Dendritic Cell Apoptosis and the Pathogenesis of Dengue
Abstract
:1. Dengue Virus

2. Dendritic Cells
3. Dendritic Cells and Dengue Virus
4. Apoptosis
5. Dengue Virus-Induced Apoptosis
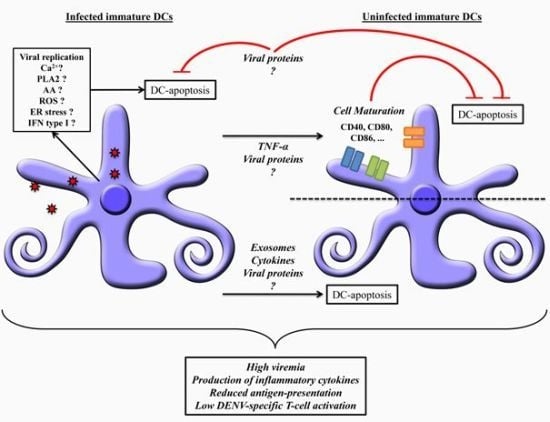
6. Dendritic Cells Apoptosis: Mechanisms and Dengue Pathogenesis

7. Concluding Remarks
Acknowledgments
Conflict of Interest
References and Notes
- OMS. Dengue and Dengue Haemorragic Fever. 2009. Available online: http://www.who.int/mediacentre/factsheets/fs117/en/ (assessed on: 10 June 2012).
- Gubler, D.J.; Meltzer, M. Impact of dengue/dengue hemorrhagic fever on the developing world. Adv. Virus Res. 1999, 53, 35–70. [Google Scholar] [CrossRef]
- Nogueria, R.M.; de Araújo, J.M.; Schatzmayr, H.G. Dengue viruses in Brazil, 1986–2006. Rev. Panam. Salud Publica. 2007, 22, 358–363. [Google Scholar] [CrossRef]
- Nogueira, R.M.R.; Schatzmayr, H.G.; Fillipis, A.M.B. Dengue virus type 3, Brazil, 2002. Emerging Infect. Dis. 2005, 11, 1376–1381. [Google Scholar] [CrossRef]
- Ministério da Saúde, MS. Balanço Dengue: Semana Epidemiológica 1 a 26 de 2011. Available online: http://portal.saude.gov.br/portal/arquivos/pdf/informe_dengue_072011.pdf (assessed on: 10 June 2012).
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef]
- Lindenbach, D.B.; Rice, C.M. Flaviviridae: The Viruses and their Replication. In Fields Virology, 4th; Knipe, D.M., Howley, P.M., Fields, B.N., Eds.; Lippincott Williams and Wilkins: New York, NY, USA, 2001; pp. 991–1042. [Google Scholar]
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. World Health Organization, 2009. Available online: http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf (assessed on: 9 October 2012).
- WHO. 2012. Available online: http://www.who.int/mediacentre/factsheets/fs117/en/ (assessed on:10 June 2012).
- Srikiatkhachorn, A. Plasma leakage in dengue haemorrhagic fever. Thromb. Haemost. 2009, 102, 1042–1049. [Google Scholar]
- Halstead, S.B.; O’Rourke, E.J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J. Exp. Med. 1977, 146, 201–217. [Google Scholar]
- Mongkolsapaya, J.; Dejnirattisai, W.; Xu, X.N.; Vasanawathana, S.; Tangthawornchaikul, N.; Chairunsri, A.; Sawasdivorn, S.; Duangchinda, T.; Dong, T.; Rowland-Jones, S.; et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 2003, 9, 921–927. [Google Scholar] [CrossRef]
- Leitmeyer, K.C.; Vaughn, D.W.; Watts, D.M.; Salas, R.; Villalobos, I.; de Chacon; Ramos, C.; Rico-Hesse, R. Dengue virus strucutural differences that correlate with pathogenesis. J. Virol. 1999, 73, 4738–4747. [Google Scholar]
- Cologna, R.; Rico-Hesse, R. American genotype structures decrease dengue virus output from human monocytes and dendritic cells. J. Virol. 2003, 77, 3929–3938. [Google Scholar] [CrossRef]
- Silveira, G.F.; Meyer, F.; Delfraro, A.; Mosimann, A.L.P.; Coluchi, N.; Vasquez, C.; Probst, C.M.; Báfica, A.; Bordignon, J.; Duarte Dos Santos, C.N. Dengue virus type-3 isolated from a fatal case with visceral complications induces enhanced pro-inflammatory responses and apoptosis of human dendritic cells. J. Virol. 2011, 85, 5374–5383. [Google Scholar] [CrossRef]
- Long, H.T.; Hibberd, M.L.; Hien, T.T.; Dung, N.M.; Van Ngoc, T.; Farrar, J.; Wills, B.; Simmons, C.P. Patterns of gene transcript abundance in the blood of children with severe or uncomplicated dengue highlight differences in disease evolution and host response to dengue virus infection. J. Infect Dis. 2009, 19, 537–546. [Google Scholar]
