Large Human Outbreak of West Nile Virus Infection in North-Eastern Italy in 2012
Abstract
:1. Introduction
2. Results and Discussion
2.1. Human Outbreak of WNV Infection in North-Eastern Italy, 2012
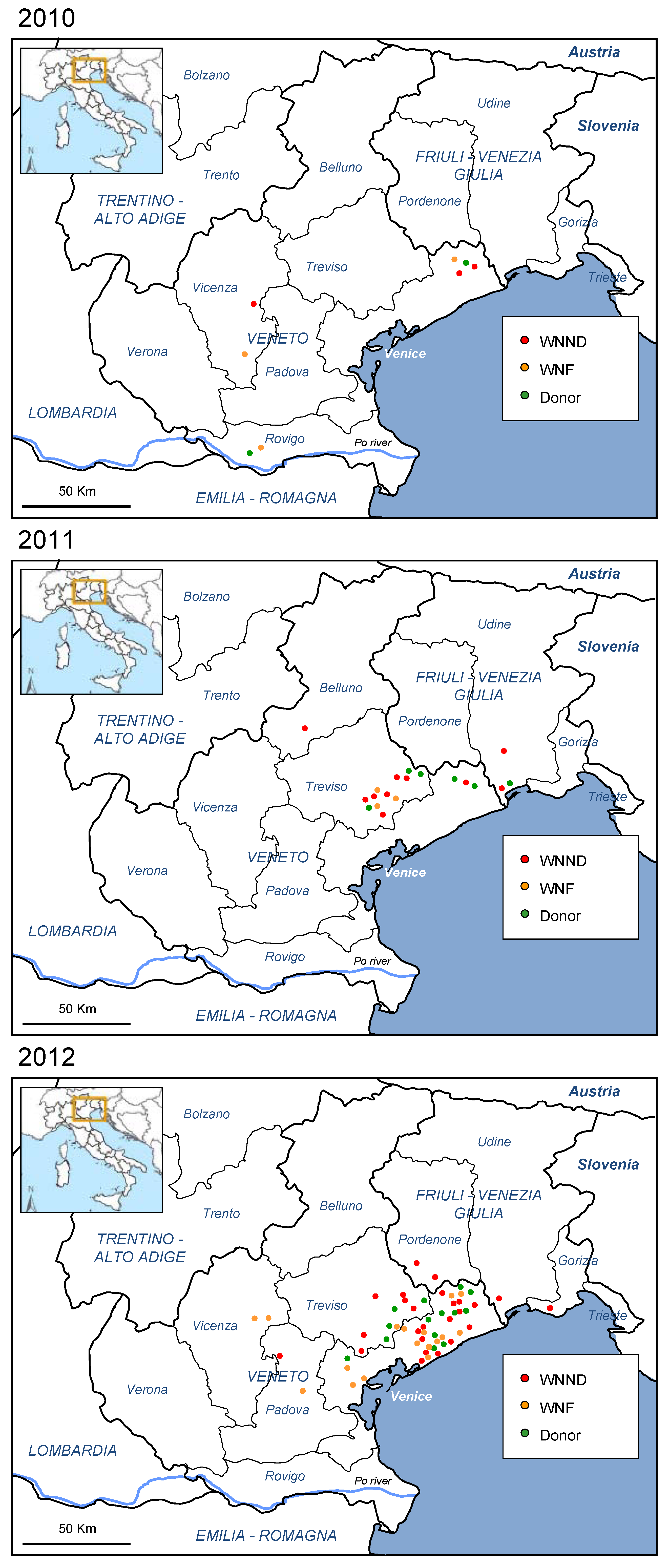

| Symptoms and signs | WNND | WNF | Blood donor |
|---|---|---|---|
| (n = 25) | (n = 17) | (n = 14) | |
| Fever | 80% | 100% | 14% |
| Headache | 68% | 71% | 21% |
| Fatigue | 60% | 59% | 64% |
| Rash | 0% | 18% | 14% |
| Artralgia | 8% | 47% | 21% |
| Myalgia | 20% | 4% | 29% |
| Lymphoadenopathy | 0% | 6% | 0% |
| Vomiting/diarrhea | 0% | 12% | 21% |
| Neurological manifestations | 68% | 0% | 7% |
| Respiratory failure | 24% | 0% | 0% |
| Encephalitis | 48% | 0% | 0% |
| Meningitis | 32% | 0% | 0% |
| Acute flaccid paresis/paralysis | 20% | 0% | 0% |
| Asymptomatic | 0% | 0% | 29% |
| WNND (n = 25) | WNF (n = 17) | Blood donors (n = 14) | |
|---|---|---|---|
| Parameter | No. Positive/No. Tested (%) | No. Positive/No. Tested (%) | No. Positive/No. Tested (%) |
| WNV RNA in plasma | 7/22 (31.8) | 3/17 (17.6) | 8/14 (57.1) |
| WNV RNA in urine | 7/16 (43.8) | 7/17 (41.1) | 2/14 (14.3) |
| WNV RNA in CSF | 1/19 (5.3) | 0/2 (0) | 0/0 |
| Serum IgM-/IgG- | 0/25 (0) | 0/17 (0) | 2/14 (14.3) |
| Serum IgM+/IgG- | 6/25 (24.0) | 3/17 (17.6) | 6/14 (42.9) |
| Serum IgM+/IgG+ | 19/25 (76.0) | 14/17 (82.3) | 6/14 (42.9) |
| CSF IgM+/IgG- | 6/19 (31.6) | 0/0 | 0/0 |
| CSF IgM+/IgG+ | 13/19 (68.4) | 0/0 | 0/0 |
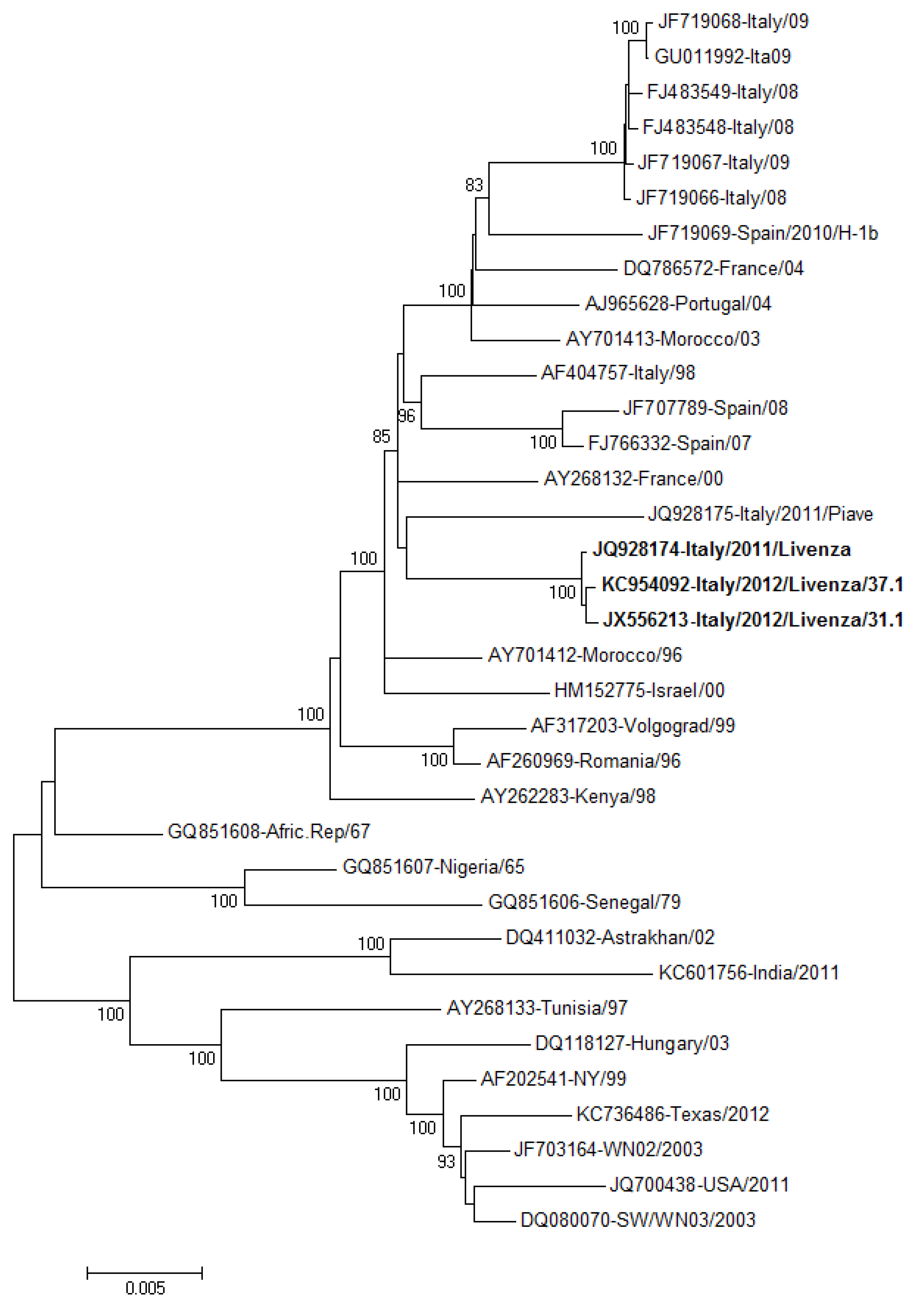
2.2. Human Cases of WNV Infection in Other Italian Regions, 2012
3. Experimental Section
3.1. National Plan for WNND Surveillance in Humans, Italy, 2012
3.2. Special Surveillance Plan for West Nile Fever in Humans, Veneto Region, Italy, 2012
3.3. Case Definition of WNND and WNF
3.4. Case Laboratory Investigations
3.5. Active Surveillance of Stable Workers and Household Contacts
3.6. Screening of Blood and Organ Donations
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ulbert, S. West Nile virus: The complex biology of an emerging pathogen. Intervirology 2011, 54, 171–184. [Google Scholar] [CrossRef]
- Kramer, L.D.; Styer, L.M.; Ebel, G.D. A global perspective on the epidemiology of West Nile virus. Annu. Rev. Entomol. 2008, 53, 61–81. [Google Scholar] [CrossRef]
