Development of Novel Vaccines against Enterovirus-71
Abstract
:1. Introduction
2. Enterovirus 71
| Enterovirus Serotypes | Clinical Manifestations |
|---|---|
| Poliovirus 1 to 3 Echovirus 4, 6, 9, 11, 30; Enterovirus 71 | Paralysis |
| Poliovirus 1 to 3; Coxsackievirus A2, A4, A7, A9, A10, B1 to B6; Echovirus 1 to 11, 13 to 23, 25, 27, 28, 30, 31; Enterovirus 70, 71 | Aseptic meningitis |
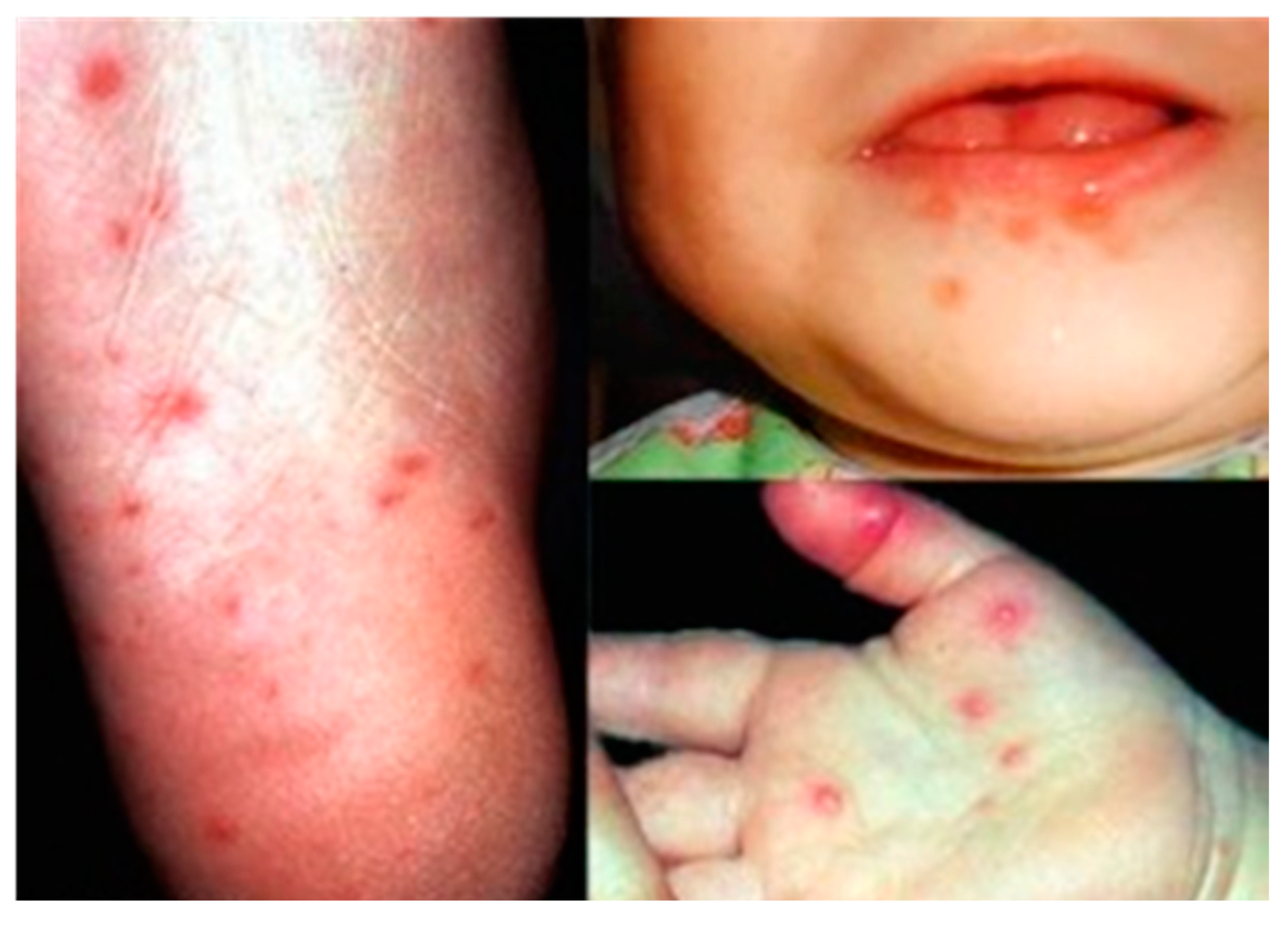
| Coxsackievirus A5, A8, A10, A16, Enterovirus 71 | Hand, foot and mouth disease (HFMD) |
| Coxsackievirus A2 to A6, A8, A10 | Herpangina |
| Coxsackievirus A24, Enterovirus 70 | Acute hemorrhagic conjunctivitis |
| Echovirus 2, 6, 9, 19 | Encephalitis |
| Coxsackievirus B1 to B5, Enterovirus 71 | Meningoencephalitis |
| Coxsackievirus B3 | Pericarditis, myocarditis |
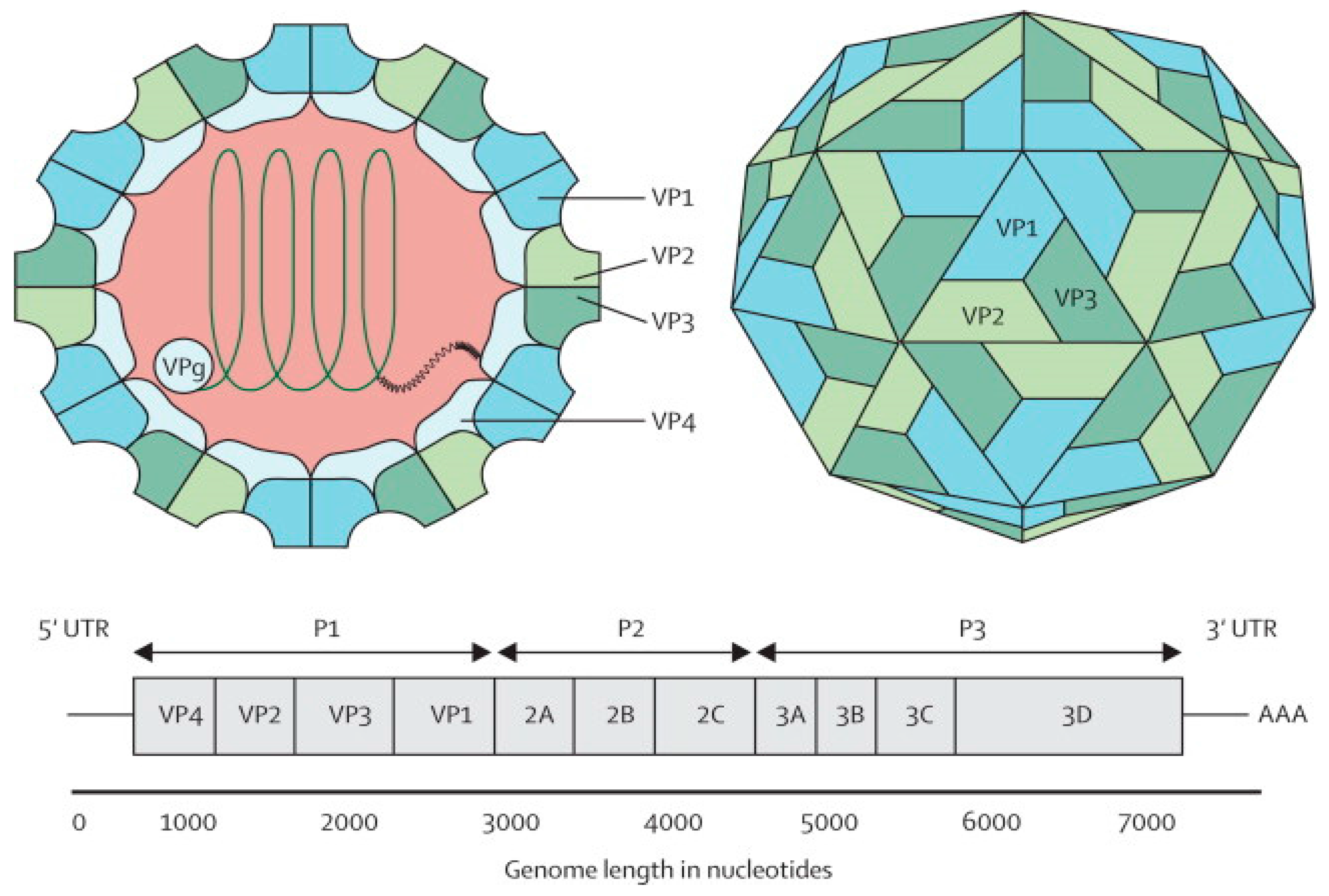
3. Potential Candidates for EV-A71 Vaccine
4. Inactivated EV-A71 Vaccines
| Organizations | Cell Lines and EV-A71 Strain | Clinical Trials Dosage (µg of EV-A71 Antigen) | Population Target | Current Status of Clinical Trial | Adjuvant | Technology for Vaccine Production |
|---|---|---|---|---|---|---|
| NHRI (Taiwan) | Vero cell and EV-A71 B4 (GMP-certified) | 5 and 10 | Young adults | Phase 1 completed | Aluminum phosphate | Roller bottles |
| Sinovac (China) | Vero cell and EV-A71 C4 | 1 | Young adults, young children and infants | Phase 3 completed 1, 2 and 3 completed | Aluminum hydroxide | Cell factory |
| Beijing Vigoo (China) | Vero cell and EV-A71 C4 | 0.8 | Young adults, young children and infants | Phase 3 completed Phase 1, 2 and 3 completed | Aluminum hydroxide | Microcarrier bioreactors, fermentation cylinder |
