Transcriptional Regulation of Latent Feline Immunodeficiency Virus in Peripheral CD4+ T-lymphocytes
Abstract
:1. Introduction
2. Viral Load
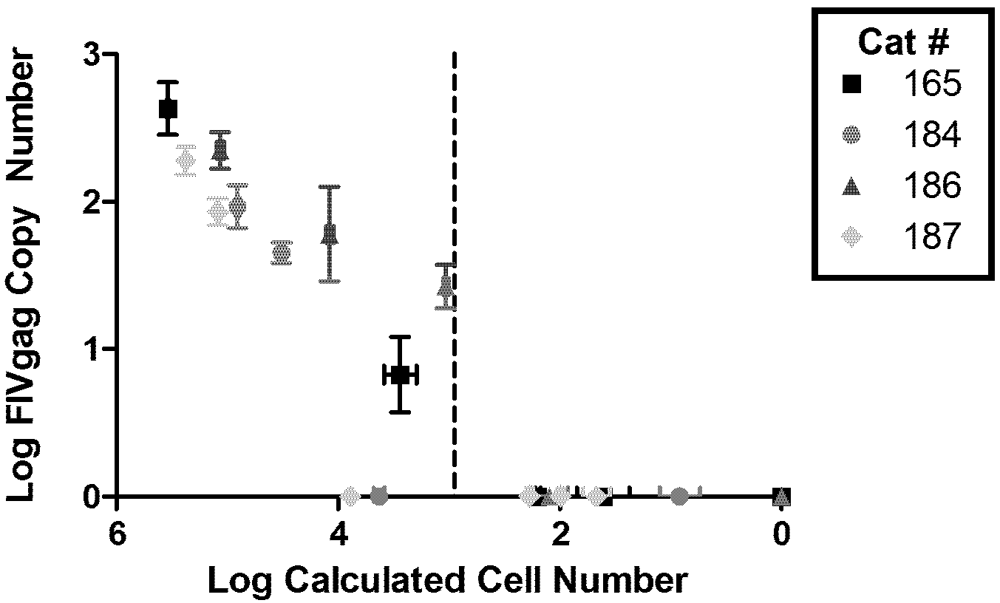
| Cat Number | Starting CD4+ T-cells | FIV gag RNA | 2-LTR Circle Junction | Infectious Supernatant | |||||
|---|---|---|---|---|---|---|---|---|---|
| d7 | d14 | d21 | d7 | d14 | d21 | d7 | d21 | ||
| 165 | 106 | + | + | + | + | + | + | + | + |
| 105 | + | + | + | + | + | + | - | + | |
| 104 | + | + | + | + | - | + | - | - | |
| 103 | - | - | - | - | - | - | - | - | |
| 102 | - | - | - | - | - | - | - | - | |
| 186 | 106 | + | + | + | + | + | + | + | + |
| 105 | + | + | + | + | + | + | - | + | |
| 104 | - | - | + | - | - | - | - | - | |
| 103 | - | - | - | - | - | - | - | - | |
| 102 | - | - | - | - | - | - | - | - | |
3. Chromatin Status

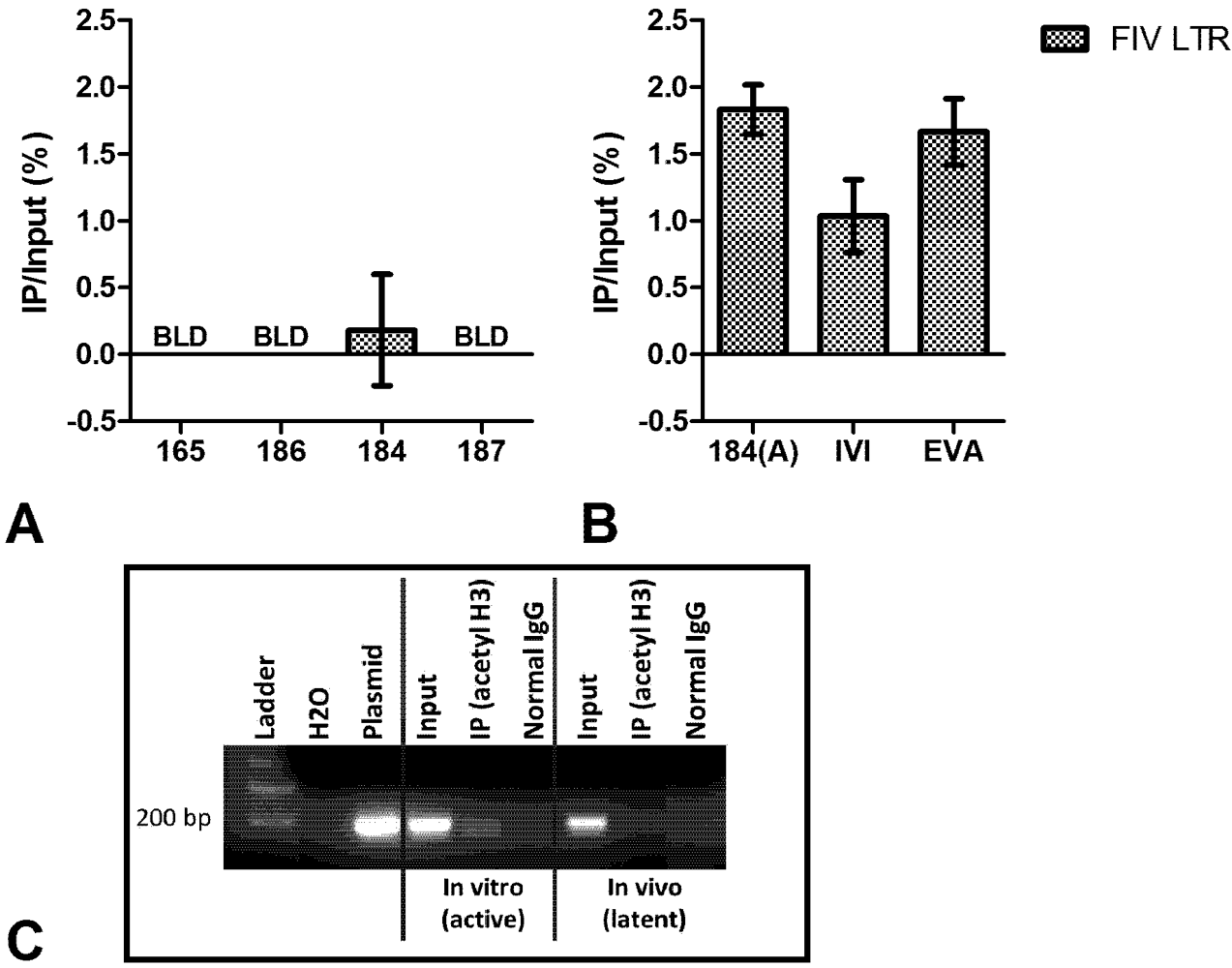
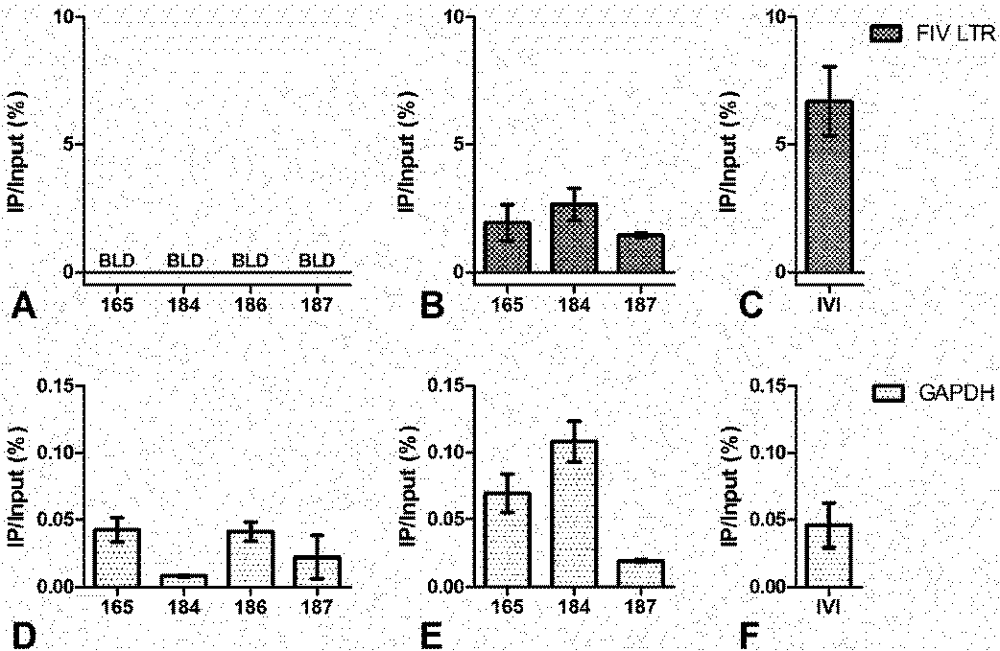
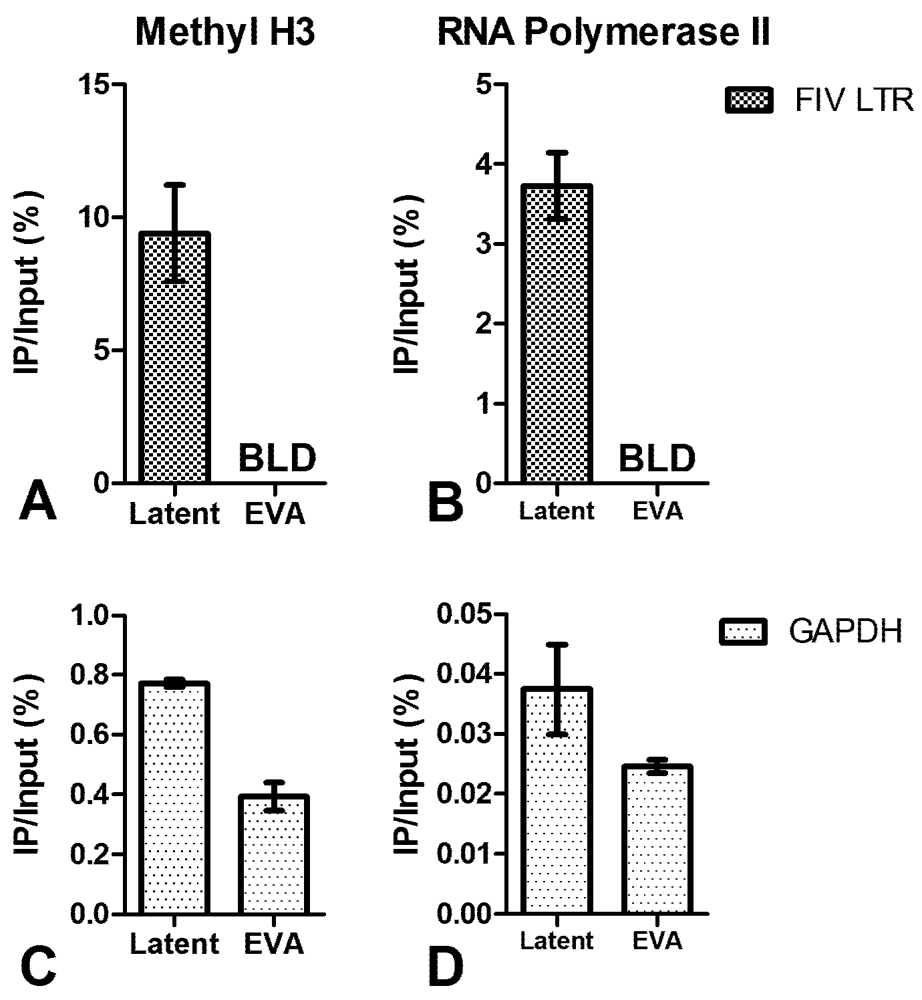
4. Short Transcripts
| Cat Number | Gag Copies | R Copies | R-U5 Copies | OrfA Copies |
|---|---|---|---|---|
| 165 | BLD | 16,000 (±2,000) | BLD | BLD |
| 184 | BLD | 30,000 (±20,000) | BLD | BLD |
| 186 | BLD | 3,900 (±900) | BLD | BLD |
| 187 | BLD | 20,000 (±10,000) | BLD | BLD |
| 184(A) | 1,600 (±100) | 690,000 (±60,000) | 1,200 (±100) | 3,100 (±300) |
5. Conclusions
Acknowledgements
Competing Interests
References
- Kanzaki, L.I.; Looney, D.J. Feline immunodeficiency virus: A concise review. Front. Biosci. 2004, 9, 370–377. [Google Scholar]