- Hayes, E.B.; Sejvar, J.J.; Zaki, S.R.; Lanciotti, R.S.; Bode, A.V.; Campbell, G.L. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg. Infect. Dis. 2005, 11, 1174–1179. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Haddad, M.B.; Tierney, B.C.; Campbell, G.L.; Marfin, A.A.; van Gerpen, J.A.; Fleischauer, A.; Leis, A.A.; Stokic, D.S.; Petersen, L.R. Neurologic manifestation and outcome of West Nile virus infection. JAMA 2003, 290, 511–515. [Google Scholar] [CrossRef]
- Lindsey, N.P.; Staples, J.E.; Lehman, J.A.; Fischer, M. Medical risk factors for severe West Nile Virus disease, United States, 2008–2010. Am. J. Trop. Med. Hyg. 2012, 87, 179–184. [Google Scholar] [CrossRef]
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Hyg. 1940, 20, 471–492. [Google Scholar]
- Murgue, B.; Murri, S.; Triki, H.; Deubel, V.; Zeller, H.G. West Nile in the Mediterranean basin: 1950–2000. Ann. N. Y. Acad. Sci. 2001, 951, 117–126. [Google Scholar]
- Tsai, T.F.; Popovici, F.; Cernescu, C.; Campbell, G.L.; Nedelcu, N.I. West Nile encephalitis epidemic in southeastern Romania. Lancet 1998, 352, 767–771. [Google Scholar] [CrossRef]
- Zeller, H.G.; Schuffenecker, I. West Nile Virus: An overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 147–156. [Google Scholar] [CrossRef]
- Platonov, A.E.; Shipulin, G.A.; Shipulina, O.Y.; Tyutyunnik, E.N.; Frolochkina, T.I.; Lanciotti, R.S.; Yazyshina, S.; Platonova, O.V.; Obukhov, I.L.; Zhukov, A.N.; et al. Outbreak of West Nile virus infection, Volgograd Region, Russia, 1999. Emerg. Infect. Dis. 2001, 7, 128–132. [Google Scholar] [CrossRef]
- Calistri, P.; Giovannini, A.; Hubalek, Z.; Ionescu, A.; Monaco, F.; Savini, G.; Lelli, R. Epidemiology of West Nile in Europe and in the Mediterranean basin. Open Virol. J. 2010, 4, 29–37. [Google Scholar]
- Sambri, V.; Capobianchi, M.; Charrel, R.; Fyodorova, M.; Gaibani, P.; Gould, E.; Niedrig, M.; Papa, A.; Pierro, A.; Rossini, G.; et al. West Nile virus in Europe: Emergence, epidemiology, diagnosis, treatment, and prevention. Clin. Microbiol. Infect. 2013, 19, 699–704. [Google Scholar] [CrossRef]
