A Polyprotein-Expressing Salmonid Alphavirus Replicon Induces Modest Protection in Atlantic Salmon (Salmo Salar) Against Infectious Pancreatic Necrosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
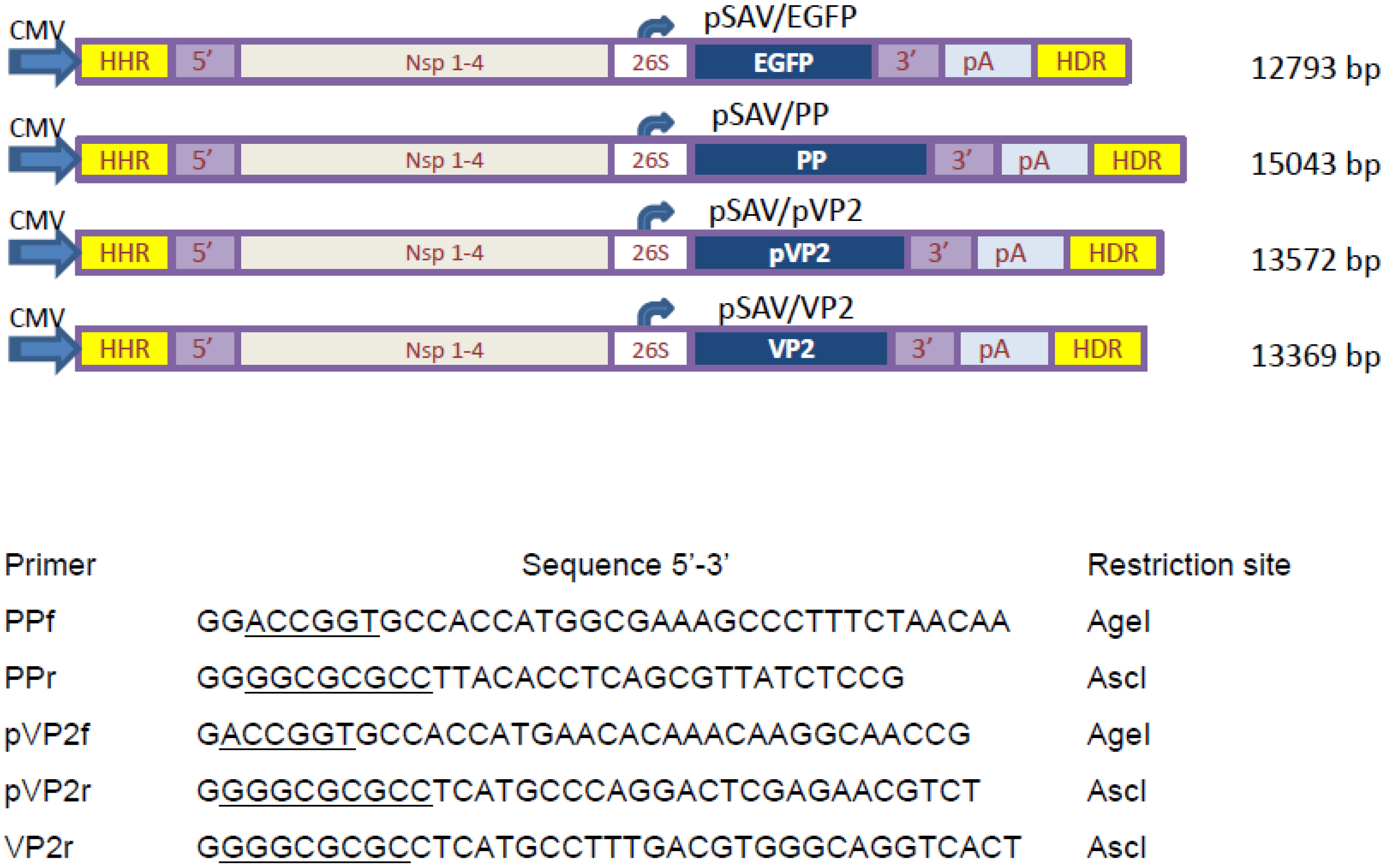
2.2. Construction of DNA-Layered SAV Replicon Vectors
2.3. Expression of Recombinant IPNV Proteins in Cell Culture
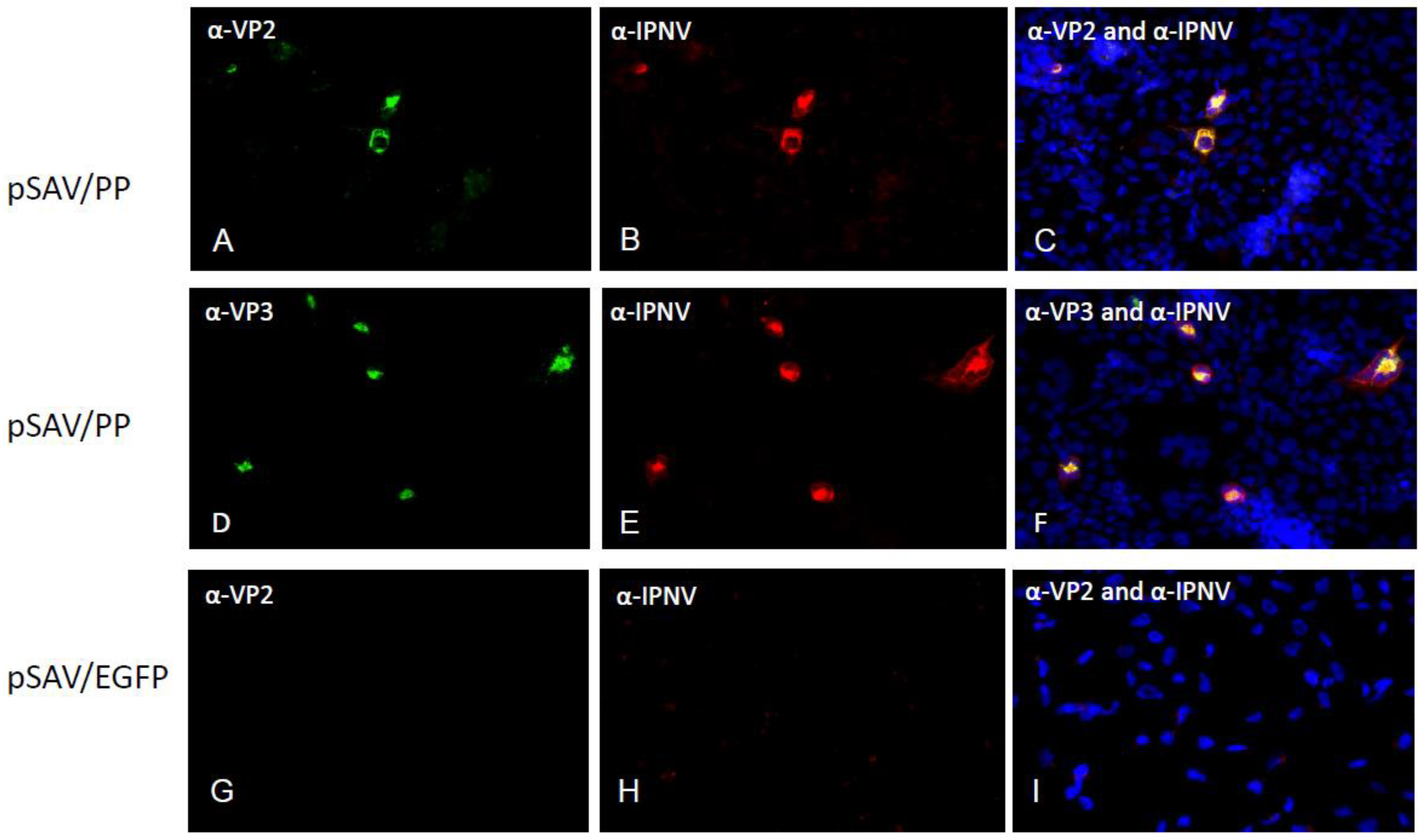
2.4. Immunofluorescence Staining
2.5. Western Blotting
2.6. Vaccination and Experimental Challenge
3. Results
3.1. Construction of SAV Replicon Vectors

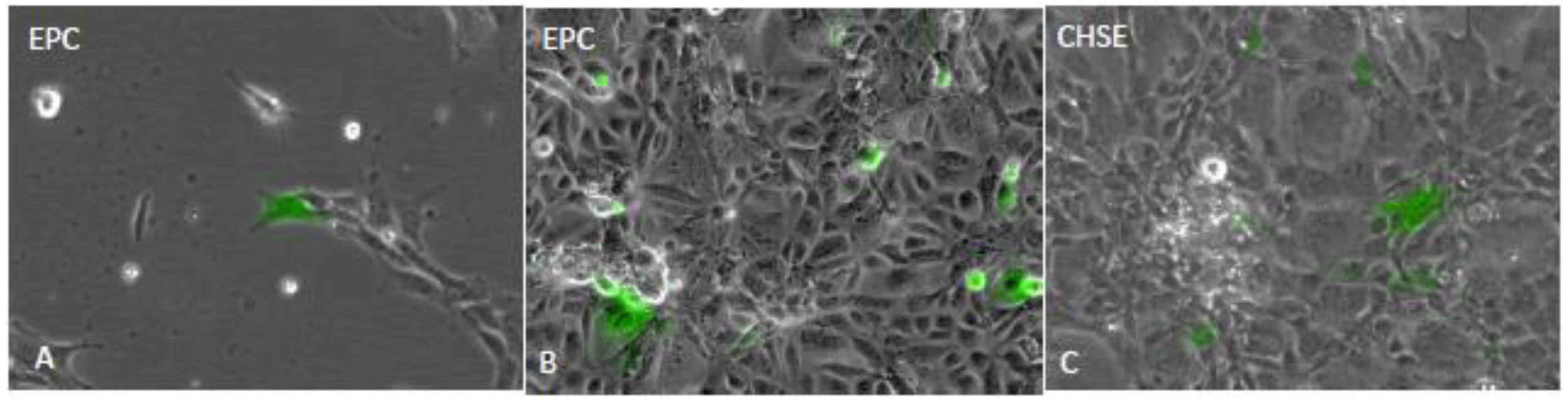
3.2. Expression of Recombinant IPNV Proteins in Cell Culture
3.3. Western Blot
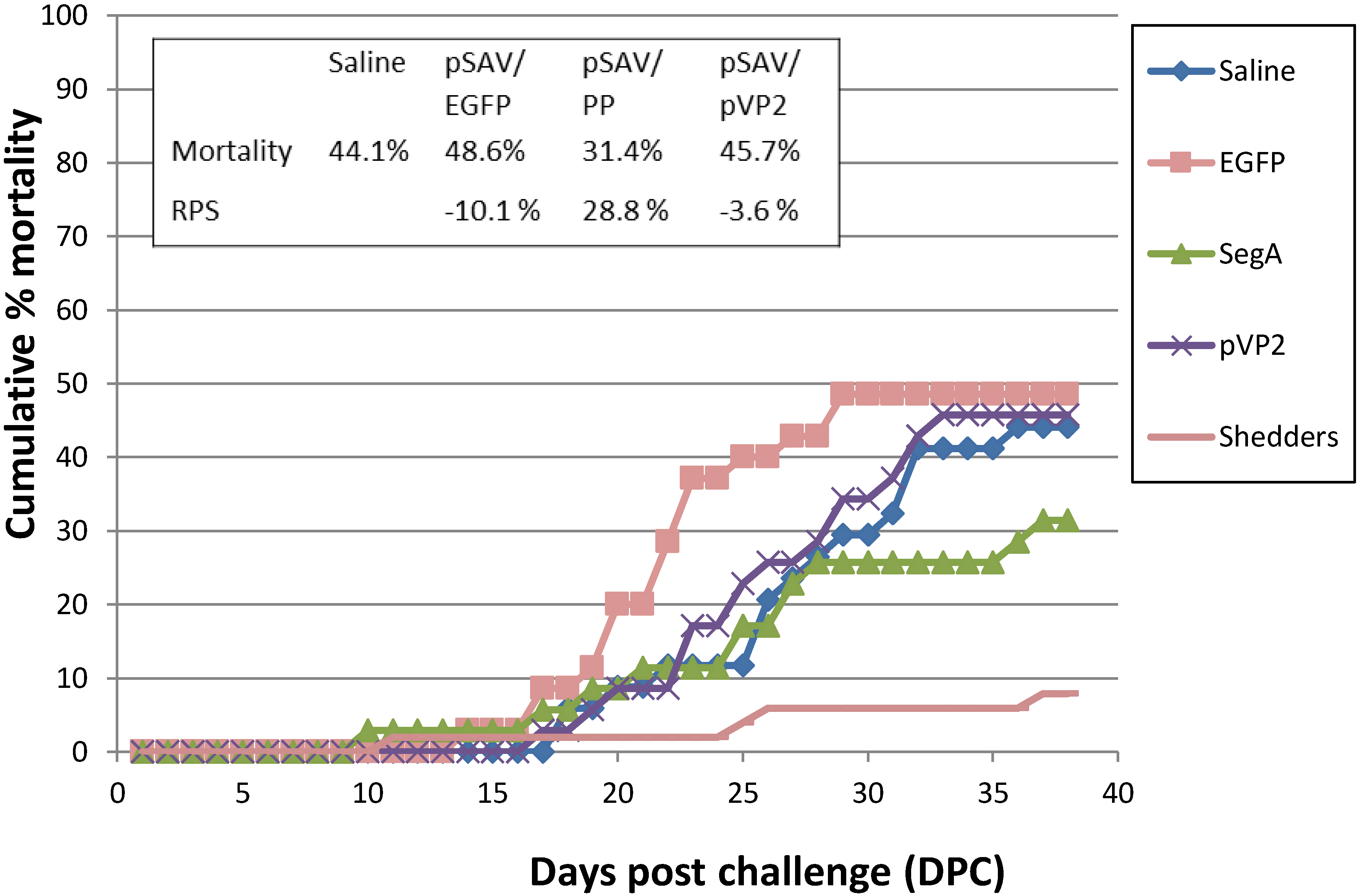
3.4. Vaccination Trial

4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hill, B.J.; Way, K. Serological classification of infectious pancreatic necrosis (IPN) virus and other aquatic birnaviruses. Annu. Rev. Fish Dis. 1995, 5, 55–77. [Google Scholar] [CrossRef]
- John, K.R.; Richards, R.H. Characteristics of a new birnavirus associated with a warmwater fish cell line. J. Gen. Virol. 1999, 80, 2061–2065. [Google Scholar] [PubMed]
- Nishizawa, T.; Kinoshita, S.; Yoshimizu, M. An approach for genogrouping of Japanese isolates of aquabirnaviruses in a new genogroup, VII, based on the VP2/NS junction region. J. Gen. Virol. 2005, 86, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Okamoto, N.; Nishimura, T. A New Viral Epizootic of Anguilla-Japonica Temminck and Schlegel. J. Fish Dis. 1981, 4, 127–139. [Google Scholar] [CrossRef]
- Wolf, K.; Snieszko, S.F.; Dunbar, C.E.; Pyle, E. Virus Nature of Infectious Pancreatic Necrosis in Trout. Pr. Soc. Exp. Biol. Med. 1960, 104, 105–108. [Google Scholar] [CrossRef]
- Hastein, T.; Krogsrud, J. Infectious Pancreatic Necrosis-1St Isolation of Virus from Fish in Norway. Acta Vet. Scand. 1976, 17, 109–111. [Google Scholar] [PubMed]