- Simmons, C.P.; Popper, S.; Dolocek, C.; Chau, T.N.; Griffiths, M.; Dung, N.T.; Long, T.H.; Hoang, D.M.; Chau, N.V.; le Thao, T.T.; et al. Patterns of host genome-wide gene transcript abundance in the peripheral blood of patients with acute dengue hemorrhagic fever. J. Infect. Dis. 2007, 195, 1097–1107. [Google Scholar] [CrossRef]
- Fernández-Mestre, M.T.; Gendzekhadze, K.; Rivas-Vetencourt, P.; Layrisse, Z. TNF-alpha-308A allele, a possible severity risk factor of hemorrhagic manifestation in dengue fever patients. Tissue Antigens 2004, 64, 469–472. [Google Scholar] [CrossRef]
- Soundravally, R.; Hoti, S.L. Polymorphisms of the TAP 1 and 2 gene may influence clinical outcome of primary dengue viral infection. Scand. J. Immunol. 2008, 67, 618–625. [Google Scholar]
- Sakuntabhai, A.; Turbpaiboon, C.; Casadémont, I.; Chuansumrit, A.; Lowhnoo, T.; Kajaste-Rudnitski, A.; Kalayanarooj, S.M.; Tangnararatchakit, K.; Tangthawornchaikul, N.; Vasanawathana, S.; et al. A variant in the CD209 promoter is associated with severity of dengue disease. Nat. Gen. 2005, 37, 507–513. [Google Scholar] [CrossRef]
- De la Sierra, B.; García, G.; Pérez, A.B.; Morier, L.; Alvarez, M.; Kourí, G.; Guzmán, M.G. Ethnicity and difference in dengue virus-specific memory T cell responses in Cuban individuals. Viral Immunol. 2006, 19, 662–668. [Google Scholar] [CrossRef]
- Egger, J.R.; Coleman, P.G. Age and clinical dengue illness. Emerg. Infect. Dis. 2007, 13, 924–927. [Google Scholar] [CrossRef]
- Pang, T.; Cardosa, M.J.; Guzman, M.G. Of cascades and perfect storms: The immunopathogenesis of dengue haemorrhagic fever-dengue shock syndrome (DHF/DSS). Immunol. Cell Biol. 2007, 85, 43–45. [Google Scholar] [CrossRef]
- Wu, S.J.; Grouard-Vogel, G.; Mascola, J.R.; Brachtel, E.; Putvatana, R.; Louder, M.K.; Filgueira, L.; Marovich, M.A.; Wong, H.K.; Blauvelt, A.; et al. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 2000, 6, 816–820. [Google Scholar] [CrossRef]
- 25. Salgado, D.M.; Eltit, J.M.; Mansfield, K.; Panqueba, C.; Castro, D.; Vega, M.R.; Xhaja, K.; Schmidt, D.; Martin, K.J.; Allen, P.D.; et al. Heart and skeletal muscle are targets of dengue virus infection. Pediatr. Infect. Dis. J. 2010, 29, 238–242. [Google Scholar] [CrossRef]
- Steinman, R.M.; Cohn, Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973, 137, 1142–1162. [Google Scholar]
- Liu, K.; Victora, G.D.; Schwickert, T.A.; Guermonprez, P.; Meredith, M.M.; Yao, K.; Chu, F.F.; Randolph, G.J.; Rudensky, A.Y.; Nussenzweig, M. In vivo analysis of dendritic cell development and homeostasis. Science 2009, 324, 392. [Google Scholar]
- Kushwah, R.; Hu, J. Complexity of dendritic cell subsets and their function in the host immune system. Immunology 2011, 133, 409–419. [Google Scholar] [CrossRef]
- Grouard, G.; Rissoan, M.C.; Filgueira, L.; Durand, I.; Banchereau, J.; Liu, Y.J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 1997, 185, 1101–1111. [Google Scholar] [CrossRef]
- Hou, W.; Gibbs, J.S.; Lu, X.; Brooke, C.B.; Roy, D.; Modlin, R.L.; Bennink, J.R.; Yewdell, J.W. Viral infection triggers rapid differentiation of human blood monocytes into dendritic cells. Blood 2012, 119, 3128–3131. [Google Scholar] [CrossRef]
- Guiducci, C.; Ghirelli, C.; Marloie-Provost, M.A.; Matray, T.; Coffman, R.L.; Liu, Y.J.; Barrat, F.J.; Soumelis, V. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN productionby human plasmacytoidpredendritic cells in response to TLR activation. J. Exp. Med. 2008, 205, 315. [Google Scholar] [CrossRef]