- Rizzo, C.; Salcuni, P.; Nicoletti, L.; Ciufolini, M.G.; Russo, F.; Masala, R.; Frongia, O.; Finarelli, A.C.; Gramegna, M.; Gallo, L.; et al. Epidemiological surveillance of West Nile neuroinvasive diseases in Italy, 2008 to 2011. Euro Surveill. 2012, 17. [Google Scholar]
- Barzon, L.; Pacenti, M.; Franchin, E.; Martello, T.; Lavezzo, E.; Squarzon, L.; Toppo, S.; Fiorin, F.; Marchiori, G.; Scotton, G.P.; et al. Clinical and virological findings in the ongoing outbreak of West Nile virus Livenza strain in northern Italy, July to September 2012. Euro Surveill. 2012, 17. [Google Scholar]
- Danis, K.; Papa, A.; Theocharopoulos, G.; Dougas, G.; Athanasiou, M.; Detsis, M.; Baka, A.; Lytras, T.; Mellou, K.; Bonovas, S.; et al. Outbreak of West Nile virus infection in Greece, 2010. Emerg. Infect. Dis. 2011, 17, 1868–1872. [Google Scholar] [CrossRef]
- Papa, A. West Nile virus infections in humans - Focus on Greece. J. Clin. Virol. 2013, 58, 351–353. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Roehrig, J.T.; Deubel, V.; Smith, J.; Parker, M.; Steele, K.; Crise, B.; Volpe, K.E.; Crabtree, M.B.; Scherret, J.H.; et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 1999, 286, 2333–2337. [Google Scholar] [CrossRef]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile virus: Review of the literature. JAMA 2013, 310, 308–315. [Google Scholar] [CrossRef]
- Sotelo, E.; Fernandez-Pinero, J.; Llorente, F.; Vázquez, A.; Moreno, A.; Agüero, M.; Cordioli, P.; Tenorio, A.; Jiménez-Clavero, M.Á. Phylogenetic relationships of Western Mediterranean West Nile virus strains (1996–2010) using full-length genome sequences: Single or multiple introductions? J. Gen. Virol. 2011, 92, 2512–2522. [Google Scholar] [CrossRef]
- May, F.J.; Davis, C.T.; Tesh, R.B.; Barrett, A.D. Phylogeography of West Nile virus: From the cradle of evolution in Africa to Eurasia, Australia, and the Americas. J. Virol. 2011, 85, 2964–2974. [Google Scholar] [CrossRef]
- Pybus, O.G.; Suchard, M.A.; Lemey, P.; Bernardin, F.J.; Rambaut, A.; Crawford, F.W.; Gray, R.R.; Arinaminpathy, N.; Stramer, S.L.; Busch, M.P.; et al. Unifying the spatial epidemiology and molecular evolution of emerging epidemics. Proc. Natl. Acad. Sci. USA 2012, 109, 15066–15071. [Google Scholar] [CrossRef]
