Abstract
Chronic infection with hepatitis C virus (HCV) is associated with significant liver disease and is therefore an important public health problem. The current standard-of-care therapy for chronic HCV infection consists of a combination of pegylated (PEG) interferon (IFN)-α and ribavirin. Although this therapy effectively generates a sustained viral response in approximately half of treated individuals, it is associated with significant hematological and neurological side effects. A new family of IFN-related proteins (IFN-λ1, 2, and 3; or alternately, IL-29, 28A, 28B, respectively) possesses properties that may make these cytokines superior to PEG-IFN-α for HCV therapy. Genetic studies have also implicated these proteins in both the natural and therapy-induced resolution of HCV infection. This review summarizes the basic aspects of IFN-λ biology, the potential role of these cytokines in HCV infection, and the outlook for their therapeutic application.
1. Structural properties of IFN-λ
The IFN-λ family of IFN-related proteins was discovered in 2003 using computational methods designed to find new proteins within the class II α-helical cytokine family [,]. Three members of this family were identified and alternatively named IFN-λ1, 2, 3, or IL-29, 28A, 28B, and are now also referred to as the “type III” IFNs to further distinguish them from IFN-α/β (type I) and IFN-γ (type II). Although the three IFN-λ family members have a high degree of amino acid identity to each other (81% between IFN-λ1 and IFN-λ2; 96% between IFN-λ2 and IFN-λ3), these proteins have low sequence homology to both IFN-α (15-19% identity, 31-33% similarity) and IL-10 (11-13% identity, 22-23% similarity) [,,]. Despite this minimal homology, conserved cysteine patterns and predicted amphipathic helix profiles indicated that the IFN-λs belong to the class II cytokine family. Furthermore, the IFN-λ genes are composed of five to six exons, an arrangement that is similar to IL-10, but is unlike the type I interferons, which are each encoded by a single exon [,]. Recently, the crystal structure of IFN-λ3 was reported to contain a bundle of four alpha helices at its core, which is similar to other class II cytokines []. Further comparison of the structure between IFN-λ and other class II cytokines found a closer association between IFN-λ and IL-10 family cytokines, in particular IL-22, than with the type I IFNs. Therefore, despite low amino acid homology between IFN-λ and the IL-10 family cytokines, there is a strong structural correlation between these two groups of proteins.
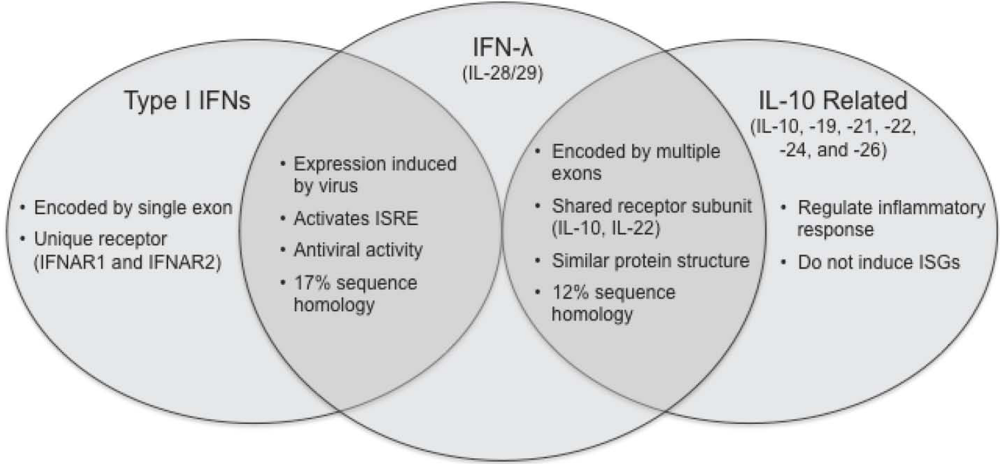
Figure 1.
Comparison of type I IFNs, IFN-λ and IL-10 related cytokines. The type III IFNs are functionally similar to type I IFNs (IFN-α/β). However, they are more structurally related to the IL-10 family cytokines.
The IFN-λs do not bind to the IFN-α/β receptor, but instead exert their activity through a distinct receptor. The IFN-λ receptor consists of two subunits: IL-28Rα and IL-10Rβ [,]. The IL-10Rβ subunit is not unique to IFN-λ, as it is also utilized by IL-10 and IL-22 [,]. While the IL-10Rβ subunit is ubiquitously expressed on many cell types [], the IL-28Rα subunit displays a more restricted profile [,], and is most strongly expressed on cells of epithelial origin [,,,]. Although the regulation of IFN-λ expression has been well studied (described in section 2 below), there is little known about the mechanisms that control expression of the IL-28Rα receptor subunit. Since the expression of the IFN-λ receptor is highly dependent on cell type, there may be tissue-specific signals required to induce IL-28Rα expression, such as specific transcription factors, DNA and histone methylation patterns, or microRNAs. Interestingly, it was also recently found that a splice variant of the IL-28Rα transcript encodes a soluble version of the receptor that inhibits IFN-λ activity in leukocytes [], further indicating that precise restriction of IFN-λ activity to specific cell types may be important for its biological function.