| CAMS (China) | Human diploid cell KMB-17 and EV-A71 C4 | 0.25 | Young adults, young children and infants | Phase 3 completed 1, 2 and 3 completed | Aluminum hydroxide, glycine | Microcarrier bioreactors |
| Inviragen (Singapore) | Vero cell and EV-A71 B3 | 0.3 and 3 | Young adults | Phase 1 completed Phase 1 Completed | Aluminum hydroxide | Cell factory |
5. Development of Viral-Like Particles as EV-A71 Vacines
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Small, J.C.; Ertl, H.C. Viruses—From pathogens to vaccine carriers. Curr. Opin. Virol. 2011, 1, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Liao, Q.; Viboud, C.; Zhang, J.; Sun, J.; Wu, J.T.; Chang, Z.; Liu, F.; Fang, V.J.; Zheng, Y.; et al. Hand, foot, and mouth disease in China, 2008–12: An epidemiological study. Lancet Infect. Dis. 2014, 14, 308–318. [Google Scholar] [CrossRef]
- Bible, J.M.; Pantelidis, P.; Chan, P.K.S.; Tong, C.Y.W. Genetic evolution of enterovirus 71: Epidemiological and pathological implications. Rev. Med. Virol. 2007, 17, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Pallansch, M.A.; Oberste, M.S.; Whitton, J.L. Enteroviruses: Polioviruses, coxsackieviruses, echoviruses and newer enteroviruses. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Cohen, J.I., Griffin, D.E., Lamb, R.A., Martin, M.A., Racaniello, V.R., Roizman, B., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003; pp. 490–530. [Google Scholar]