- Evensen, O.; Rimstad, E. Immunohistochemical identification of infectious pancreatic necrosis virus in paraffin-embedded tissues of Atlantic salmon (Salmo salar). J. Vet. Diagn. Invest. 1990, 2, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Petit, S.; Lejal, N.; Huet, J.C.; Delmas, B. Active residues and viral substrate cleavage sites of the protease of the birnavirus infectious pancreatic necrosis virus. J. Virol. 2000, 74, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Dobos, P. The molecular biology of infectious pancreatic necrosis virus. Annu. Rev. Fish Dis. 1995, 5, 25–54. [Google Scholar] [CrossRef]
- Villanueva, R.A.; Galaz, J.L.; Valdes, J.A.; Jashes, M.M.; Sandino, A.M. Genome assembly and particle maturation of the birnavirus infectious pancreatic necrosis virus. J. Virol. 2004, 78, 13829–13838. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Aravena, A.; Cortez-San Martin, M.; Galaz, J.; Imarai, M.; Miranda, D.; Spencer, E.; Sandino, A. Evaluation of the immune response against immature viral particles of infectious pancreatic necrosis virus (IPNV): A new model to develop an attenuated vaccine. Vaccine 2012, 30, 5110–5117. [Google Scholar] [CrossRef] [PubMed]
- Santi, N.; Vakharia, V.N.; Evensen, O. Identification of putative motifs involved in the virulence of infectious pancreatic necrosis virus. Virology 2004, 322, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.W.; Sarin, L.; Graham, S.C.; Pang, J.; Bamford, D.H.; Stuart, D.I.; Grimes, J.M. Structure of a VP1-VP3 Complex Suggests How Birnaviruses Package the VP1 Polymerase. J. Virol. 2013, 87, 3229–3236. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.; Skjesol, A.; Jorgensen, J.B. VP3, a structural protein of infectious pancreatic necrosis virus, interacts with RNA-dependent RNA polymerase VP1 and with double-stranded RNA. J. Virol. 2007, 81, 6652–6663. [Google Scholar] [CrossRef] [PubMed]