- Zehender, G.; Ebranati, E.; Bernini, F.; Lo Presti, A.; Rezza, G.; Delogu, M.; Galli, M.; Ciccozzi, M. Phylogeography and epidemiological history of West Nile virus genotype 1a in Europe and the Mediterranean basin. Infect. Genet. Evol. 2011, 11, 646–653. [Google Scholar] [CrossRef]
- Barzon, L.; Pacenti, M.; Franchin, E.; Squarzon, L.; Lavezzo, E.; Toppo, S.; Martello, T.; Cattai, M.; Cusinato, R.; Palù, G. Novel West Nile virus lineage 1a full genome sequences from human cases of infection in north-eastern Italy, 2011. Clin. Microbiol. Infect. 2012, 18, E541–E544. [Google Scholar]
- Savini, G.; Capelli, G.; Monaco, F.; Polci, A.; Russo, F.; di Gennaro, A.; Marini, V.; Teodori, L.; Montarsi, F.; Pinoni, C.; et al. Evidence of West Nile virus lineage 2 circulation in Northern Italy. Vet. Microbiol. 2012, 158, 267–273. [Google Scholar] [CrossRef]
- Magurano, F.; Remoli, M.E.; Baggieri, M.; Fortuna, C.; Marchi, A.; Fiorentini, C.; Bucci, P.; Benedetti, E.; Ciufolini, M.G.; Rizzo, C.; et al. Circulation of West Nile virus lineage 1 and 2 during an outbreak in Italy. Clin. Microbiol. Infect. 2012, 18, E545–E547. [Google Scholar]
- Rossini, G.; Cavrini, F.; Pierro, A.; Macini, P.; Finarelli, A.C.; Po, C.; Peroni, G.; di Caro, A.; Capobianchi, M.; Nicoletti, L.; et al. First human case of West Nile virus neuroinvasive infection in Italy, September 2008 – case report. Euro Surveill. 2008, 13. [Google Scholar]
- Barzon, L.; Squarzon, L.; Cattai, M.; Franchin, E.; Pagni, S.; Cusinato, R.; Palù, G. West Nile virus infection in Veneto region, Italy, 2008–2009. Euro Surveill. 2009, 14. [Google Scholar]
- Rizzo, C.; Vescio, F.; Declich, S.; Finarelli, A.C.; Macini, P.; Mattivi, A.; Rossini, G.; Piovesan, C.; Barzon, L.; Palù, G.; et al. West Nile virus transmission with human cases in Italy, August–September 2009. Euro Surveill. 2009, 14. [Google Scholar]
- Barzon, L.; Franchin, E.; Squarzon, L.; Lavezzo, E.; Toppo, S.; Martello, T.; Bressan, S.; Pagni, S.; Cattai, M.; Piazza, A.; et al. Genome sequence analysis of the first human West Nile virus isolated in Italy in 2009. Euro Surveill. 2009, 14. [Google Scholar]
- Angelini, P.; Tamba, M.; Finarelli, A.C.; Bellini, R.; Albieri, A.; Bonilauri, P.; Cavrini, F.; Dottori, M.; Gaibani, P.; Martini, E.; et al. West Nile virus circulation in Emilia-Romagna, Italy: The integrated surveillance system 2009. Euro Surveill. 2010, 15. [Google Scholar]