- Burkhard, M.J.; Dean, G.A. Transmission and immunopathogenesis of fiv in cats as a model for hiv. Curr. HIV Res. 2003, 1, 15–29. [Google Scholar]
- Elder, J.H.; Lin, Y.C.; Fink, E.; Grant, C.K. Feline immunodeficiency virus (fiv) as a model for study of lentivirus infections: Parallels with hiv. Curr. HIV Res. 2010, 8, 73–80. [Google Scholar]
- Diehl, L.J.; Mathiason-Dubard, C.K.; O’Neil, L.L.; Obert, L.A.; Hoover, E.A. Induction of accelerated feline immunodeficiency virus disease by acute-phase virus passage. J. Virol. 1995, 69, 6149–6157. [Google Scholar]
- Murphy, B.; Vapniarsky, N.; Hillman, C.; Castillo, D.; McDonnel, S.; Moore, P.; Luciw, P.A.; Sparger, E.E. Fiv establishes a latent infection in feline peripheral blood cd4+ t lymphocytes in vivo during the asymptomatic phase of infection. Retrovirology 2012, 9. [Google Scholar] [CrossRef]
- Chun, T.W.; Carruth, L.; Finzi, D.; Shen, X.; DiGiuseppe, J.A.; Taylor, H.; Hermankova, M.; Chadwick, K.; Margolick, J.; Quinn, T.C.; et al. Quantification of latent tissue reservoirs and total body viral load in hiv-1 infection. Nature 1997, 387, 183–188. [Google Scholar]
- Josefsson, L.; King, M.S.; Makitalo, B.; Brannstrom, J.; Shao, W.; Maldarelli, F.; Kearney, M.F.; Hu, W.S.; Chen, J.; Gaines, H.; et al. Majority of cd4+ t cells from peripheral blood of hiv-1-infected individuals contain only one hiv DNA molecule. Proc. Natl. Acad. Sci. USA 2011, 108, 11199–11204. [Google Scholar]