- Liu, S.L.; Pan, H.; Liu, P.; Amer, S.; Chan, T.C.; Zhan, J.; Huo, X.; Liu, Y.; Teng, Z.; Wang, L.; Zhuang, H. Comparative epidemiology and virology of fatal and nonfatal cases of hand, foot and mouth disease in mainland China from 2008 to 2014. Rev. Med. Virol. 2015, 25, 115–28. [Google Scholar] [CrossRef] [PubMed]
- Melnick, J.L. Poliovirus and other enteroviruses. In Viral Infections of Humans; Evans, A.S., Kaslow, R.A., Eds.; Plenum: New York, NY, USA, 1997; pp. 583–663. [Google Scholar]
- Ooi, M.H.; Wong, S.C.; Lewthwaite, P.; Cardosa, M.J.; Solomon, T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010, 9, 1097–1105. [Google Scholar] [CrossRef]
- Ho, M.; Chen, E.R.; Hsu, K.H.; Twu, S.J.; Chen, K.T.; Tsai, S.F.; Wang, J.R.; Shih, S.R. An epidemic of enterovirus 71 infection in Taiwan. N. Engl. J. Med. 1999, 341, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Su, L.; Cao, L.; Zhong, H.; Dong, N.; Xu, J. Enterovirus genotypes causing hand foot and mouth disease in Shanghai, China: A molecular epidemiological analysis. BMC Infect. Dis. 2013, 13, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.A.; Pallansch, M.A. Complete nucleotide sequence of Enterovirus 71 is distinct from poliovirus. Virus Res. 1995, 39, 195–205. [Google Scholar] [CrossRef]
- Tee, K.K.; Lam, T.T.; Chan, Y.F.; Bible, J.M.; Kamarulzaman, A.; Tong, C.Y.; Takebe, Y.; Pybus, O.G. Evolutionary genetics of human enterovirus 71: Origin, population dynamics, natural selection, and seasonal periodicity of the VP1 gene. J. Virol. 2010, 84, 3339–3350. [Google Scholar] [CrossRef] [PubMed]
- Picornaviridae. Available online: http://viralzone.expasy.org/all_by_species/33.html (accessed on 12 October 2014).