- Capobianchi, M.R.; Sambri, V.; Castilletti, C.; Pierro, A.M.; Rossini, G.; Gaibani, P.; Cavrini, F.; Selleri, M.; Meschi, S.; Lapa, D.; et al. Retrospective screening of solid organ donors in Italy, 2009, reveals unpredicted circulation of West Nile virus. Euro Surveill. 2010, 15. [Google Scholar]
- Pezzotti, P.; Piovesan, C.; Barzon, L.; Cusinato, R.; Cattai, M.; Pacenti, M.; Piazza, A.; Franchin, E.; Pagni, S.; Bressan, S.; et al. Prevalence of IgM and IgG antibodies to West Nile virus among blood donors in an affected area of north-eastern Italy, summer 2009. Euro Surveill. 2011, 16. [Google Scholar]
- Busani, L.; Capelli, G.; Cecchinato, M.; Lorenzetto, M.; Savini, G.; Terregino, C.; Vio, P.; Bonfanti, L.; Pozza, M.D.; Marangon, S. West Nile virus circulation in Veneto region in 2008–2009. Epidemiol. Infect. 2011, 139, 818–825. [Google Scholar] [CrossRef]
- Gobbi, F.; Barzon, L.; Capelli, G.; Angheben, A.; Pacenti, M.; Napoletano, G.; Piovesan, C.; Montarsi, F.; Martini, S.; Rigoli, R.; et al. Surveillance for West Nile, dengue, and chikungunya virus infections, Veneto Region, Italy, 2010. Emerg. Infect. Dis. 2012, 18, 671–673. [Google Scholar]
- Barzon, L.; Pacenti, M.; Cusinato, R.; Cattai, M.; Franchin, E.; Pagni, S.; Martello, T.; Bressan, S.; Squarzon, L.; Cattelan, A.; et al. Human cases of West Nile Virus infection in north-eastern Italy, 15 June to 15 November 2010. Euro Surveill. 2011, 16. [Google Scholar]
- Bagnarelli, P.; Marinelli, K.; Trotta, D.; Monachetti, A.; Tavio, M.; del Gobbo, R.; Capobianchi, M.; Menzo, S.; Nicoletti, L.; Magurano, F.; et al. Human case of autochthonous West Nile virus lineage 2 infection in Italy, September 2011. Euro Surveill. 2011, 16. [Google Scholar]
- Barzon, L.; Pacenti, M.; Franchin, E.; Lavezzo, E.; Martello, T.; Squarzon, L.; Toppo, S.; Fiorin, F.; Marchiori, G.; Russo, F.; et al. New endemic West Nile virus lineage 1a in northern Italy, July 2012. Euro Surveill. 2012, 17. [Google Scholar]
- Istituto Nazionale di Statistica. Available online: http://www.istat.it.
