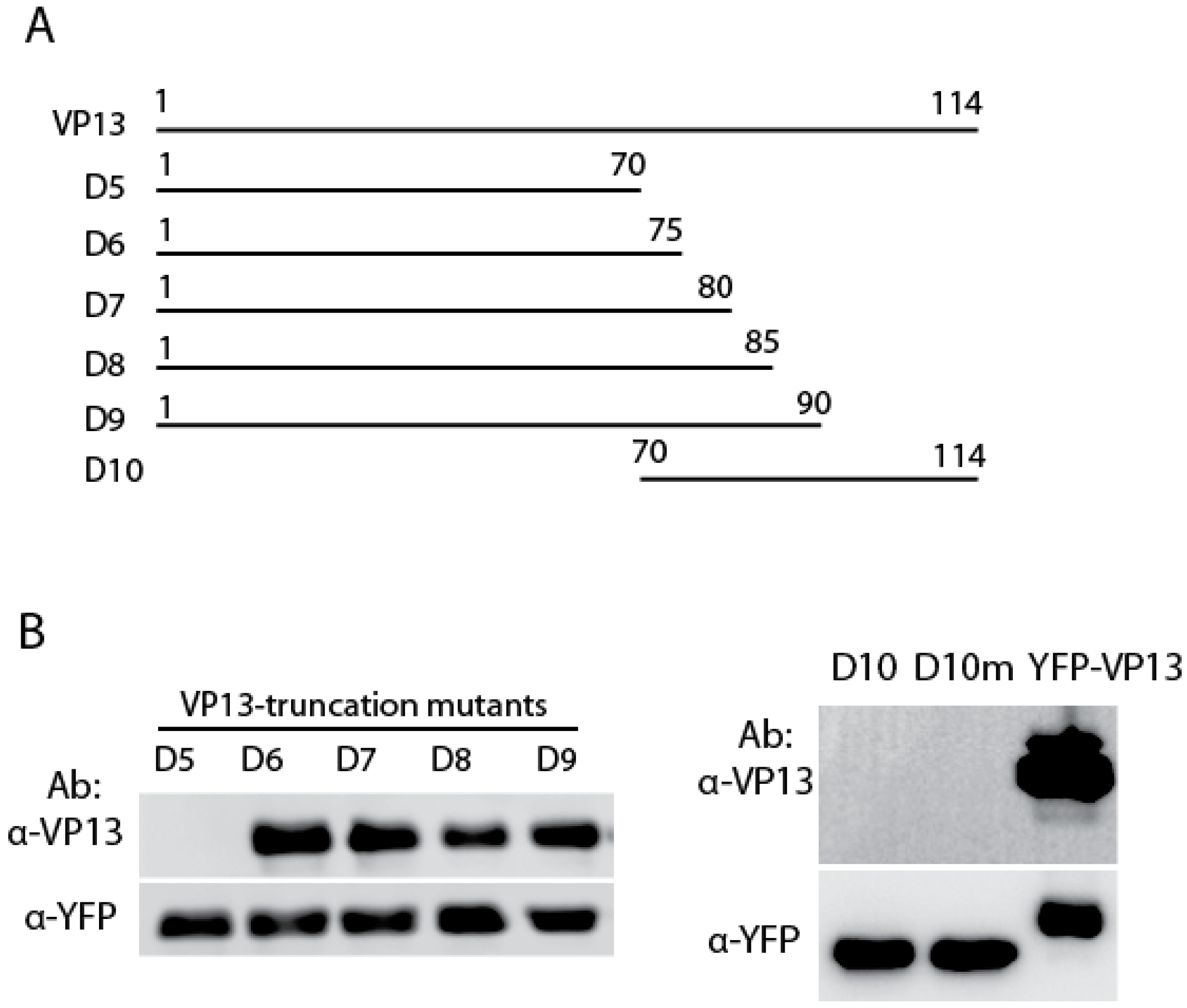
3.2. The Linear Epitope Spans Residues aa66-75 of VP13 Protein
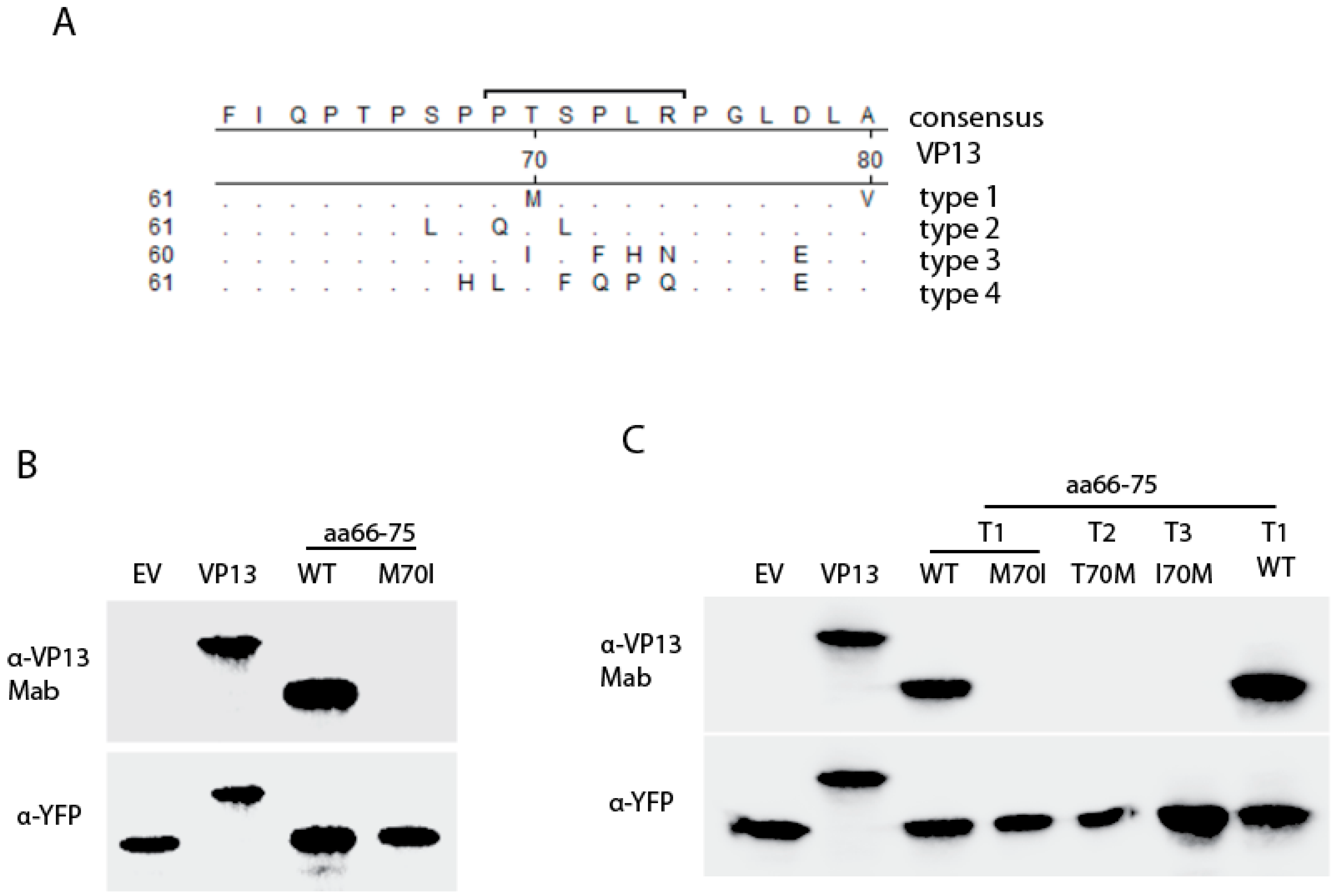
3.3. The Residue Methionine 70 Is Essential for the Interaction of the Linear Epitope with the Mab
3.4. IFA Detection of the Linear Epitope in HEV-Infected Cells Using the Mab
3.5. The Peptide aa66–75 Is Genotype-Specific in Reacting with HEV-Positive Human Serum Samples
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Emerson, S.; Anderson, D.; Arankalle, V.A.; Meng, X.-J.; Purdy, M.; Schlauder, G.G.; Tsarev, S. Hepevirus, Virus Taxonomy; VIIIth Report of the ICTV; Fauquest, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., Ball, L.A., Eds.; Elseiver/Academic Press: London, UK, 2004. [Google Scholar]
- Khuroo, M.S. Discovery of hepatitis E: The epidemic non-A, non-B hepatitis 30 years down the memory lane. Virus Res. 2011, 161, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Dalton, H.R.; Abravanel, F.; Izopet, J. Hepatitis E virus infection. Clin. Microbiol. Rev. 2014, 27, 116–138. [Google Scholar] [CrossRef] [PubMed]
- Jameel, S. Molecular biology and pathogenesis of hepatitis E virus. Expert Rev. Mol. Med. 1999, 1–16. [Google Scholar] [CrossRef]
- Kumar, S.; Subhadra, S.; Singh, B.; Panda, B.K. Hepatitis E virus: the current scenario. Int. J. Infect. Dis. 2013, 17, e228–e233. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.W.; Smith, M.M.; Guerra, M.E.; Huang, C.C.; Bradley, D.W.; Fry, K.E.; Reyes, G.R. Hepatitis E virus (HEV): Molecular cloning and sequencing of the full-length viral genome. Virology 1991, 185, 120–131. [Google Scholar] [CrossRef]
- Graff, J.; Nguyen, H.; Yu, C.; Elkins, W.R.; St Claire, M.; Purcell, R.H.; Emerson, S.U. The open reading frame 3 gene of hepatitis E virus contains a cis-reactive element and encodes a protein required for infection of macaques. J. Virol. 2005, 79, 6680–6689. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Opriessnig, T.; Halbur, P.G.; Meng, X.J. Initiation at the third in-frame AUG codon of open reading frame 3 of the hepatitis E virus is essential for viral infectivity in vivo. J. Virol. 2007, 81, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.; Torian, U.; Nguyen, H.; Emerson, S.U. A bicistronic subgenomic mRNA encodes both the ORF2 and ORF3 proteins of hepatitis E virus. J. Virol. 