- Belsham, G.J.; Sonenberg, N. RNA-protein interactions in regulation of picornavirus RNA translation. Microbiol. Rev. 1996, 60, 499–511. [Google Scholar] [PubMed]
- Toyoda, H.; Nicklin, M.J.H.; Murray, M.G.; Anderson, C.W.; Dunn, J.J.; Studier, F.W.; Wimmer, E. A second virus-encoded proteinase involved in proteolytic processing of poliovirus polyprotein. Cell 1986, 45, 761–770. [Google Scholar] [CrossRef]
- Shen, M.; Reitman, Z.J.; Zhao, Y.; Moustafa, I.; Wang, Q.; Arnold, J.J.; Pathak, H.B.; Cameron, C.E. Picornavirus genome replication: Identification of the surface of the poliovirus (PV) 3C dimer that interacts with PV 3Dpol during VPg uridylylation and construction of a structural model for the PV 3C2–3Dpol complex. J. Biol. Chem. 2008, 283, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Kuo, R.L.; Kung, S.H.; Hsu, Y.Y.; Liu, W.T. Infection with enterovirus 71 or expression of its 2A protease induces apoptotic cell death. J. Gen. Virol. 2002, 83, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Li, M.L.; Hsu, T.A.; Chen, T.C.; Chang, S.C.; Lee, J.C.; Chen, C.C.; Stollar, V.; Shih, S.R. The 3C protease activity of Enterovirus 71 induces human neural cell apoptosis. Virology 2002, 293, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.; Lennette, E.; Ho, H. An apparently new enterovirus isolated from patients with disease of the central nervous system. J. Infect. Dis. 1974, 129, 304–309. [Google Scholar] [CrossRef] [PubMed]
- The Dermatologic Image Database, Department of Dermatology, University of Iowa College of Medicine, USA. 1996. Available online: http://tray.dermatology.uiowa.edu/ImageBase (accessed on 1 September 2015).