2. Activation of IFN-λ expression
IFN-λ expression has been detected in primary neuronal cells, alveolar epithelial cells, hepatocytes, and a variety of cell lines [,,]. However, like IFN-α, the primary producers of IFN-λ appear to be dendritic cells (DCs) [,,,,]. Similar to the type I IFNs, IFN-λ expression is induced following viral infection or activation of Toll-like receptors (TLRs). Stimulation of the cytoplasmic receptor RIG-I, which detects cytoplasmic viral RNA, activates IFN-λ expression [,]. While many cell types may produce IFN-λ following TLR activation, DCs and DC-derived cell lines are the best characterized, as they produce relatively high levels of IFN-λ [,,,]. Activation of TLRs-3, -4, -7 and -9 all increase IFN-λ expression in DCs [,]. TLR-3, -7 and -9 are typically localized in endosomes and detect viral pathogen-associated molecular patterns such as doubled stranded RNA (dsRNA), single stranded RNA, and non-methylated double-stranded CpG-rich DNA []. Stimulation of these receptors ultimately leads to the activation of transcription factors such as interferon-regulatory factor (IRF)-3, IRF-7 and NF-κB. Though IFN-λ is typically activated by viral infections, activation of TLR-4 by bacterial LPS has been shown to induce IFN-λ in DCs [,], suggesting that IFN-λ may have additional functions in the modulation of the immune response [].
Binding sites for IRF-3, IRF-7, and NF-κB have all been identified in the promoter region for IFN-λ and are essential for induction of expression []. However, there are differences in the regulation of IFN-λ1 and IFN-λ2/3 transcription. IFN-λ1 is activated by both IRF-3 and -7, whereas IFN-λ2/3 is primarily regulated by IRF-7 []. In contrast to the type I IFNs, which are not induced by IFN treatment, IFN-λ mRNA expression can be induced by stimulating cells with IFN-α or IFN-λ alone, indicating that IFN-λ is in fact also an interferon-stimulated gene (ISG) []. Furthermore, IFN-α may play a role in regulating TLR-induced activation of IFN-λ expression [], demonstrating additional cross-talk between the type I and type III interferon responses.
The extent to which IFN-λ is induced in a natural HCV infection is unclear, as HCV has evolved multiple mechanisms to inhibit the IFN-α/β response in infected hepatocytes. The HCV NS3/4A protease inhibits IRF-3 activation and cleaves the RIG-I and TLR signaling adapters IPS-1 and TRIF [,,]. A second HCV protein (NS2) also blocks activation of IFN-β through an uncharacterized mechanism that is distinct from that of NS3/4A [], and the HCV NS5A protein is yet another factor capable of inhibiting IFN-α/β expression []. Because IFN-α/β and IFN-λ are activated through a common molecular mechanism [] by identical types of stimuli [], the viral immunomodulatory mechanisms that HCV has evolved to inhibit IFN-α/β expression also likely block IFN-λ production. In fact, when over-expressed in cell culture, NS3/4A prevents the induction of both IFN-α/β as well as IFN-λ []. Furthermore, like IFN-α/β, IFN-λ is expressed in PBMC but not in the liver of chronic HCV patients [].
3. IFN-λ-induced signaling and gene expression
The signaling pathways induced by IFN-λ are very similar to those induced by the type I IFNs (Figure 2) []. The intracellular domains of the IFN-λ receptor subunits, IL-28Rα and IL-10Rβ, interact with the receptor-associated tyrosine kinases Jak1 and Tyk2. These kinases in turn phosphorylate STAT proteins, which then dimerize and act as transcription factors. Binding of IFN-λ to its receptor induces phosphorylation of STAT-1, -2, -3, and -5 through a process that requires two key tyrosine residues on IL-28Rα [,,,,,,]. In most cell types, IFN-λ induces phosphorylation of STAT-1 and STAT-2, which form a heterodimer that interacts with IRF-9 to form the transcription factor interferon-stimulated gene factor-3 (ISGF-3) []. ISGF-3 preferentially binds to promoters containing ISREs, which are found in the upstream regions of ISGs. Like IFN-α, IFN-λ-induced STAT activation is negatively regulated by the suppressor of cytokine signaling (SOCS) proteins [].
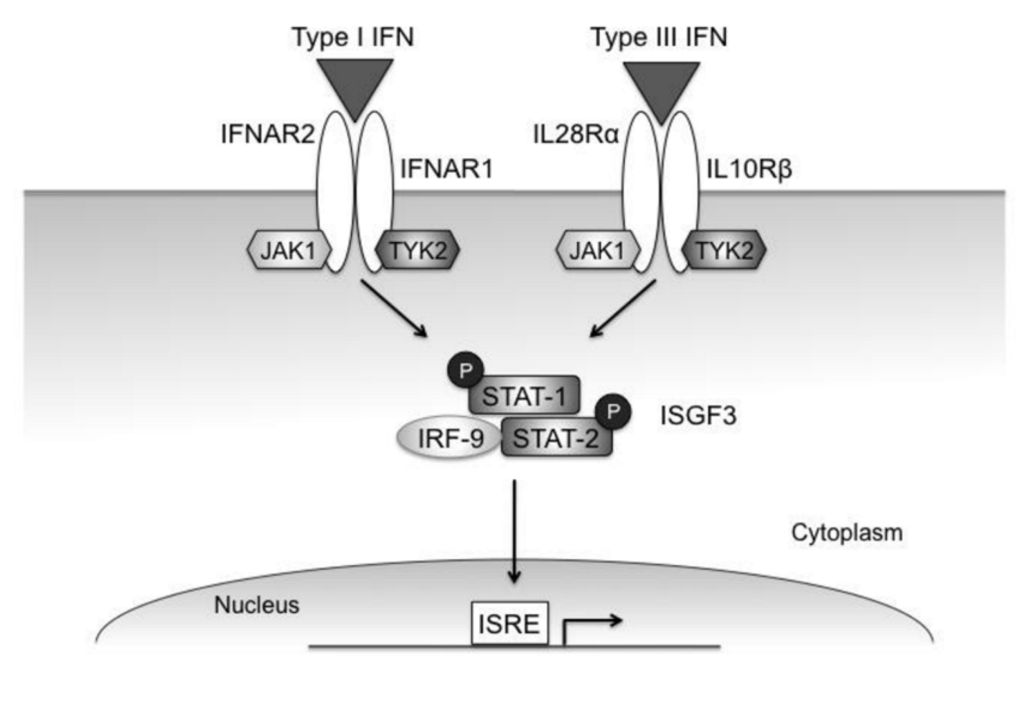
Figure 2.