- Jego, G.; Palucka, A.K.; Blanck, J.P.; Chalouni, C.; Pascual, V.; Banchereau, J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 2003, 19, 225–234. [Google Scholar] [CrossRef]
- Irla, M.; Kupfer, N.; Suter, T.; Lissilaa, R.; Benkhoucha, M.; Skupsky, J.; Lalive, P.H.; Fontana, A.; Reith, W.; Hugues, S. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. J. Exp. Med. 2010, 207, 1891–1905. [Google Scholar] [CrossRef]
- Hopkins, R.A.; Connolly, J.E. The specialized roles of immature and mature dendritic cells in antigen cross-presentation. Immunol. Res. 2012, 53, 91–107. [Google Scholar] [CrossRef]
- Sallusto, F.; Schaerli, P.; Loetscher, P.; Schaniel, C.; Lenig, D.; Mackay, C.R.; Qin, S.; Lanzavecchia, A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 1998, 28, 2760–2769. [Google Scholar] [CrossRef]
- Sozzani, S.; Luini, W.; Borsatti, A.; Polentarutti, N.; Zhou, D.; Piemonti, L.; D’Amico, G.; Power, C.A.; Wells, T.N.; Gobbi, M.; et al. Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J. Immunol. 1997, 159, 1993–2000. [Google Scholar]
- Watarai, H.; Hinohara, A.; Nagafune, J.; Nakayama, T.; Taniguchi, M.; Yamaguchi, Y. Plasma membrane-focused proteomics: dramatic changes in surface expression during the maturation of human dendritic cells. Proteomics 2005, 5, 4001–4011. [Google Scholar] [CrossRef]
- Weck, M.M.; Grünebach, F.; Werth, D.; Sinzger, C.; Bringmann, A.; Brossart, P. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood 2007, 109, 3890–3894. [Google Scholar] [CrossRef]
- Groettrup, M.; Soza, A.; Kuckelkorn, U.; Kloetzel, P.M. Peptide antigen production by the proteasome: Complexity provides efficiency. Immunol. Today 1996, 17, 429–435. [Google Scholar] [CrossRef]
- Kloetzel, P.M.; Ossendorp, F. Proteasome and peptidase function in MHC-class-I-mediated antigen presentation. Curr. Opin. Immunol. 2004, 16, 76–81. [Google Scholar] [CrossRef]
- Navarro-Sánchez, E.; Dèspres, P.; Cedillo-Barrón, L. Innate immune response to dengue virus. Arch. Med. Res. 2005, 36, 273–277. [Google Scholar] [CrossRef]
- Taweechaisupapong, S.; Sriurairatana, S.; Angsubhakorn, S.; Yoksan, S.; Khin, M.M.; Sahaphong, S.; Bhamarapravati, N. Langerhans cell density and serological changes following intradermal immunisation of mice with dengue 2 virus. J. Med. Microbiol. 1996, 45, 138–145. [Google Scholar] [CrossRef]
- Satthaporn, S.; Eremin, O. Dendritic cells (I): Biological functions. J. Roy. Coll. Surg. Edinb. 2001, 46, 9–20. [Google Scholar]
- Bowie, A.G.; Unterholzner, L. Viral evasion and subversion of pattern-recognition receptor signaling. Nat. Rev. Immunol. 2008, 8, 911–922. [Google Scholar] [CrossRef]
- Libraty, D.H.; Pichyangkul, S.; Ajariyakhajorn, C.; Endy, T.P.; Ennis, F.A. Human dendritic cells are activated by dengue virus infection: Enhancement by gamma interferon and implications for disease pathogenesis society. J. Virol. 2001, 75, 3501–3508. [Google Scholar] [CrossRef]
- Lechmann, M.; Berchtold, S.; Hauber, J.; Steinkasserer, A. CD83 on dendritic cells: More than just a marker for maturation. Trends Immunol. 2002, 23, 273–275. [Google Scholar] [CrossRef]
- Inaba, K.; Metlay, J.P.; Crowley, M.T.; Steinman, R.M. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J. Exp. Med. 1990, 172, 631–640. [Google Scholar] [CrossRef]