- Lin, T.Y.; Chang, L.Y.; Hsia, S.H.; Huang, Y.C.; Chiu, C.H.; Hsueh, C.; Shih, S.R.; Liu, C.C.; Wu, M.H. The 1998 Enterovirus 71 outbreak in Taiwan: Pathogenesis and management. Clin. Infect. Dis. 2002, 34, S52–S57. [Google Scholar] [CrossRef] [PubMed]
- Sabanathan, S.; Tan, L.V.; Thwaites, L.; Wills, B.; Qui, P.T.; van Doorn, H.R. Enterovirus 71 related severe hand, foot and mouth disease outbreaks in South-East Asia: Current situation and ongoing challenges. J. Epidemiol. Commun. Health 2014, 0, 1–3. [Google Scholar] [CrossRef] [PubMed]
- WHO Emerging Disease Surveillance and Response: Hand, Foot and Mouth Disease (HFMD). Available online: http://www.wpro.who.int/emerging_diseases/HFMD/en/ (accessed on 22 February 2015).
- Xu, W.; Liu, C.F.; Yan, L.; Li, J. J.; Wang, L.J.; Qi, Y.; Cheng, R.B.; Xiong, X.Y. Distribution of enteroviruses in hospitalized children with hand, foot and mouth disease and relationship between pathogens and nervous system complications. Virol. J. 2012, 9, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.L.; Mao, Q.Y.; Wang, Y.P.; Zhu, F.C.; Li, J.X.; Yao, X.; Gao, F.; Wu, X.; Xu, M.; Wang, J.Z. Progress on the research and development of inactivated EV71 whole-virus vaccines. Hum. Vaccines Immunother. 2013, 9, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.N.; Lin, Y.C.; Fann, C.; Liao, N.S.; Shih, S.R.; Ho, M.S. Protection against lethal Enterovirus 71 infection in newborn mice by passive immunization with subunit VP1 vaccines and inactivated virus. Vaccine 2001, 20, 895–904. [Google Scholar] [CrossRef]
- Arita, M.; Shimizu, H.; Nagata, N.; Ami, Y.; Suzaki, Y.; Sata, T.; Iwasaki, T.; Miyamura, T. Temperature-sensitive mutants of enterovirus 71 show attenuation in cynomolgus monkeys. J. Gen. Virol. 2005, 86, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Nagata, N.; Iwata, N.; Ami, Y.; Suzaki, Y.; Mizuta, K.; Iwasaki, T.; Sata, T.; Wakita, T.; Shimizu, H. An attenuated strain of enterovirus 71 belonging to genotype A showed a broad spectrum of antigenicity with attenuated neurovirulence in cynomolgus monkeys. J. Virol. 2007, 81, 9386–9395. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Han, J.F.; Qin, C.F.; Chen, R. Virus-like particles for enterovirus 71 produced from Saccharomyces cerevisiae potently elicits protective immune responses in mice. Vaccine 2013, 31, 3281–3287. [Google Scholar] [CrossRef] [PubMed]
- Foo, D.G.W.; Alonso, S.; Chow, V.T.K.; Poh, C.L. Passive protection against lethal enterovirus 71 infection in newborn mice by neutralizing antibodies elicited by a synthetic peptide. Microbes Infect. 2007, 9, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K.; Poh, C.L.; Fecondo, J.; Pourianfar, H.; Shaw, J.; Grollo, L. Cross-reactive neutralizing antibody epitopes against enterovirus 71 identified by an in silico approach. Vaccine 2012, 30, 7105–7110. [Google Scholar] [CrossRef] [PubMed]
- Tung, W.; Bakar, S.; Sekawi, Z.; Rosli, R. DNA vaccine constructs against Enterovirus 71 elicit immune response in mice. Genet. Vaccines Ther. 2007, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xu, W.; Xia, J.; Liang, Z.; Liu, Y.; Zhang, X.; Tan, X.; Wang, L.; Mao, Q.; Wu, J.; et al. Efficacy, safety, and immunogenicity of an Enterovirus 71 vaccine in China. N. Engl. J. Med. 2014, 370, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.C.; Meng, F.Y.; Li, J.X.; Li, X.L.; Mao, Q.Y.; Tao, H.; Zhang, Y.T.; Yao, X.; Chu, K.; Chen, Q.H.; et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant Enterovirus 71 vaccine in children in china: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013, 381, 2024–2032. [Google Scholar] [CrossRef]