- Imajoh, M.; Goto, T.; Oshima, S. Characterization of cleavage sites and protease activity in the polyprotein precursor of Japanese marine aquabirnavirus and expression analysis of generated proteins by a VP4 protease activity in four distinct cell lines. Arch. Virol. 2007, 152, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.H.; Do, J.W.; Cha, S.J.; Bang, J.D.; Park, M.A.; Yoo, D.J.; Lee, J.M.; Kim, H.G.; Chung, D.K.; Park, J.W. Comparison of the immunogenicity of recombinant VP2 and VP3 of infectious pancreatic necrosis virus and marine birnavirus. Arch. Virol. 2004, 149, 2059–2068. [Google Scholar] [PubMed]
- Gadan, K.; Marjara, I.S.; Sundh, H.; Sundell, K.; Evensen, O. Slow release cortisol implants result in impaired innate immune responses and higher infection prevalence following experimental challenge with infectious pancreatic necrosis virus in Atlantic salmon (Salmo salar) parr. Fish Shellfish Immun. 2012, 32, 637–644. [Google Scholar] [CrossRef]
- Guy, D.; Bishop, S.; Brotherstone, S.; Hamilton, A.; Roberts, R.; McAndrew, B.; Woolliams, J. Analysis of the incidence of infectious pancreatic necrosis mortality in pedigreed Atlantic salmon, Salmo salar L., populations. J. Fish Dis. 2006, 29, 637–647. [Google Scholar] [CrossRef]
- Ronneseth, A.; Wergeland, H.I.; Devik, M.; Evensen, O.; Pettersen, E.F. Mortality after IPNV challenge of Atlantic salmon (Salmo salar L.) differs based on developmental stage of fish or challenge route. Aquaculture 2007, 271, 100–111. [Google Scholar] [CrossRef]
- Frost, P.; Ness, A. Vaccination of Atlantic salmon with recombinant VP2 of infectious pancreatic necrosis virus (IPNV), added to a multivalent vaccine, suppresses viral replication following IPNV challenge. Fish Shellfish Immun. 1997, 7, 609–619. [Google Scholar] [CrossRef]
- Gomez-Casado, E.; Estepa, A.; Coll, J.M. A comparative review on European-farmed finfish RNA viruses and their vaccines. Vaccine 2011, 29, 2657–2671. [Google Scholar] [CrossRef] [PubMed]
- Bootland, L.M.; Dobos, P.; Stevenson, R.M.W. Experimental Induction of the Carrier State in Yearling Brook Trout-A Model Challenge Protocol for Ipnv Immunization. Vet. Immun. Immunopathol. 1986, 12, 365–372. [Google Scholar] [CrossRef]