Type I and type III IFN signaling pathways. Though the type I and type III receptors are distinct, both cytokines induce STAT phosphorylation through the Jak kinases associated with the respective receptor subunits. Both IFN-α and IFN-λ primarily activate STAT-1 and -2, which complex with IRF-9 to form the transcription factor ISGF-3. This complex induces expression of genes with ISREs in their promoters.
Though the signaling pathway induced by IFN-λ is nearly identical to that of IFN-α, the kinetics and magnitude of the responses can be subtly different. In Huh-7 hepatocellular carcinoma cells, IFN-λ induces STAT-1 and STAT-2 more rapidly than IFN-α []. Additionally, IFN-λ induces STAT-1 and STAT-2 phosphorylation for a longer period of time in HaCaT keratinocytes compared to IFN-α []. Furthermore, although the subsequent transcriptional response is slightly delayed, the increase in ISG expression induced by IFN-λ is stronger and more prolonged than the response activated by IFN-α [,]. Nevertheless, with their signaling patterns being nearly identical, IFN-α and IFN-λ induce very similar patterns of gene expression [,,]. Consistent with the convergence of the two signaling pathways to a similar transcriptional response, combinations of IFN-λ and IFN-α together appear to have no more than a dose-dependent additive effect on HCV replication [,].
Although Jak/STAT signaling mediates the primary functions of IFN-λ, other pathways are also activated by the receptor. One study found that IFN-λ activated ERK-1/2, mitogen activated protein kinase (MAPK) and Akt in intestinal epithelial and colorectal cancer-derived cell lines []. This activity led to increased IL-8 expression, a chemokine that is associated with the inflammatory response []. Additionally, activation of MAPKs was also observed in Raji cells following treatment with IFN-λ []. These results indicate that IFN-λ can induce multiple signaling pathways that may contribute to its activity as an antiviral and immunomodulatory cytokine.
4. Functions of IFN-λ
4.2. IFN-λ immunomodulatory activity
IFN-λ clearly has direct inhibitory effects on the replication of most viruses, and therefore may be an important component of the innate immune response, at least in certain contexts. However, a number of studies have also demonstrated that IFN-λ also plays a role in antiviral immunity through modulation of both the maturation and differentiation of immune cells. Though monocytes express low levels of the IFN-λ receptor, differentiation of these cells into DCs leads to an upregulation of IL-28Rα and an increased ability to express IFN-λ [,,]. Conversely, monocytes that differentiate into macrophages show reduced expression of IFN-λ []. Additionally, when DCs are subsequently exposed to IFN-λ, increased maturation and migration capacity are induced []. These changes are due in large part to changes of cell surface molecules on DCs that alter both the stimulation and homing of these antigen-presenting cells []. In turn, IFN-λ influences the effects that DCs have when interacting with T cells. DCs treated with IFN-λ preferentially expand regulatory T cells, which are critical for negative regulation of the immune response, as well as promoting self-tolerance [].
By altering the maturation and differentiation of other immune cells, particularly T cells, IFN-λ also alters the expression of other cytokines and chemokines. The role of IFN-λ appears to be primarily focused on biasing T cell differentiation against Th2 development and Th2 cytokine secretion [,]. IFN-λ inhibits IL-4, IL-5 and IL-13 expression in T cells independently of IL-10 [,], and modulates both cytokine and chemokine expression in peripheral blood mononuclear cells (PBMCs), reducing IL-13 as well as IL-6, IL-8 and IL-10 production []. Additionally, the chemokines MIG, IP-10 and I-TAC, which are antimicrobial chemoattractants for mononuclear cells, were found to be upregulated by IFN-λ in PBMCs []. In total, these studies indicate a potential important role for IFN-λ in both the regulation and development of the adaptive immune response.
5. IL28B polymorphisms and HCV infection/therapy outcome
Recent genome-wide association studies have found a strong genetic link between HCV infection, treatment outcome, and IFN-λ. Both spontaneous HCV clearance and a sustained viral response following PEG-IFN-α plus ribavirin therapy correlate with single nucleotide polymorphisms (SNPs) found in the IL28B gene locus, which encodes the IFN-λ3 protein [,,,,]. The rs12979860 SNP resides 3 kb upstream of the IL28B gene, and variations at this position are associated with approximately 2-fold differences in spontaneous clearance and response to treatment [,,]. The C/C genotype is associated with better outcomes, and the T/T genotype, worse outcomes. The rs8099917 SNP is located within an intergenic region between the IL28A and IL28B genes, and is similarly associated with a 2-3 fold difference in spontaneous clearance and response to therapy [,,]. The polymorphisms associated with poor response to therapy are found at a higher frequency in African populations compared to European populations, consistent with the lower response rates of PEG-IFN-α plus ribavirin treatment in African-Americans [].