- Dubois, B.; Vanbervliet, B.; Fayette, J.; Massacrier, C.; Van Kooten, C.; Brière, F.; Banchereau, J.; Caux, C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J. Exp. Med. 1997, 185, 941–951. [Google Scholar] [CrossRef]
- Dubois, B.; Massacrier, C.; Vanbervliet, B.; Fayette, J.; Brière, F.; Banchereau, J.; Caux, C. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J. Immunol. 1998, 161, 2223–2231. [Google Scholar]
- Wykes, M.; Pombo, A.; Jenkins, C.; MacPherson, G.G. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J. Immunol. 1998, 161, 1313–1319. [Google Scholar]
- Herrera, O.B.; Golshayan, D.; Tibbott, R.; SalcidoOchoa, F.; James, M.J.; Marelli-Berg, F.M.; Lechler, R.I. A novel pathway of alloantigen presentation by dendritic cells. J. Immunol. 2004, 173, 4828–4837. [Google Scholar]
- Palmer, D.R.; Sun, P.; Celluzzi, C.; Bisbing, J.; Pang, S.; Sun, W.; Marovich, M.A.; Burgess, T.; Cross, A.R.; Foundation, H.M.J. Differential effects of dengue virus on infected and bystander dendritic cells. J. Virol. 2005, 79, 2432–2439. [Google Scholar] [CrossRef]
- Ho, L.J.; Wang, J.J.; Shaio, M.F.; Kao, C.L.; Chang, D.M.; Han, S.W.; Lai, J.H. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J. Immunol. 2001, 166, 1499–1506. [Google Scholar]
- Dejnirattisai, W.; Duangchinda, T.; Lin, C.S.; Vasanawathana, S.; Jones, M.; Jacobs, M.; Malasit, P.; Xu, X.; Screaton, G.; Mongkolsapaya, J. A complex interplay among virus, dendritic cells, T cells, and cytokines in dengue virus infections. J. Immunol. 2008, 181, 5865–5874. [Google Scholar]
- Nightingale, Z.D.; Patkar, C.; Rothman, A.L. Viral replication and paracrine effects result in distinct, functional responses of dendritic cells following infection with dengue 2 virus. J. Leukoc. Biol. 2008, 84, 1028–1038. [Google Scholar] [CrossRef]
- Cardier, J.E.; Maríno, E.; Romano, E.; Taylor, P.; Liprandi, F.; Bosch, N.; Rothman, A.L.; Cardier, E.; Marin, E. Proinflammatory factors present in sera from patients with acute dengue infection induce activation and apoptosis of human microvascular endothelial cells: Possible role of TNF-alpha in endothelial cell damage in dengue. Cytokine 2005, 30, 359–365. [Google Scholar] [CrossRef]
- Ashour, J.; Laurent-Rolle, M.; Shi, P.Y.; García-Sastre, A. NS5 of dengue virus mediates STAT2 binding and degradation. J. Virol. 2009, 83, 5408–5418. [Google Scholar] [CrossRef]
- Muñoz-Jordán, J.L.; Laurent-Rolle, M.; Ashour, J.; Martínez-Sobrido, L.; Ashok, M.; Lipkin, WI.; García-Sastre, A. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J. Virol. 2005, 79, 8004–8013. [Google Scholar]
- Rodriguez-Madoz, J.R.; Belicha-Villanueva, A.; Bernal-Rubio, D.; Ashour, J.; Ayllon, J.; Fernandez-Sesma, A. Inhibition of the type I interferon response in human dendritic cells by dengue virus infection requires a catalytically active NS2B3 complex. J. Virol. 2010, 84, 9760–9774. [Google Scholar]
- Luplerdlop, N.; Missé, D.; Bray, D.; Deleuze, V.; Gonzalez, V.L.; Yssel, H.; Veas, F. Dengue virus infected dendritic cells trigger vascular leakage through metalloproteinase overproduction. EMBO Rep. 2006, 7, 1176–1181. [Google Scholar] [CrossRef]
- Portt, L.; Norman, G.; Clapp, C.; Greenwood, M.; Greenwood, M.T. Anti-apoptosis and cell survival: A review. Biochim. Biophys. Acta 2011, 1813, 238–259. [Google Scholar]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Chicheportiche, Y.; Bourdon, P.R.; Xu, H.; Hsu, Y.M.; Scott, H.; Hession, C.; Garcia, I.; Browning, J.L. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J. Biol. Chem. 1997, 272, 32401–32410. [Google Scholar]