- Mikalsen, A.B.; Torgersen, J.; Alestrom, P.; Hellemann, A.L.; Koppang, E.O.; Rimstad, E. Protection of Atlantic salmon Salmo salar against infectious pancreatic necrosis after DNA vaccination. Dis. Aquatic. Org. 2004, 60, 11–20. [Google Scholar] [CrossRef]
- Frost, P.; Ness, A.; Maaseide, N.P.; Knappskog, D.H.; Rodseth, O.M. Efficacy of a recombinant vaccine against infectious pancreatic necrosis in Atlantic salmon post-smolt. Fish Vaccinol. 1997, 90, 460. [Google Scholar] [CrossRef]
- Ramstad, A.; Romstad, A.B.; Knappskog, D.H.; Midtlyng, P.J. Field validation of experimental challenge models for IPN vaccines. J. Fish Dis. 2007, 30, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Munang’andu, H.M.; Fredriksen, B.N.; Mutoloki, S.; Brudeseth, B.; Kuo, T.Y.; Marjara, I.S.; Dalmo, R.A.; Evensen, O. Comparison of vaccine efficacy for different antigen delivery systems for infectious pancreatic necrosis virus vaccines in Atlantic salmon (Salmo salar L.) in a cohabitation challenge model. Vaccine 2012, 30, 4007–4016. [Google Scholar] [CrossRef]
- Rayner, J.O.; Dryga, S.A.; Kamrud, K.I. Alphavirus vectors and vaccination. Rev. Med. Virol. 2002, 12, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Frolov, I.; Hoffman, T.A.; Pragai, B.M.; Dryga, S.A.; Huang, H.V.; Schlesinger, S.; Rice, C.M. Alphavirus-based expression vectors: Strategies and applications. Proc. Natl. Acad. Sci. USA 1996, 93, 11371–11377. [Google Scholar] [CrossRef] [PubMed]
- Perri, S.; Greer, C.E.; Thudium, K.; Doe, B.; Legg, H.; Liu, H.; Romero, R.E.; Tang, Z.Q.; Bin, Q.; Dubensky, T.W.; et al. An alphavirus replicon particle chimera derived from Venezuelan equine encephalitis and Sindbis viruses is a potent gene-based vaccine delivery vector. J. Virol. 2003, 77, 10394–10403. [Google Scholar] [CrossRef] [PubMed]
- Erdman, M.; Kamrud, K.I.; Harris, D.; Smith, J. Alphavirus replicon particle vaccines developed for use in humans induce high levels of antibodies to influenza virus hemagglutinin in swine: Proof of concept. Vaccine 2010, 28, 594–596. [Google Scholar] [CrossRef]