Although the relationship between HCV infection outcome and therapy response with IL28B variation is now well established, the molecular mechanisms behind this association have not yet been identified. The rs12979860 SNP is associated with two other polymorphisms found in the IFN-λ3 transcription initiation and coding regions, which may potentially alter the expression or activity of the cytokine [,]. Individuals harboring the rs8099917 minor allele were found to have reduced IFN-λ3 expression levels in PBMC, indicating that this variant may be located within a transcriptional regulatory element [,]. Because IFN-α upregulates IFN-λ expression [,], IFN-λ may amplify interferon-stimulated gene expression following administration of PEG-IFN-α. As other host factors have also been associated with therapy outcome, the interplay between these various factors needs to be better defined. For example, patients who fail to achieve a sustained viral response after PEG-IFN-α therapy have a high pre-therapy level of intrahepatic ISG expression [,], and it has not yet been addressed whether this observation is also related to IL28B variation. Elucidation of these mechanisms will be important for understanding the role of IFN-λ in chronic HCV infection and in IFN-based therapies.
6. IFN-λ and HCV Therapy
It was immediately recognized after its discovery that due to the relatively restricted expression of the IFN-λ receptor, the type III IFNs could potentially be useful therapeutically for chronic HCV infection [,]. Because the receptor is expressed at low levels on T cells and NK cells, and is not expressed on hematopoetic precursor cells, it was predicted that therapeutic use of IFN-λ would not cause the hematological side effects associated with PEG-IFN-α therapy []. This prediction has largely been proven true by a recently reported Phase 1b clinical trial of PEG-IFN-λ1 for genotype 1 chronic HCV infection []. In this study, the antiviral efficacy and side effects of PEG-IFN-λ1 were measured in IFN-α relapse patients as a single agent, and in relapse and treatment-naïve patients in combination with ribavirin.
The majority of patients in this study achieved significant reductions in HCV RNA levels after 4 weeks of treatment with PEG-IFN-λ1, both as a single agent alone and when administered together with ribavirin. More specifically, in individuals who received weekly doses of 1.5 µg/kg or greater, 96% (23 of 24) of treatment-relapse patients and 86% (6 of 7) of treatment-naïve patients had a > 2 log10 decline in viral RNA []. Furthermore, a subset of these patients (17% of relapse and 29% of naïve) attained undetectable levels of HCV RNA. While not directly compared in this study, the biphasic kinetics of viral decline were found to be similar to the pattern typically observed with IFN-α therapy. This study also demonstrated that PEG-IFN-λ1 administration did not cause the significant reductions in neutrophil counts, platelet counts, or hemoglobin levels that can be associated with PEG-IFN-α therapy []. IFN-λ1 therapy was generally otherwise well-tolerated, with adverse events such as fatigue, nausea, myalgia, fever, or irritability being relatively rare and mild [,]. Therefore, PEG-IFN-λ1 may have fewer of the safety and tolerability issues that can limit PEG-IFN-α efficacy.
While the results from the Phase 1 trial are very promising, a number of questions remain regarding the clinical utility of IFN-λ for chronic HCV that can only be resolved through additional larger studies. First, will IFN-λ therapy successfully generate a long-term sustained viral response after cessation of therapy? Second, how will the response to IFN-λ be influenced by the IL28B polymorphisms that affect PEG-IFN-α therapy? Third, will the efficacy of IFN-λ1 extend to HCV genotypes other than genotype 1? Fourth, will long-term IFN-λ administration cause the same neurological side effects that can accompany IFN-α therapy? Despite these unresolved questions, the encouraging results obtained thus far give reason to be optimistic that the clinical potential of IFN-λ will be realized not only for chronic HCV infection, but also for other diseases that respond to IFN-α therapy, such as chronic HBV infection and melanoma.
Acknowledgments
M.D.R. is supported by NIH grants CA131133, CA137067, and the Charles Dana Foundation Neuroimmunology Program. N.E.P. was supported by NIH training grants T32 AI055403, T32 AI007640, and a fellowship from the Anna Fuller Fund. Portions of this review were modified from a dissertation submitted to the Yale University Graduate School of Arts and Sciences in partial fulfillment of the requirements for the degree of Doctor of Philosophy (N.E.P.).