2006, 80, 5919–5926. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, C.; Hagedorn, C.H. Phylogenetic analysis of global hepatitis E virus sequences: Genetic diversity, subtypes and zoonosis. Rev. Med. Virol. 2006, 16, 5–36. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Simmonds, P.; Jameel, S.; Emerson, S.U.; Harrison, T.J.; Meng, X.J.; Okamoto, H.; van der Poel, W.H.; Purdy, M.A. Consensus proposals for classification of the family Hepeviridae. J. Gen. Virol. 2014, 95 Pt 10, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.J. Recent advances in Hepatitis E virus. J. Viral. Hepat. 2010, 17, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Holla, R.P.; Jameel, S. Molecular virology of hepatitis E virus. Virus Res. 2011, 161, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Kannan, H.; Fan, S.; Patel, D.; Bossis, I.; Zhang, Y.J. The hepatitis E virus open reading frame 3 product interacts with microtubules and interferes with their dynamics. J. Virol. 2009, 83, 6375–6382. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Takahashi, M.; Jirintai, S.; Tanaka, T.; Nishizawa, T.; Yasuda, J.; Okamoto, H. Tumour susceptibility gene 101 and the vacuolar protein sorting pathway are required for the release of hepatitis E virions. J. Gen. Virol. 2011, 92 Pt 12, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Takahashi, M.; Jirintai; Tanaka, T.; Yamada, K.; Nishizawa, T.; Okamoto, H. A PSAP motif in the ORF3 protein of hepatitis E virus is necessary for virion release from infected cells. J. Gen. Virol. 2011, 92 Pt 2, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Kenney, S.P.; Pudupakam, R.S.; Huang, Y.W.; Pierson, F.W.; LeRoith, T.; Meng, X.J. The PSAP motif within the ORF3 protein of an avian strain of the hepatitis E virus is not critical for viral infectivity in vivo but plays a role in virus release. J. Virol. 2012, 86, 5637–5646. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Ma, Z.; Wang, R.; Yu, Y.; Kannan, H.; Fredericksen, B.; Zhang, Y.J. Enhancement of Interferon Induction by ORF3 Product of Hepatitis E Virus. J. Virol. 2014, 88, 8696–8705. [Google Scholar] [CrossRef] [PubMed]