- Pedersen, N.C.; Leutenegger, C.M.; Woo, J.; Higgins, J. Virulence differences between two field isolates of feline immunodeficiency virus (fiv-apetaluma and fiv-cpgammar) in young adult specific pathogen free cats. Vet. Immunol. Immunopathol. 2001, 79, 53–67. [Google Scholar] [CrossRef]
- Pace, M.J.; Agosto, L.; Graf, E.H.; O’Doherty, U. Hiv reservoirs and latency models. Virology 2011, 411, 344–354. [Google Scholar]
- Colin, L.; van Lint, C. Molecular control of hiv-1 postintegration latency: Implications for the development of new therapeutic strategies. Retrovirology 2009, 6. [Google Scholar] [CrossRef]
- Liu, G.; Xia, T.; Chen, X. The activation domains, the proline-rich domain, and the c-terminal basic domain in p53 are necessary for acetylation of histones on the proximal p21 promoter and interaction with p300/creb-binding protei. J. Biol. Chem. 2003, 278, 17557–17565. [Google Scholar]
- Zhang, Z.; Klatt, A.; Gilmour, D.S.; Henderson, A.J. Negative elongation factor nelf represses human immunodeficiency virus transcription by pausing the rna polymerase II complex. J. Biol. Chem. 2007, 282, 16981–16988. [Google Scholar]
- Lenasi, T.; Contreras, X.; Peterlin, B.M. Transcriptional interference antagonizes proviral gene expression to promote hiv latency. Cell Host Microbe 2008, 4, 123–133. [Google Scholar]
- Peterlin, B.M.; Price, D.H. Controlling the elongation phase of transcription with p-tefb. Mol. Cell 2006, 23, 297–305. [Google Scholar]
- Adams, M.; Sharmeen, L.; Kimpton, J.; Romeo, J.M.; Garcia, J.V.; Peterlin, B.M.; Groudine, M.; Emerman, M. Cellular latency in human immunodeficiency virus-infected individuals with high cd4 levels can be detected by the presence of promoter-proximal transcripts. Proc. Natl. Acad. Sci. USA 1994, 91, 3862–3866. [Google Scholar]