References
- Kotenko, S.V.; Gallagher, G.; Baurin, V.V.; Lewis-Antes, A.; Shen, M.; Shah, N.K.; Langer, J. A.; Sheikh, F.; Dickensheets, H.; Donnelly, R.P. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 2003, 4, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, P.; Kindsvogel, W.; Xu, W.; Henderson, K.; Schlutsmeyer, S.; Whitmore, T.E.; Kuestner, R.; Garrigues, U.; Birks, C.; Roraback, J.; Ostrander, C.; Dong, D.; Shin, J.; Presnell, S.; Fox, B.; Haldeman, B.; Cooper, E.; Taft, D.; Gilbert, T.; Grant, F.J.; Tackett, M.; Krivan, W.; McKnight, G.; Clegg, C.; Foster, D.; Klucher, K.M. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 2003, 4, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.H.; Dellgren, C.; Hamming, O.J.; Vends, S.; Paludan, S.R.; Hartmann, R. Interferon-lambda Is Functionally an Interferon but Structurally Related to the Interleukin-10 Family. J. Biol. Chem. 2009, 284, 20869–20875. [Google Scholar] [CrossRef] [PubMed]
- Kotenko, S.V.; Izotova, L.S.; Mirochnitchenko, O.V.; Esterova, E.; Dickensheets, H.; Donnelly, R.P.; Pestka, S. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta ) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J. Biol. Chem. 2001, 276, 2725–2732. [Google Scholar] [CrossRef] [PubMed]
- Kotenko, S.V.; Krause, C.D.; Izotova, L.S.; Pollack, B.P.; Wu, W.; Pestka, S. Identification and functional characterization of a second chain of the interleukin-10 receptor complex. EMBO J. 1997, 16, 5894–5903. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Kunz, S.; Asadullah, K.; Sabat, R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J. Immunol. 2002, 168, 5397–5402. [Google Scholar] [PubMed]
- Sommereyns, C.; Paul, S.; Staeheli, P.; Michiels, T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008, 4, e1000017. [Google Scholar] [CrossRef] [PubMed]
- Witte, K.; Gruetz, G.; Volk, H.D.; Looman, A.C.; Asadullah, K.; Sterry, W.; Sabat, R.; Wolk, K. Despite IFN-lambda receptor expression, blood immune cells, but not keratinocytes or melanocytes, have an impaired response to type III interferons: implications for therapeutic applications of these cytokines. Genes Immun. 2009, 10, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Mordstein, M.; Kochs, G.; Dumoutier, L.; Renauld, J.C.; Paludan, S.R.; Klucher, K.; Staeheli, P. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses . PLoS Pathog. 2008, 4, e1000151. [Google Scholar] [CrossRef] [PubMed]
- Mordstein, M.; Neugebauer, E.; Ditt, V.; Jessen, B.; Rieger, T.; Falcone, V.; Sorgeloos, F.; Ehl, S.; Mayer, D.; Kochs, G.; Schwemmle, M.; Gunther, S.; Drosten, C.; Michiels, T.; Staeheli, P. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J. Virol. 2010, 84, 5670–5677. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Oberley-Deegan, R.; Wang, S.; Nikrad, M.; Funk, C.J.; Hartshorn, K.L.; Mason, R.J. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-lambda1) in response to influenza A infection. J. Immunol. 2009, 182, 1296–1304. [Google Scholar] [PubMed]
- Ank, N.; Iversen, M.B.; Bartholdy, C.; Staeheli, P.; Hartmann, R.; Jensen, U.B.; Dagnaes-Hansen, F.; Thomsen, A.R.; Chen, Z.; Haugen, H.; Klucher, K.; Paludan, S.R. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J. Immunol. 2008, 180, 2474–2485. [Google Scholar] [PubMed]
- Iversen, M.B.; Ank, N.; Melchjorsen, J.; Paludan, S.R. Expression of type III interferon (IFN) in the vaginal mucosa is mediated primarily by dendritic cells and displays stronger dependence on NF-kappaB than type I IFNs. J. Virol. 2010, 84, 4579–4586. [Google Scholar] [CrossRef] [PubMed]
- Coccia, E.M.; Severa, M.; Giacomini, E.; Monneron, D.; Remoli, M.E.; Julkunen, I.; Cella, M.; Lande, R.; Uze, G. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur. J. Immunol. 2004, 34, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, P.; Veckman, V.; Siren, J.; Klucher, K. M.; Hiscott, J.; Matikainen, S.; Julkunen, I. Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells. J. Virol. 2005, 79, 9608–9617. [Google Scholar] [CrossRef] [PubMed]
- Onoguchi, K.; Yoneyama, M.; Takemura, A.; Akira, S.; Taniguchi, T.; Namiki, H.; Fujita, T. Viral infections activate types I and III interferon genes through a common mechanism. J. Biol. Chem. 2007, 282, 7576–7581. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, X.; Wang, Y.J.; Zhou, Y.; Hu, S.; Ye, L.; Hou, W.; Li, H.; Ho, W.Z. Activation of toll-like receptor-3 induces interferon-lambda expression in human neuronal cells. Neuroscience 2009, 159, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Jouanguy, E.; Sancho-Shimizu, V.; von Bernuth, H.; Yang, K.; Abel, L.; Picard, C.; Puel, A.; Casanova, J.L. Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses . Immunol. Rev. 2007, 225–236. [Google Scholar] [CrossRef]