- ECDC. West Nile Fever Maps. Situation Update. Transmission Season 2012; Latest Update 30/11/2012. Available online: http://www.ecdc.europa.eu/en/healthtopics/west_nile_fever/West-Nile-fever-maps/Pages/index.aspx (accessed on 20 November 2013).
- CDC. Nile Virus (WNV) Human Infections Reported to ArboNET, by State, United States, 2012. Available online: http://www.cdc.gov/westnilestatsMaps/finalMapsData/index.html (accessed on 20 November 2013).
- Barzon, L.; Pacenti, M.; Palù, G. West Nile virus and kidney disease. Expert Rev. Anti Infect. Ther. 2013, 11, 479–487. [Google Scholar] [CrossRef]
- Barzon, L.; Pacenti, M.; Franchin, E.; Pagni, S.; Martello, T.; Cattai, M.; Cusinato, R.; Palù, G. Excretion of West Nile virus in urine during acute infection. J. Infect. Dis. 2013, 208, 1086–1092. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar]
- Capelli, G.; Ravagnan, S.; Montarsi, F.; Ciocchetta, S.; Cazzin, S.; Bonfanti, L.; di Gennaro, A.; Portanti, O.; Mulatti, P.; Monne, I.; et al. Further evidence of lineage 2 West Nile Virus in Culex pipiens of North-Eastern Italy. Vet. Ital. 2013. [Google Scholar] [CrossRef]
- Rossini, G.; Carletti, F.; Bordi, L.; Cavrini, F.; Gaibani, P.; Landini, M.P.; Pierro, A.; Capobianchi, M.R.; di Caro, A.; Sambri, V. Phylogenetic analysis of West Nile virus isolates, Italy, 2008–2009. Emerg. Infect. Dis. 2011, 17, 903–906. [Google Scholar] [CrossRef]
- Savini, G.; Puggioni, G.; di Gennaro, A.; di Francesco, G.; Rocchigiani, A.M.; Polci, A.; Marini, V.; Pinoni, C.; Rolesu, S.; Marruchella, G.; et al. West Nile virus lineage 2 in Sardinian wild birds in 2012: A further threat to public health. Epidemiol. Infect. 2013, 141, 2313–2316. [Google Scholar] [CrossRef]
- Ministero della Salute. Sorveglianza dei casi umani delle malattie trasmesse da vettori con particolare riferimento alla chikungunya, dengue e West Nile disease - Aggiornamento 2012. [Ministry of Health. Surveillance of human cases of vector-borne diseases with a particular focus on chikungunya, dengue and West Nile disease – 2012 Update]. Rome: Ministry of Health; DGPRE 0012922-P-12/06/2012 I.4.c.a.9/2011/24. Document in Italian. Available online: http://www.trovanorme.salute.gov.it/renderNormsanPdf?anno=0&codLeg=42895&parte=1%20&serie (accessed on 20 November 2013).