- Olsen, C.M.; Pemula, A.K.; Braaen, S.; Sankaran, K.; Rimstad, E. Salmonid alphavirus replicon is functional in fish, mammalian and insect cells and in vivo in shrimps (Litopenaeus vannamei). Vaccine 2013, 31, 5672–5679. [Google Scholar] [CrossRef]
- Karlsen, M.; Villoing, S.; Rimstad, E.; Nylund, A. Characterization of untranslated regions of the salmonid alphavirus 3 (SAV3) genome and construction of a SAV3 based replicon. Virol. J. 2009, 6, 173. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, M.; Villoing, S.; Ottem, K.F.; Rimstad, E.; Nylund, A. Development of infectious cDNA clones of Salmonid alphavirus subtype 3. BMC Res. Notes 2010, 3, 241. [Google Scholar] [CrossRef] [PubMed]
- Hikke, M.C.; Braaen, S.; Villoing, S.; Hodneland, K.; Geertsema, C.; Verhagen, L.; Frost, P.; Vlak, J.M.; Rimstad, E.; Pijlman, G.P. Salmonid alphavirus glycoprotein E2 requires low temperature and E1 for virion formation and induction of protective immunity. Vaccine 2014, 32, 6206–6212. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Hodneland, K.; Frost, P.; Braaen, S.; Rimstad, E. A hemagglutinin-esterase-expressing salmonid alphavirus replicon protects Atlantic salmon (Salmo salar) against infectious salmon anemia (ISA). Vaccine 2013, 31, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Rimstad, E.; Krona, R.F.; Hornes, E.; FAU-Olsvik, O.F.; Hyllseth, B. Detection of infectious pancreatic necrosis virus (IPNV) RNA by hybridization with an oligonucleotide DNA probe. Vet. Microbiol. 1990, 23, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Ramly, R.B.; Olsen, C.M.; Braaen, S.; Rimstad, E. Infectious salmon anaemia virus nuclear export protein is encoded by a spliced gene product of genomic segment 7. Virus Res. 2013, 177, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Winton, J.; Batts, W.F.; de Kinkelin, P.F.; LeBerre, M.F.; Bremont, M.F.; Fijan, N. Current lineages of the