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef]
- Sakahira, H.; Enari, M.; Nagata, S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 1998, 391, 96–99. [Google Scholar] [CrossRef]
- Kothakota, S.; Azuma, T.; Reinhard, C.; Klippel, A.; Tang, J.; Chu, K.; McGarry, T.J.; Kirschner, M.W.; Koths, K.; Kwiatkowski, D.J.; et al. Caspase-3-generated fragment of gelsolin: Effector of morphological change in apoptosis. Science 1997, 278, 294–298. [Google Scholar]
- Fugier-Vivier, I.; Servet-Delprat, C.; Rivailler, P.; Rissoan, M.C.; Liu, Y.J.; Rabourdin-Combe, C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 1997, 186, 813–823. [Google Scholar] [CrossRef]
- Jin, H.; Xiao, C.; Zhao, G.; Du, X.; Yu, Y.; Kang, Y.; Wang, B. Induction of immature dendritic cell apoptosis by foot and mouth disease virus is an integrin receptor mediated event before viral infection. J. Cell. Biochem. 2007, 102, 980–991. [Google Scholar] [CrossRef]
- Kushwah, R.; Oliver, J.R.; Zhang, J.; Siminovitch, K.A.; Hu, J. Apoptotic dendritic cells induce tolerance in mice through suppression of dendritic cell maturation and induction of antigen-specific regulatory T cells. J. Immunol. 2009, 183, 7104–7118. [Google Scholar] [CrossRef]
- Kessel, A.; Bamberger, E.; Masalha, M.; Toubi, E. The role of T regulatory cells in human sepsis. J. Autoimmun. 2009, 32, 211–215. [Google Scholar] [CrossRef]
- Kushwah, R.; Hu, J. Dendritic cell apoptosis: regulation of tolerance versus immunity. J. Immun. 2010, 185, 795–802. [Google Scholar] [CrossRef]
- Halstead, S.B.; O’Rourke, E.J.; Allison, A.C. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J. Exp. Med. 1977, 146, 218–229. [Google Scholar] [CrossRef]
- King, A.D.; Nisalak, A.; Kalayanrooj, S.; Myint, K.S.; Pattanapanyasat, K.; Nimmannitya, S.; Innis, B.L. B cells are the principal circulating mononuclear cells infected by dengue virus. Southeast Asian J. Trop. Med. Public Health 1999, 30, 718–728. [Google Scholar]
- Imbert, J.L.; Guevara, P.; Ramos-Castañeda, J.; Ramos, C.; Sotelo, J. Dengue virus infects mouse cultured neurons but not astrocytes. J. Med. Virol. 1994, 42, 228–233. [Google Scholar] [CrossRef]
- Huang, Y.H.; Lei, H.Y.; Liu, H.S.; Lin, Y.S.; Liu, C.C.; Yeh, T.M. Dengue virus infects human endothelial cells and induces IL-6 and IL-8 production. Am. J. Trop. Med. Hyg. 2000, 63, 71–75. [Google Scholar]
- Thepparit, C.; Smith, D.R. Serotype-specific entry of dengue virus into liver cells: Identification of the 37-kilodalton/67-kilodalton high-affinity lamin receptor as a dengue virus serotype 1 receptor. J. Virol. 2004, 78, 12647–12656. [Google Scholar] [CrossRef]
- Anderson, R. Manipulation of cell surface macromolecules by flaviviruses. Adv. Virus Res. 2003, 59, 229–274. [Google Scholar] [CrossRef]
- Limonta, D.; Capó, V.; Torres, G.; Pérez, A.B.; Guzmán, M.G. Apoptosis in tissues from fatal dengue shock syndrome. J. Clin. Virol. 2007, 40, 50–54. [Google Scholar] [CrossRef]
- Courageot, M.P.; Catteau, A.; Desprès, P. Mechanisms of dengue virus-induced cell death. Adv. Virus Res. 2003, 60, 157–186. [Google Scholar] [CrossRef]
- Marianneau, P.; Cardona, A.; Edelman, L.; Deubel, V.; Desprès, P. Dengue virus replication in human hepatoma cells activates NF-kappaB which in turn induces apoptotic cell death. J. Virol. 1997, 71, 3244–3249. [Google Scholar]