- Chong, P.; Liu, C.C.; Chow, Y.H.; Chou, A.H.; Klein, M. Review of Enterovirus 71 vaccines. Clin. Infect. Dis. 2015, 60, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.F.; Chang, M.H.; Chiang, B.L.; Jeng, S.T. Oral immunization of mice using transgenic tomato fruit expressing VP1 protein from Enterovirus 71. Vaccine 2006, 24, 2944–2951. [Google Scholar] [CrossRef] [PubMed]
- Han, J.F.; Cao, R.Y.; Deng, Y.Q.; Tian, X.; Jiang, T.; Qin, E.D.; Qin, C.F. Antibody dependent enhancement infection of Enterovirus 71 in vitro and in vivo. Virol. J. 2011, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Palmer, P.; Charley, B.; Rombaut, B.; Daeron, M.; Lebon, P. Antibody-dependent induction of type I interferons by poliovirus in human mononuclear blood cells requires the type II Fcγ receptor (CD32). Virology 2000, 278, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Girn, J.; Kavoosi, M.; Chantler, J. Enhancement of coxsackievirus B3 infection by antibody to a different coxsackievirus strain. J. Gen. Virol. 2002, 83, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wang, J. EV71 vaccine, an invaluable gift for children. Clin. Trans. Immunol. 2014, 3, e28. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Mo, Z.; Liang, Z.; Zhang, Y.; Li, R.; Ong, K.C.; Wong, K.T.; Yang, E.; Che, Y.; Wang, J.; et al. Immunity and clinical efficacy of an inactivated Enterovirus 71 vaccine in healthy Chinese children: A report of further observations. BMC Med. 2015, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Dong, C.; Li, X.; Gao, Q.; Guo, Z.; Yao, X.; Wang, Y.; Gao, F.; Li, F.; Xu, M.; et al. Comparative analysis of the immunogenicity and protective effects of inactivated EV71 vaccines in mice. PLoS ONE 2012, 7, e46043. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.; Hsieh, S.Y.; Liu, C.C.; Chou, A.H.; Chang, J.Y.; Wu, S.C.; Liu, S.J.; Chow, Y.H.; Su, I.J.; Klein, M. Production of EV71 vaccine candidates. Hum. Vaccines Immunother. 2012, 8, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Bek, E.J.; Hussain, K.M.; Phuektes, P.; Kok, C.C.; Gao, Q.; Cai, F.; Gao, Z.; McMinn, P.C. Formalin-inactivated vaccine provokes cross-protective immunity in a mouse model of human enterovirus 71 infection. Vaccine 2011, 29, 4829–4838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; An, D.; Liu, W.; Mao, Q.; Jin, J.; Xu, L.; Sun, S.; Jiang, L.; Li, X.; Shao, J.; et al. Analysis of cross-reactive neutralizing antibodies in human HFMD serum with an EV71 pseudovirus-based assay. PLoS ONE 2014, 9, e100545. [Google Scholar] [CrossRef] [PubMed]
- Lu, S. EV71 vaccines: A milestone in the history of global vaccine development. Emerg. Microbes Infect. 2014, 3, e27. [Google Scholar] [CrossRef] [PubMed]
- Cereghino, J.L.; Cregg, J.M. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol. Rev. 2000, 24, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Roldão, A.; Mellado, M.C.; Castilho, L.R.; Carrondo, M.J.T.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ku, Z.; Liu, Q.; Wang, X.; Chen, T.; Ye, X.; Li, D.; Jin, X.; Huang, Z. High-yield production of recombinant virus-like particles of Enterovirus 71 in Pichia pastoris and their protective efficacy against oral viral challenge in mice. Vaccine 2015, 33, 2335–2341. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.H.; Liu, C.C.; Chang, J.Y.; Lien, S.P.; Guo, M.S.; Tasi, H.P.; Hsiao, K.N.; Liu, S.J.; Sia, C.; Wu, S.C.; et al. Immunological evaluation and comparison of different EV71 vaccine candidates. Clin. Dev. Immunol. 2012, 2012, 831282. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Yeh, C.T.; Li, W.H.; Yu, C.P.; Lin, W.C.; Yang, J.Y.; Wu, H.L.; Hu, Y.C. Enhanced enterovirus 71 virus-like particle yield from a new baculovirus design. Biotechnol. Bioeng. 2015, 112, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.L.; Lin, Y.W.; Shao, H.Y.; Yu, S.L.; Wu, S.R.; Lin, H.Y.; Liu, C.C.; Huang, C.; Chong, P.; Chow, Y.H. Recombinant adeno-vaccine expressing enterovirus 71-like particles against hand, foot, and mouth disease. PLoS Negl. Trop. Dis. 2015, 9, e0003692. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, H.Y.; Han, J.F.; Deng, Y.Q.; Zhu, S.Y.; Li, X.F.; Yang, H.Q.; Li, Y.X.; Zhang, Y.; Qin, E.D. Novel recombinant chimeric virus-like particle is immunogenic and protective against both Enterovirus 71 and Coxsackievirus A16 in mice. Sci. Rep. 2015, 5, 7878–7886. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; He, D.; Yang, L.; Li, Z.; Ye, X.; Yu, H.; Zhao, H.; Li, S.; Yuan, L.; Qian, H.; et al. A broadly cross-protective vaccine presenting the neighboring epitopes within the VP1 GH loop and VP2 EF loop of Enterovirus 71. Sci. Rep. 2015, 5, 12973–12985. [Google Scholar] [CrossRef] [PubMed]
- Lyu, K.; Wang, G.C.; He, Y.L.; Han, J.F.; Ye, Q.; Qin, C.F.; Chen, R. Crystal structures of EV71 recombinant virus particles provide insights into vaccine design. J. Biol. Chem. 2014, 290, 3198–3208. [Google Scholar] [CrossRef] [PubMed]
- Lyu, K.; He, Y.L.; Li, H.Y.; Chen, R. Crystal structures of yeast-produced enterovirus 71 and enterovirus 71/coxsackievirus A16 chimeric virus-like particles provide the structural basis for novel vaccine design against hand-foot-and-mouth disease. J. Virol. 2015, 89, 6196–6208. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cao, R.Y.; Chen, R.; Mo, L.; Han, J.F.; Wang, X.; Xu, X.; Jiang, T.; Deng, Y.Q.; Lyu, K.; et al. Rational design of thermostable vaccines by engineered peptide-induced virus self-biomineralization under physiological conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 7619–7624. [Google Scholar] [CrossRef] [PubMed]
- Plevka, P.; Perera, R.; Cardosa, J.; Kuhn, R.; Rossman, M.G. Crystal structure of human Enterovirus 71. Science 2012, 336, 1274–1276. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, W.; Ren, J.; Hu, Z.; Xu, J.; Lou, Z.; Li, X.; Yin, W.; Shen, X.; Porta, C.; et al. A sensor-adaptor mechanism for enterovirus uncoating from structures of EV71. Nat. Struct. Mol. Biol. 2012, 19, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Zolla-Pazner, S.; Cardozo, T. Structure-function relationships of HIV-1 envelope sequence-variable regions refocus vaccine design. Nat. Rev. Immunol. 2010, 10, 527–535. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yee, P.T.I.; Poh, C.L. Development of Novel Vaccines against Enterovirus-71. Viruses 2016, 8, 1. https://doi.org/10.3390/v8010001
Yee PTI, Poh CL. Development of Novel Vaccines against Enterovirus-71. Viruses. 2016; 8(1):1. https://doi.org/10.3390/v8010001
Chicago/Turabian StyleYee, Pinn Tsin Isabel, and Chit Laa Poh. 2016. "Development of Novel Vaccines against Enterovirus-71" Viruses 8, no. 1: 1. https://doi.org/10.3390/v8010001