- Thomson, S.J.; Goh, F.G.; Banks, H.; Krausgruber, T.; Kotenko, S.V.; Foxwell, B.M.; Udalova, I.A. The role of transposable elements in the regulation of IFN-lambda1 gene expression. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 11564–11569. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, P.I.; Pietila, T.E.; Veckman, V.; Kotenko, S.V.; Julkunen, I. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J. Immunol. 2007, 179, 3434–3442. [Google Scholar] [PubMed]
- Ank, N.; West, H.; Bartholdy, C.; Eriksson, K.; Thomsen, A.R.; Paludan, S.R. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J. Virol. 2006, 80, 4501–4509. [Google Scholar] [CrossRef] [PubMed]
- Siren, J.; Pirhonen, J.; Julkunen, I.; Matikainen, S. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J. Immunol. 2005, 174, 1932–1937. [Google Scholar] [PubMed]
- Foy, E.; Li, K.; Wang, C.; Sumpter Jr., R.; Ikeda, M.; Lemon, S.M.; Gale Jr. , M. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease . Science 2003, 300, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Foy, E.; Li, K.; Sumpter, R.; Loo, Y.M.; Johnson, C.L.; Wang, C.; Fish, P.M.; Yoneyama, M.; Fujita, T.; Lemon, S.M.; Gale Jr., M. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling . Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 2986–2991. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Foy, E.; Ferreon, J.C.; Nakamura, M.; Ferreon, A.C.; Ikeda, M.; Ray, S.C.; Gale Jr., M.; Lemon, S.M. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF . Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 2992–2997. [Google Scholar] [CrossRef] [PubMed]
- Kaukinen, P.; Sillanpaa, M.; Kotenko, S.; Lin, R.; Hiscott, J.; Melen, K.; Julkunen, I. Hepatitis C virus NS2 and NS3/4A proteins are potent inhibitors of host cell cytokine/chemokine gene expression. Virol. J. 2006, 3, 66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lin, R.T.; Li, Y.; Douglas, S.D.; Maxcey, C.; Ho, C.; Lai, J. P.; Wang, Y.J.; Wan, Q.; Ho, W.Z. Hepatitis C virus inhibits intracellular interferon alpha expression in human hepatic cell lines. Hepatology 2005, 42, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Mihm, S.; Frese, M.; Meier, V.; Wietzke-Braun, P.; Scharf, J.G.; Bartenschlager, R.; Ramadori, G. Interferon type I gene expression in chronic hepatitis C. Lab. Invest. 2004, 84, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Dumoutier, L.; Tounsi, A.; Michiels, T.; Sommereyns, C.; Kotenko, S.V.; Renauld, J.C. Role of the Interleukin (IL)-28 Receptor Tyrosine Residues for Antiviral and Antiproliferative Activity of IL-29/Interferon-lambda1: Similarities with Type I Interferon Signaling. J. Biol. Chem. 2004, 279, 32269–32274. [Google Scholar] [CrossRef] [PubMed]
- Marcello, T.; Grakoui, A.; Barba-Spaeth, G.; Machlin, E.S.; Kotenko, S.V.; MacDonald, M. R.; Rice, C. M. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 2006, 131, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.E.; Schreckhise, H.; Khuu-Duong, K.; Henderson, K.; Rosler, R.; Storey, H.; Yao, L.; Liu, H.; Barahmand-pour, F.; Sivakumar, P.; Chan, C.; Birks, C.; Foster, D.; Clegg, C. H.; Wietzke-Braun, P.; Mihm, S.; Klucher, K.M. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology 2006, 44, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Lasfar, A.; Lewis-Antes, A.; Smirnov, S.V.; Anantha, S.; Abushahba, W.; Tian, B.; Reuhl, K.; Dickensheets, H.; Sheikh, F.; Donnelly, R.P.; Raveche, E.; Kotenko, S. V. Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma. Cancer Res. 2006, 66, 4468–4477. [Google Scholar] [CrossRef] [PubMed]
- Meager, A.; Visvalingam, K.; Dilger, P.; Bryan, D.; Wadhwa, M. Biological activity of interleukins-28 and -29: comparison with type I interferons. Cytokine 2005, 31, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, K.; Brand, S.; Baehs, S.; Goke, B.; Meinecke, J.; Spottl, G.; Meyer, H.; Auernhammer, C.J. Novel interferon-lambdas induce antiproliferative effects in neuroendocrine tumor cells. Biochem. Biophys. Res. Commun. 2006, 344, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Stark, G.R.; Kerr, I.M.; Williams, B.R.; Silverman, R.H.; Schreiber, R.D. How cells respond to interferons. Annu. Rev. Biochem. 1998, 67, 227–264. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.; Zitzmann, K.; Dambacher, J.; Beigel, F.; Olszak, T.; Vlotides, G.; Eichhorst, S.T.; Goke, B.; Diepolder, H.; Auernhammer, C.J. SOCS-1 inhibits expression of the antiviral proteins 2',5'-OAS and MxA induced by the novel interferon-lambdas IL-28A and IL-29. Biochem. Biophys. Res. Commun. 2005, 331, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.G.; Sheikh, F.; Scarzello, A.J.; Romero-Weaver, A.L.; Baker, D.P.; Donnelly, R.P.; Gamero, A.M. IFNalpha and IFNlambda differ in their antiproliferative effects and duration of JAK/STAT signaling activity. Cancer Biol. Ther. 2008, 7, 1109–1115. [Google Scholar] [PubMed]