- Sparks, A.B.; Quilliam, L.A.; Thorn, J.M.; Der, C.J.; Kay, B.K. Identification and characterization of Src SH3 ligands from phage-displayed random peptide libraries. J. Biol. Chem. 1994, 269, 23853–23856. [Google Scholar] [PubMed]
- Simon, J.A.; Schreiber, S.L. Grb2 SH3 binding to peptides from Sos: Evaluation of a general model for SH3-ligand interactions. Chem. Biol. 1995, 2, 53–60. [Google Scholar] [CrossRef]
- Shukla, P.; Nguyen, H.T.; Torian, U.; Engle, R.E.; Faulk, K.; Dalton, H.R.; Bendall, R.P.; Keane, F.E.; Purcell, R.H.; Emerson, S.U. Cross-species infections of cultured cells by hepatitis E virus and discovery of an infectious virus-host recombinant. Proc. Natl. Acad. Sci. USA 2011, 108, 2438–2443. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Yu, Y.; Ma, Z.; Khattar, S.K.; Fredericksen, B.; Zhang, Y.J. Hepatitis E Virus Inhibits Type I Interferon Induction by ORF1 Product. J. Virol. 2014, 88, 11924–11932. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Yang, Y.; Yu, Y.; Xiao, Y.; Zhang, Y. Enhancing Solubilization of Hydrophobic ORF3 Product of Hepatitis E Virus in Bacterial Expression System. J. Vaccine Immunotechnol. 2014, 1, 5. [Google Scholar]
- Zhang, Y.; Sharma, R.D.; Paul, P.S. Monoclonal antibodies against conformationally dependent epitopes on porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 1998, 63, 125–136. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, K.Y.; Stein, D.A.; Patel, D.; Watkins, R.; Moulton, H.M.; Iversen, P.L.; Matson, D.O. Inhibition of replication and transcription activator and latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus by morpholino oligomers. Antivir. Res. 2007, 73, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Purcell, R.H.; Emerson, S.U. Identification of the 5′ terminal sequence of the SAR-55 and MEX-14 strains of hepatitis E virus and confirmation that the genome is capped. J. Med. Virol. 2001, 65, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Bhattacharyya, R.P.; Lim, W.A. The structure and function of proline recognition domains. Sci. STKE 2003, 2003, RE8. [Google Scholar] [CrossRef] [PubMed]