- Desprès, P.; Flamand, M.; Ceccaldi, P.E.; Deubel, V. Human isolates of dengue type 1 virus induce apoptosis in mouse neuroblastoma cells. J. Virol. 1996, 70, 4090–4096. [Google Scholar]
- Bhamarapravati, N.; Tuchinda, P.; Boonyapaknavik, V. Pathology of Thailand haemorrhagic fever: A study of 100 autopsy cases. Ann. Trop. Med. Parasitol. 1967, 61, 500–510. [Google Scholar]
- Kuo, C.H.; Tai, D.I.; Chang-Chien, C.S.; Lan, C.K.; Chiou, S.S.; Liaw, Y.F. Liver biochemical tests and dengue fever. Am. J. Trop. Med. Hyg. 1992, 47, 265–270. [Google Scholar]
- Nguyen, T.L.; Nguyen, T.H.; Tieu, N.T. The impact of dengue haemorrhagic fever on liver function. Res Virol. 1997, 148, 273–277. [Google Scholar] [CrossRef]
- Nagila, A.; Netsawang, J.; Srisawat, C.; Noisakran, S.; Morchang, A.; Yasamut, U.; Puttikhunt, C.; Kasinrerk, W.; Malasit, P.; Yenchitsomanus, P.T.; et al. Role of CD137 signaling in dengue virus-mediated apoptosis. Biochem. Biophys. Res. Commun. 2011, 410, 428–433. [Google Scholar] [CrossRef]
- Netsawang, J.; Noisakran, S.; Puttikhunt, C.; Kasinrerk, W.; Wongwiwat, W.; Malasit, P.; Yenchitsomanus, P.T.; Limjindaporn, T. Nuclear localization of dengue virus capsid protein is required for DAXX interaction and apoptosis. Virus Res. 2010, 147, 275–283. [Google Scholar] [CrossRef]
- Kim, J.O.; Kim, H.W.; Baek, K.M.; Kang, C.Y. NF-kappaB and AP-1 regulate activation-dependent CD137 (4-1BB) expression in T cells. FEBS Lett. 2003, 541, 163–170. [Google Scholar] [CrossRef]
- Bram, R.J.; Crabtree, G.R. Calcium signalling in T cells stimulated by a cyclophilin B-binding protein. Nature 1994, 371, 355–358. [Google Scholar] [CrossRef]
- Li, J.; Huang, R.; Liao, W.; Chen, Z.; Zhang, S.; Huang, R. Dengue virus utilizes calcium modulating cyclophilin-binding ligand to subvert apoptosis. Biochem. Biophys. Res. Commun. 2012, 418, 622–627. [Google Scholar] [CrossRef]
- Axelroid, J.; Burch, R.M.; Jelsema, C.L. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: Arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988, 1, 117–123. [Google Scholar]
- Jaattela, M.; Benedict, M.; Tewari, M.; Shayman, J.A.; Dixit, V.W. Bcl-x and Bcl-2 inhibit TNF and Fas-induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene 1995, 10, 2297–2305. [Google Scholar]
- Malewicz, B.; Parthsarathy, S.; Jenkin, H.M.; Baumann, W.J. Rapid phospholipase A2 stimulation and diacylglycerolcholinephosphotransferase inhibition in baby hamster kidney cells during initiation of dengue virus infection. Biochem. Biophys. Res. Commun. 1981, 101, 404–410. [Google Scholar] [CrossRef]
- Nevalainen, T.J.; Losacker, W. Serum phospholipase A2 in dengue. J. Infect. 1997, 35, 251–252. [Google Scholar] [CrossRef]
- Jan, J.T.; Chen, B.H.; Ma, S.H.; Liu, C.I.; Tsai, H.P.; Wu, H.C.; Jiang, S.Y.; Yang, K.D.; Shaio, M.F. Potential dengue virus-triggered apoptotic pathway in human neuroblastoma cells: Arachidonic acid, superoxide anion, and NF-kappa B are sequentially involved. J. Virol. 2000, 74, 8680–8691. [Google Scholar] [CrossRef]
- Xiao, C.; Ghosh, S. NF-kappaB, an evolutionarily conserved mediator of immune and inflammatory responses. Adv. Exp. Med. Biol. 2005, 560, 41–45. [Google Scholar] [CrossRef]
- Catteau, A.; Kalinina, O.; Wagner, M.; Deubel, V.; Courageot, M.; Philippe, D. Dengue virus M protein contains a proapoptotic sequence referred to as ApoptoM. J. Gen. Virol. 2003, 84, 2781–2793. [Google Scholar] [CrossRef]