epithelioma papulosum cyprini (EPC) cell line are contaminated with fathead minnow, Pimephales promelas, cells. J. Fish Dis. 2010, 33, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.T.; Mcloughlin, M.F.; Rowley, H.M.; Platten, M.A.; Mccormick, J.I. Isolation of A Toga-Like Virus from Farmed Atlantic Salmon Salmo-Salar with Pancreas Disease. Dis. Aquatic Org. 1995, 22, 25–32. [Google Scholar] [CrossRef]
- Chiu, C.L.; Wu, J.L.; Her, G.M.; Chou, Y.L.; Hong, J.R. Aquatic birnavirus capsid protein, VP3, induces apoptosis via the Bad-mediated mitochondria pathway in fish and mouse cells. Apoptosis 2010, 15, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Guo, T.C.; Mutoloki, S.; Haugland, O.; Marjara, I.S.; Evensen, O. Alpha Interferon and Not Gamma Interferon Inhibits Salmonid Alphavirus Subtype 3 Replication In Vitro. J. Virol. 2010, 84, 8903–8912. [Google Scholar] [CrossRef] [PubMed]
- De Las Heras, A.I.; Prieto, S.I.P.; Saint-Jean, S.R. In vitro and in vivo immune responses induced by a DNA vaccine encoding the VP2 gene of the infectious pancreatic necrosis virus. Fish Shellfish Immun. 2009, 27, 120–129. [Google Scholar] [CrossRef]
- Shivappa, R.B.; McAllister, P.E.; Edwards, G.H.; Santi, N.; Evensen, O.; Vakharia, V.N. Development of a subunit vaccine for infectious pancreatic necrosis virus using a baculovirus insect/larvae system. Dev. Biol. 2005, 121, 165–174. [Google Scholar]
- Bowden, T.J.; Smail, D.A.; Ellis, A.E. Development of a reproducible infectious pancreatic necrosis virus challenge model for Atlantic salmon, Salmo salar L. J. Fish Dis. 2002, 25, 555–563. [Google Scholar] [CrossRef]
- Cuesta, A.; Chaves-Pozo, E.; de las Heras, A.I.; Rodriguez Saint-Jean, S.; Perez-Prieto, S.; Tafalla, C. An active DNA vaccine against infectious pancreatic necrosis virus (IPNV) with a different mode of action than fish rhabdovirus DNA vaccines. Vaccine 2010, 28, 3291–3300. [Google Scholar] [CrossRef] [PubMed]