- Chatterji, U.; de Parseval, A.; Elder, J.H. Feline immunodeficiency virus orfa is distinct from other lentivirus transactivators. J. Virol. 2002, 76, 9624–9634. [Google Scholar]
- Gemeniano, M.C.; Sawai, E.T.; Leutenegger, C.M.; Sparger, E.E. Feline immunodeficiency virus orf-ais required for virus particle formation and virus infectivity. J. Virol. 2003, 77, 8819–8830. [Google Scholar]
- Trono, D.; Van Lint, C.; Rouzioux, C.; Verdin, E.; Barre-Sinoussi, F.; Chun, T.W.; Chomont, N. Hiv persistence and the prospect of long-term drug-free remissions for hiv-infected individuals. Science 2010, 329, 174–180. [Google Scholar]
- Chomont, N.; El-Far, M.; Ancuta, P.; Trautmann, L.; Procopio, F.A.; Yassine-Diab, B.; Boucher, G.; Boulassel, M.R.; Ghattas, G.; Brenchley, J.M.; et al. Hiv reservoir size and persistence are driven by t cell survival and homeostatic proliferation. Nat. Med. 2009, 15, 893–900. [Google Scholar]
- Blankson, J.N.; Persaud, D.; Siliciano, R.F. The challenge of viral reservoirs in hiv-1 infection. Annu. Rev. Med. 2002, 53, 557–593. [Google Scholar]
- Chun, T.W.; Stuyver, L.; Mizell, S.B.; Ehler, L.A.; Mican, J.A.; Baseler, M.; Lloyd, A.L.; Nowak, M.A.; Fauci, A.S. Presence of an inducible hiv-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 1997, 94, 13193–13197. [Google Scholar]
- Margolis, D.M. Mechanisms of hiv latency: An emerging picture of complexity. Curr. HIV/AIDS Rep. 2010, 7, 37–43. [Google Scholar] [CrossRef]