- Duarte dos Santos, C.N.; Frenkiel, M.P.; Courageot, M.P.; Rocha, C.F.; Vazeille-Falcoz, M.C.; Wien, M.W.; Rey, F.A.; Deubel, V.; Desprès, P. Determinants in the envelope E protein and viral RNA helicase NS3 that influence the induction of apoptosis in response to infection with dengue virus type-1. Virology 2000, 274, 292–308. [Google Scholar] [CrossRef]
- Avirutnan, P.; Malasit, P.; Seliger, B.; Bhakdi, S.; Husmann, M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J. Immunol. 1998, 161, 6338–6346. [Google Scholar]
- Avirutnan, P.; Punyadee, N.; Noisakran, S.; Komoltri, C.; Thiemmeca, S.; Auethavornanan, K.; Jairungsri, A.; Kanlaya, R.; Tangthawornchaikul, N.; Puttikhunt, C.; et al. Vascular leakage in severe dengue virus infections: A potential role for the nonstructural viral protein NS1 and complement. J. Infect. Dis. 2006, 193, 1078–1088. [Google Scholar] [CrossRef]
- Flamand, M.; Megret, F.; Mathieu, M.; Lepault, J.; Rey, F.A.; Deubel, V. Dengue virus type 1 nonstructural glycoprotein NS1 is secreted from mammalian cells as soluble hexamer in a glycosylation-dependent fashion. J. Virol. 1999, 73, 6104–6110. [Google Scholar]
- Winkler, G.; Maxwell, S.E.; Ruemmler, C.; Stollar, V. Newly synthesized dengue-2 virus nonstructural ürotein NSl is a soluble protein but becomes partially hydrophobic and membrane-associated after dimerization pages. Virology 1989, 305, 302–305. [Google Scholar]
- Avirutnan, P.; Fuchs, A.; Hauhart, R.E.; Somnuke, P.; Youn, S.; Diamond, M.S.; Atkinson, J.P. Antagonism of the complement component C4 by flavivirus nonstructural protein NS1. J. Exp. Med. 2010, 207, 793–806. [Google Scholar] [CrossRef]
- Morchang, A.; Yasamut, U.; Netsawang, J.; Noisakran, S.; Wongwiwat, W.; Songprakhon, P.; Srisawat, C.; Puttikhunt, C.; Kasinrerk, W.; Malasit, P.; et al. Cell death gene expression profile: Role of RIPK2 in dengue virus-mediated apoptosis. Virus Res. 2011, 156, 25–34. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J. Regulation of immune responses by spontaneous and T cell-mediated dendritic cell death. J. Clin. Cell Immunol. 2011, 11. [Google Scholar] [CrossRef]
- Kingston, D.; Schmid, M.A.; Onai, N.; Obata-Onai, A.; Baumjohann, D.; Manz, M.G. The concerted action of GM-CSF and Flt3-ligand on in vivo dendritic cell homeostasis. Blood 2009, 114, 835–843. [Google Scholar] [CrossRef]
- Chang, W.L.; Baumgarth, N.; Eberhardt, M.K.; Lee, C.Y.; Baron, C.A.; Gregg, J.P.; Barry, P.A. Exposure of myeloid dendritic cells to exogenous or endogenous IL-10 during maturation determines their longevity. J. Immunol. 2007, 178, 7794–7804. [Google Scholar]
- Borkowski, T.A.; Nelson, A.J.; Farr, A.G.; Udey, M.C. Expression of gp40, the murine homologue of human epithelial cell adhesion molecule (Ep-CAM), by murine dendritic cells. Eur. J. Immunol. 1996, 26, 110–114. [Google Scholar] [CrossRef]
- Ito, M.; Minamiya, Y.; Kawai, H.; Saito, S.; Saito, H.; Nakagawa, T.; Imai, K.; Hirokawa, M.; Ogawa, J. Tumor-derived TGFbeta-1 induces dendritic cell apoptosis in the sentinel lymph node. J. Immunol. 2006, 176, 5637–5643. [Google Scholar]
- Krishnadas, D.K.; Ahn, J.S.; Han, J.; Kumar, R.; Agrawal, B. Immunomodulation by hepatitis C virus-derived proteins: targeting human dendritic cells by multiple mechanisms. Int. Immunol. 2010, 22, 491–502. [Google Scholar] [CrossRef]
- Swiecki, M.; Wang, Y.; Vermi, W.; Gilfillan, S.; Schreiber, R.D.; Colonna, M. Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. J. Exp. Med. 2011, 208, 2367–2374. [Google Scholar] [CrossRef]