- Caswellreno, P.; Reno, P.W.; Nicholson, B.L. Monoclonal-Antibodies to Infectious Pancreatic Necrosis Virus-Analysis of Viral Epitopes and Comparison of Different Isolates. J. Gen. Virol. 1986, 67, 2193–2205. [Google Scholar] [CrossRef] [PubMed]
- Christie, K.E.; Ness, S.; Djupvik, H.O. Infectious Pancreatic Necrosis Virus in Norway-Partial Serotyping by Monoclonal-Antibodies. J. Fish Dis. 1990, 13, 323–327. [Google Scholar] [CrossRef]
- Frost, P.; Havarstein, L.S.; Lygren, B.; Stahl, S.; Endresen, C.; Christie, K.E. Mapping of Neutralization Epitopes on Infectious Pancreatic Necrosis Viruses. J. Gen. Virol. 1995, 76, 1165–1172. [Google Scholar] [CrossRef]
- Tarrab, E.; Berthiaume, L.; Grothe, S.; Oconnormccourt, M.; HeppelL, J.; Lecomte, J. Evidence of A Major Neutralizable Conformational Epitope Region on Vp2 of Infectious Pancreatic Necrosis Virus. J. Gen. Virol. 1995, 76, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, F.; Chevalier, C.; Delmas, B.; Rey, F.A. Crystal Structure of an Aquabirnavirus Particle: Insights into Antigenic Diversity and Virulence Determinism. J. Virol. 2010, 84, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Song, H.C.; Santi, N.; Evensen, O.; Vakharia, V.N. Molecular determinants of infectious pancreatic necrosis virus virulence and cell culture adaptation. J. Virol. 2005, 79, 10289–10299. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, N.A.; Rodriguez St-Jean, S.; Perez-Prieto, S.I. Food pellets as an effective delivery method for a DNA vaccine against infectious pancreatic necrosis virus in rainbow trout (Oncorhynchus mykiss, Walbaum). Fish Shellfish Immun. 2014, 37, 220–228. [Google Scholar] [CrossRef]
- Ona, A.; Luque, D.; Abaitua, F.; Maraver, A.; Caston, J.R.; Rodriguez, J.F. The C-terminal domain of the pVP2 precursor is essential for the interaction between VP2 and VP3, the capsid polypeptides of infectious bursal disease virus. Virology 2004, 322, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, M.L.; Mbewe-Mvula, A.; Rosario, M.; Johansson, D.X.; Kakoulidou, M.; Bridgeman, A.; Reyes-Sandoval, A.; Nicosia, A.; Ljungberg, K.; Hanke, T.; et al. Superior Induction of T Cell Responses to Conserved HIV-1 Regions by Electroporated Alphavirus Replicon DNA Compared to That with Conventional Plasmid DNA Vaccine. J. Virol. 