- Zhou, Z.; Hamming, O.J.; Ank, N.; Paludan, S.R.; Nielsen, A.L.; Hartmann, R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J. Virol. 2007, 81, 7749–7758. [Google Scholar] [CrossRef] [PubMed]
- Pagliaccetti, N.E.; Eduardo, R.; Kleinstein, S.H.; Mu, X.J.; Bandi, P.; Robek, M.D. Interleukin-29 functions cooperatively with interferon to induce antiviral gene expression and inhibit hepatitis C virus replication. J. Biol. Chem. 2008, 283, 30079–30089. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.; Beigel, F.; Olszak, T.; Zitzmann, K.; Eichhorst, S.T.; Otte, J.M.; Diebold, J.; Diepolder, H.; Adler, B.; Auernhammer, C.J.; Goke, B.; Dambacher, J. IL-28A and IL-29 mediate antiproliferative and antiviral signals in intestinal epithelial cells and murine CMV infection increases colonic IL-28A expression . Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G960–G968. [Google Scholar] [CrossRef] [PubMed]
- Mukaida, N.; Harada, A.; Matsushima, K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998, 9, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Puel, A.; Zhang, S.; Eidenschenk, C.; Ku, C. L.; Casrouge, A.; Picard, C.; von Bernuth, H.; Senechal, B.; Plancoulaine, S.; Al-Hajjar, S.; Al-Ghonaium, A.; Marodi, L.; Davidson, D.; Speert, D.; Roifman, C.; Garty, B. Z.; Ozinsky, A.; Barrat, F.J.; Coffman, R.L.; Miller, R.L.; Li, X.; Lebon, P.; Rodriguez-Gallego, C.; Chapel, H.; Geissmann, F.; Jouanguy, E.; Casanova, J.L. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda Is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity 2005, 23, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Butera, M.; Nelson, D.R.; Liu, C. Novel type I interferon IL-28A suppresses hepatitis C viral RNA replication. Virol. J. 2005, 2, 80. [Google Scholar] [CrossRef]
- Hou, W.; Wang, X.; Ye, L.; Zhou, L.; Yang, Z.Q.; Riedel, E.; Ho, W.Z. Lambda interferon inhibits human immunodeficiency virus type 1 infection of macrophages. J. Virol. 2009, 83, 3834–3842. [Google Scholar] [CrossRef] [PubMed]
- Robek, M.D.; Boyd, B.S.; Chisari, F.V. Lambda Interferon Inhibits Hepatitis B and C Virus Replication. J. Virol. 2005, 79, 3851–3854. [Google Scholar] [CrossRef] [PubMed]
- Pagliaccetti, N.E.; Chu, E.N.; Bolen, C.R.; Kleinstein, S.H.; Robek, M.D. Lambda and alpha interferons inhibit hepatitis B virus replication through a common molecular mechanism but with different in vivo activities. Virology 2010, 401, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.M.; de Oliveira, D.B.; Magalhaes, C.L.; Bonjardim, C.A.; Ferreira, P.C.; Kroon, E. G. Antiviral activity of type I interferons and interleukins 29 and 28a (type III interferons) against Apeu virus. Antiviral Res. 2008, 80, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Cho, O.; Kim, K.; Shin, H.J.; Kotenko, S.V.; Park, S. Effect of interferon-lambda on replication of hepatitis B virus in human hepatoma cells. Virus Res. 2007, 126, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Jiang, D.; Qing, M.; Weidner, J.M.; Qu, X.; Guo, H.; Chang, J.; Gu, B.; Shi, P.Y.; Block, T.M.; Guo, J.T. Antiviral effect of interferon lambda against West Nile virus. Antiviral Res. 2009, 83, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Stoltz, M.; Ahlm, C.; Lundkvist, A.; Klingstrom, J. Lambda Interferon (IFN-λ) in Serum Is Decreased in Hantavirus-Infected Patients, and In Vitro-Established Infection Is Insensitive to Treatment with All IFNs and Inhibits IFN-γ-Induced Nitric Oxide Production. J. Virol. 2007, 81, 8685–8691. [Google Scholar] [CrossRef] [PubMed]
- Baize, S.; Pannetier, D.; Faure, C.; Marianneau, P.; Marendat, I.; Georges-Courbot, M.C.; Deubel, V. Role of interferons in the control of Lassa virus replication in human dendritic cells and macrophages. Microbes Infect. 2006, 8, 1194–1202. [Google Scholar] [CrossRef]
- Bartlett, N.W.; Buttigieg, K.; Kotenko, S.V.; Smith, G.L. Murine interferon lambdas (type III interferons) exhibit potent antiviral activity in vivo in a poxvirus infection model. J. Gen. Virol. 2005, 86, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Bandi, P.; Pagliaccetti, N.E.; Robek, M.D. Inhibition of type III interferon activity by orthopoxvirus immunomodulatory proteins. J. Interferon Cytokine Res. 2010, 30, 123–34. [Google Scholar] [CrossRef] [PubMed]
- Megjugorac, N.J.; Gallagher, G.E.; Gallagher, G. Modulation of human plasmacytoid DC function by IFN-lambda1 (IL-29). J. Leuko. Biol. 2009, 86, 1359–1363. [Google Scholar] [CrossRef]