- Jamin, A.; Gorin, S.; Cariolet, R.; Le Potier, M.F.; Kuntz-Simon, G. Classical swine fever virus induces activation of plasmacytoid and conventional dendritic cells in tonsil, blood, and spleen of infected pigs. Vet. Res. 2008, 39, 7. [Google Scholar] [CrossRef]
- Carrasco, C.P.; Rigden, R.C.; Vincent, I.E.; Balmelli, C.; Ceppi, M.; Bauhofer, O.; Tâche, V.; Hjertner, B.; McNeilly, F.; Van Gennip, H.G.; et al. Interaction of classical swine fever virus with dendritic cells. J. Gen. Virol. 2004, 85, 1633–1641. [Google Scholar] [CrossRef]
- Kaminskyy, V.; Zhivotovsky, B. To kill or be killed: How viruses interact with the cell death machinery. J. Intern. Med. 2010, 267, 473–482. [Google Scholar] [CrossRef]
- De Carvalho Bittencourt, M.; Martial, J.; Cabié, A.; Thomas, L.; Césaire, R. Decreased peripheral dendritic cell numbers in dengue virus infection. J. Clin. Immunol. 2012, 32, 161–172. [Google Scholar] [CrossRef]
- Galluzzi, L.; Brenner, C.; Morselli, E.; Touat, Z.; Kroemer, G. Viral control of mitochondrial apoptosis. PLoS Pathog. 2008, 4, e1000018. [Google Scholar] [CrossRef]
- Reed, D.S.; Hensley, L.E.; Geisbert, J.B.; Jahrling, P.B.; Geisbert, T.W. Depletion of peripheral blood T lymphocytes and NK cells during the course of ebola hemorrhagic fever in cynomolgusmacaques. Viral Immunol. 2004, 17, 390–400. [Google Scholar] [CrossRef]
- Lundqvist, A.; Nagata, T.; Kiessling, R.; Pisa, P. Mature dendritic cells are protected from Fas/CD95-mediated apoptosis by up-regulation of Bcl-Xl. Cancer Immunol. Immunother. 2002, 51, 139–144. [Google Scholar] [CrossRef]
- Servet-Delprat, C.; Vidalain, P.O.; Azocar, O.; Le Deist, F.; Fischer, A.; Rabourdin-Combe, C. Consequences of Fas-mediated human dendritic cell apoptosis induced by measles virus. J Virol. 2000, 74, 4387–4393. [Google Scholar] [CrossRef]
- Marriott, H.M.; Dockrell, D.H. Streptococcus pneumoniae: The role of apoptosis in host defense and pathogenesis. Int. J. Biochem. Cell Biol. 2006, 38, 1848–1854. [Google Scholar] [CrossRef]
- Colino, J.; Snapper, C.M. Two distinct mechanisms for induction of dendritic cell apoptosis in response to intact Streptococcus pneumoniae. J. Immunol. 2003, 171, 2354–2365. [Google Scholar]
- Woodberry, T.; Minigo, G.; Piera, K.A.; Amante, F.H.; Pinzon-Charry, A.; Good, M.F.; Lopez, J.A.; Engwerda, C.R.; McCarthy, J.S.; Anstey, N.M. Low-level Plasmodium falciparum blood-stage infection causes dendritic cell apoptosis and dysfunction in healthy volunteers. J. Infect. Dis. 2012, 206, 333–340. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Martins, S.D.T.; Silveira, G.F.; Alves, L.R.; Dos Santos, C.N.D.; Bordignon, J. Dendritic Cell Apoptosis and the Pathogenesis of Dengue. Viruses 2012, 4, 2736-2753. https://doi.org/10.3390/v4112736
Martins SDT, Silveira GF, Alves LR, Dos Santos CND, Bordignon J. Dendritic Cell Apoptosis and the Pathogenesis of Dengue. Viruses. 2012; 4(11):2736-2753. https://doi.org/10.3390/v4112736
Chicago/Turabian StyleMartins, Sharon De T., Guilherme F. Silveira, Lysangela R. Alves, Claudia Nunes Duarte Dos Santos, and Juliano Bordignon. 2012. "Dendritic Cell Apoptosis and the Pathogenesis of Dengue" Viruses 4, no. 11: 2736-2753. https://doi.org/10.3390/v4112736
APA StyleMartins, S. D. T., Silveira, G. F., Alves, L. R., Dos Santos, C. N. D., & Bordignon, J. (2012). Dendritic Cell Apoptosis and the Pathogenesis of Dengue. Viruses, 4(11), 2736-2753. https://doi.org/10.3390/v4112736