- ECDC. West Nile Fever. EU Case Definition. Available online: http://www.ecdc.europa.eu/en/healthtopics/west_nile_fever/EU-case-definition/Pages/EU-case-definition.aspx (accessed on 20 November 2013).
- Lanciotti, R.S.; Kerst, A.J.; Nasci, R.S.; Godsey, M.S.; Mitchell, C.J.; Savage, H.M.; Komar, N.; Panella, N.A.; Allen, B.C.; Volpe, K.E.; et al. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 2000, 38, 4066–4071. [Google Scholar]
- Linke, S.; Ellerbrok, H.; Niedrig, M.; Nitsche, A.; Pauli, G. Detection of West Nile virus lineages 1 and 2 by real-time PCR. J. Virol. Methods 2007, 146, 355–358. [Google Scholar] [CrossRef]
- Scaramozzino, N.; Crance, J.M.; Jouan, A.; DeBriel, D.A.; Stoll, F.; Garin, D. Comparison of flavivirus universal primer pairs and development of a rapid, highly sensitive heminested reverse transcription-PCR assay for detection of flaviviruses targeted to a conserved region of the NS5 gene sequences. J. Clin. Microbiol. 2001, 39, 1922–1927. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barzon, L.; Pacenti, M.; Franchin, E.; Pagni, S.; Lavezzo, E.; Squarzon, L.; Martello, T.; Russo, F.; Nicoletti, L.; Rezza, G.; et al. Large Human Outbreak of West Nile Virus Infection in North-Eastern Italy in 2012. Viruses 2013, 5, 2825-2839. https://doi.org/10.3390/v5112825
Barzon L, Pacenti M, Franchin E, Pagni S, Lavezzo E, Squarzon L, Martello T, Russo F, Nicoletti L, Rezza G, et al. Large Human Outbreak of West Nile Virus Infection in North-Eastern Italy in 2012. Viruses. 2013; 5(11):2825-2839. https://doi.org/10.3390/v5112825
Chicago/Turabian StyleBarzon, Luisa, Monia Pacenti, Elisa Franchin, Silvana Pagni, Enrico Lavezzo, Laura Squarzon, Thomas Martello, Francesca Russo, Loredana Nicoletti, Giovanni Rezza, and et al. 2013. "Large Human Outbreak of West Nile Virus Infection in North-Eastern Italy in 2012" Viruses 5, no. 11: 2825-2839. https://doi.org/10.3390/v5112825
APA StyleBarzon, L., Pacenti, M., Franchin, E., Pagni, S., Lavezzo, E., Squarzon, L., Martello, T., Russo, F., Nicoletti, L., Rezza, G., Castilletti, C., Capobianchi, M. R., Salcuni, P., Cattai, M., Cusinato, R., & Palù, G. (2013). Large Human Outbreak of West Nile Virus Infection in North-Eastern Italy in 2012. Viruses, 5(11), 2825-2839. https://doi.org/10.3390/v5112825