- Korkaya, H.; Jameel, S.; Gupta, D.; Tyagi, S.; Kumar, R.; Zafrullah, M.; Mazumdar, M.; Lal, S.K.; Xiaofang, L.; Sehgal, D.; et al. The ORF3 protein of hepatitis E virus binds to Src homology 3 domains and activates MAPK. J. Biol. Chem. 2001, 276, 42389–42400. [Google Scholar] [CrossRef] [PubMed]



| Primer a | Sequences (5′ to 3′) b | Used for |
|---|---|---|
| VP13-F1 | GCTCGAGGTTCGCGACCATGCGCCCTC | Cloning of VP13-D5 |
| VP13-R1 | CGAATTCTTACATCGGGGGCGAAGGGGTTG | Cloning of VP13-D5 |
| VP13-R2 | CGAATTCTTACGGCCGCAGCGGTGACATCG | Cloning of VP13-D6 |
| VP13-R3 | CGAATTCTTACACGAGGTCCAGCCCCGGCC | Cloning of VP13-D7 |
| VP13-R4 | CGAATTCTTAGGGCGGGTTGGCGAACACG | Cloning of VP13-D8 |
| VP13-R5 | CGAATTCTTACGGAGCCGAGTGGTCGGGCG | Cloning of VP13-D9 |
| VP13-F3 | GCTCGAGGTATGTCACCGCTGCGGCCGG | Cloning of VP13-D10 |
| VP13-R6 | CGAATTCTTAGCGGCGCGGCCCCAGC | Cloning of VP13-D10 |
| VP13-F2 | GCTCGAGGTATGCCACCGCTGCGGCCGG | Cloning of VP13-D10 mutant |
| H3F30 | AATTCCCTTCGCCCCCGATGTCACCGCTGCGGCCGC | Cloning aa66–75 |
| H3R27 | TCGAGCGGCCGCAGCGGTGACATCGGGGGCGAAGGG | Cloning aa66–75 |
| H3F31 | AATTCCCTTCGCCCCCGATCTCACCGCTGCGGCCGC | Cloning aa66–75 with mutation M70I |
| H3R28 | TCGAGCGGCCGCAGCGGTGAGATCGGGGGCGAAGGG | Cloning aa66–75 with mutation M70I |
| T2H3F3 | AATTCCCTTTGCCCCAGATGTTGCCGCTGCGTCCGC | Cloning aa66–75 of type 2 VP13 with mutation T70M |
| T2H3R3 | TCGAGCGGACGCAGCGGCAACATCTGGGGCAAAGGG | Cloning aa66–75 of type 2 VP13 with mutation T70M |
| KH3F5 | AATTCCCTTCGCCGCCGATGTCGTTTCACAATCCGC | Cloning aa66–75 of type 3 VP13 with mutation I70M |
| KH3R6 | TCGAGCGGATTGTGAAACGACATCGGCGGCGAAGGG | Cloning aa66–75 of type 3 VP13 with mutation I70M |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Lin, S.; Nan, Y.; Ma, Z.; Yang, L.; Zhang, Y. A Linear Surface Epitope in a Proline-Rich Region of ORF3 Product of Genotype 1 Hepatitis E Virus. Viruses 2016, 8, 227. https://doi.org/10.3390/v8080227
Yang Y, Lin S, Nan Y, Ma Z, Yang L, Zhang Y. A Linear Surface Epitope in a Proline-Rich Region of ORF3 Product of Genotype 1 Hepatitis E Virus. Viruses. 2016; 8(8):227. https://doi.org/10.3390/v8080227
Chicago/Turabian StyleYang, Yonglin, Shaoli Lin, Yuchen Nan, Zexu Ma, Liping Yang, and Yanjin Zhang. 2016. "A Linear Surface Epitope in a Proline-Rich Region of ORF3 Product of Genotype 1 Hepatitis E Virus" Viruses 8, no. 8: 227. https://doi.org/10.3390/v8080227