2012, 86, 4082–4090. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, M.; Yousaf, M.N.; Villoing, S.; Nylund, A.; Rimstad, E. The amino terminus of the salmonid alphavirus capsid protein determines subcellular localization and inhibits cellular proliferation. Arch. Virol. 2010, 155, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Moen, T.; Baranski, M.; Sonesson, A.K.; Kjoglum, S. Confirmation and fine-mapping of a major QTL for resistance to infectious pancreatic necrosis in Atlantic salmon (Salmo salar): Population-level associations between markers and trait. BMC Genomics 2009, 10. [Google Scholar] [CrossRef] [PubMed]
- Gheyas, A.; Houston, R.; Mota-Velasco, J.; Guy, D.; Tinch, A.; Haley, C.; Woolliams, J. Segregation of infectious pancreatic necrosis resistance QTL in the early life cycle of Atlantic Salmon (Salmo salar). Anim. Genet. 2010, 41, 531–536. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, A.; Olsen, C.M.; Hodneland, K.; Rimstad, E. A Polyprotein-Expressing Salmonid Alphavirus Replicon Induces Modest Protection in Atlantic Salmon (Salmo Salar) Against Infectious Pancreatic Necrosis. Viruses 2015, 7, 252-267. https://doi.org/10.3390/v7010252
Abdullah A, Olsen CM, Hodneland K, Rimstad E. A Polyprotein-Expressing Salmonid Alphavirus Replicon Induces Modest Protection in Atlantic Salmon (Salmo Salar) Against Infectious Pancreatic Necrosis. Viruses. 2015; 7(1):252-267. https://doi.org/10.3390/v7010252
Chicago/Turabian StyleAbdullah, Azila, Christel M. Olsen, Kjartan Hodneland, and Espen Rimstad. 2015. "A Polyprotein-Expressing Salmonid Alphavirus Replicon Induces Modest Protection in Atlantic Salmon (Salmo Salar) Against Infectious Pancreatic Necrosis" Viruses 7, no. 1: 252-267. https://doi.org/10.3390/v7010252