- Imai, K.; Togami, H.; Okamoto, T. Involvement of histone h3 lysine 9 (h3k9) methyltransferase g9a in the maintenance of hiv-1 latency and its reactivation by bix01294. J. Biol. Chem. 2010, 285, 16538–16545. [Google Scholar]
- Margolis, D.M. Histone deacetylase inhibitors and hiv latency. Curr. Opin. HIV AIDS 2011, 6, 25–29. [Google Scholar]
- Friedman, J.; Cho, W.K.; Chu, C.K.; Keedy, K.S.; Archin, N.M.; Margolis, D.M.; Karn, J. Epigenetic silencing of hiv-1 by the histone h3 lysine 27 methyltransferase enhancer of zeste 2. J. Virol. 2011, 85, 9078–9089. [Google Scholar]
- Geeraert, L.; Kraus, G.; Pomerantz, R.J. Hide-and-seek: The challenge of viral persistence in hiv-1 infection. Annu. Rev. Med. 2008, 59, 487–501. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
McDonnel, S.J.; Sparger, E.E.; Luciw, P.A.; Murphy, B.G. Transcriptional Regulation of Latent Feline Immunodeficiency Virus in Peripheral CD4+ T-lymphocytes. Viruses 2012, 4, 878-888. https://doi.org/10.3390/v4050878
McDonnel SJ, Sparger EE, Luciw PA, Murphy BG. Transcriptional Regulation of Latent Feline Immunodeficiency Virus in Peripheral CD4+ T-lymphocytes. Viruses. 2012; 4(5):878-888. https://doi.org/10.3390/v4050878
Chicago/Turabian StyleMcDonnel, Samantha J., Ellen E. Sparger, Paul A. Luciw, and Brian G. Murphy. 2012. "Transcriptional Regulation of Latent Feline Immunodeficiency Virus in Peripheral CD4+ T-lymphocytes" Viruses 4, no. 5: 878-888. https://doi.org/10.3390/v4050878