- Mennechet, F.J.; Uze, G. Interferon-lambda-treated dendritic cells specifically induce proliferation of FOXP3-expressing suppressor T cells. Blood 2006, 107, 4417–4423. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Witte, K.; Witte, E.; Proesch, S.; Schulze-Tanzil, G.; Nasilowska, K.; Thilo, J.; Asadullah, K.; Sterry, W.; Volk, H. D.; Sabat, R. Maturing dendritic cells are an important source of IL-29 and IL-20 that may cooperatively increase the innate immunity of keratinocytes. J. Leukocyte Biol. 2008, 83, 1181–1193. [Google Scholar] [CrossRef]
- Jordan, W.J.; Eskdale, J.; Srinivas, S.; Pekarek, V.; Kelner, D.; Rodia, M.; Gallagher, G. Human interferon lambda-1 (IFN-lambda1/IL-29) modulates the Th1/Th2 response. Genes Immun. 2007, 8, 254–261. [Google Scholar] [CrossRef]
- Dai, J.; Megjugorac, N.J.; Gallagher, G.E.; Yu, R.Y.; Gallagher, G. IFN-lambda1 (IL-29) inhibits GATA3 expression and suppresses Th2 responses in human naive and memory T cells. Blood 2009, 113, 5829–5838. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.; Dai, J.; Eskdale, J.; Gallagher, G.E.; Megjugorac, N.J.; Gallagher, G. Interferon-lambda1 (interleukin-29) preferentially down-regulates interleukin-13 over other T helper type 2 cytokine responses in vitro. Immunology 2008, 125, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Jordan, W.J.; Eskdale, J.; Boniotto, M.; Rodia, M.; Kellner, D.; Gallagher, G. Modulation of the human cytokine response by interferon lambda-1 (IFN-lambda1/IL-29). Genes Immun. 2007, 8, 13–20. [Google Scholar] [CrossRef]
- Pekarek, V.; Srinivas, S.; Eskdale, J.; Gallagher, G. Interferon lambda-1 (IFN-lambda1/IL-29) induces ELR(-) CXC chemokine mRNA in human peripheral blood mononuclear cells, in an IFN-gamma-independent manner. Genes Immun. 2007, 8, 177–180. [Google Scholar] [CrossRef]
- Rauch, A.; Kutalik, Z.; Descombes, P.; Cai, T.; di Iulio, J.; Mueller, T.; Bochud, M.; Battegay, M.; Bernasconi, E.; Borovicka, J.; Colombo, S.; Cerny, A.; Dufour, J.F.; Furrer, H.; Gunthard, H.F.; Heim, M.; Hirschel, B.; Malinverni, R.; Moradpour, D.; Mullhaupt, B.; Witteck, A.; Beckmann, J.S.; Berg, T.; Bergmann, S.; Negro, F.; Telenti, A.; Bochud, P.Y. Genetic variation in IL28B Is Associated with Chronic Hepatitis C and Treatment Failure - A Genome-Wide Association Study. Gastroenterology 2009, 138, 1338–1345. [Google Scholar] [CrossRef]
- Suppiah, V.; Moldovan, M.; Ahlenstiel, G.; Berg, T.; Weltman, M.; Abate, M.L.; Bassendine, M.; Spengler, U.; Dore, G.J.; Powell, E.; Riordan, S.; Sheridan, D.; Smedile, A.; Fragomeli, V.; Muller, T.; Bahlo, M.; Stewart, G.J.; Booth, D.R.; George, J. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat. Genet. 2009, 41, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nishida, N.; Sugiyama, M.; Kurosaki, M.; Matsuura, K.; Sakamoto, N.; Nakagawa, M.; Korenaga, M.; Hino, K.; Hige, S.; Ito, Y.; Mita, E.; Tanaka, E.; Mochida, S.; Murawaki, Y.; Honda, M.; Sakai, A.; Hiasa, Y.; Nishiguchi, S.; Koike, A.; Sakaida, I.; Imamura, M.; Ito, K.; Yano, K.; Masaki, N.; Sugauchi, F.; Izumi, N.; Tokunaga, K.; Mizokami, M. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat. Genet. 2009, 41, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.L.; Thio, C.L.; Martin, M. P.; Qi, Y.; Ge, D.; O'Huigin, C.; Kidd, J.; Kidd, K.; Khakoo, S.I.; Alexander, G.; Goedert, J.J.; Kirk, G.D.; Donfield, S. M.; Rosen, H.R.; Tobler, L.H.; Busch, M.P.; McHutchison, J.G.; Goldstein, D.B.; Carrington, M. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009, 461, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Fellay, J.; Thompson, A.J.; Simon, J.S.; Shianna, K.V.; Urban, T.J.; Heinzen, E.L.; Qiu, P.; Bertelsen, A.H.; Muir, A.J.; Sulkowski, M.; McHutchison, J.G.; Goldstein, D.B. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009, 461, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Muir, A.J.; Sulkowski, M.S.; Ge, D.; Fellay, J.; Shianna, K.V.; Urban, T.; Afdhal, N.H.; Jacobson, I.M.; Esteban, R.; Poordad, F.; Lawitz, E.J.; McCone, J.; Shiffman, M. L.; Galler, G.W.; Lee, W.M.; Reindollar, R.; King, J.W.; Kwo, P.Y.; Ghalib, R.H.; Freilich, B.; Nyberg, L.M.; Zeuzem, S.; Poynard, T.; Vock, D.M.; Pieper, K.S.; Patel, K.; Tillmann, H.L.; Noviello, S.; Koury, K.; Pedicone, L.D.; Brass, C.A.; Albrecht, J.K.; Goldstein, D.B.; McHutchison, J.G. IL28B polymorphism improves viral kinetics and is the strongest pre-treatment predictor of sustained virologic response in genotype 1 hepatitic C virus. Gastroenterology 2010, 139, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Conjeevaram, H.S.; Fried, M.W.; Jeffers, L.J.; Terrault, N.A.; Wiley-Lucas, T.E.; Afdhal, N.; Brown, R.S.; Belle, S.H.; Hoofnagle, J.H.; Kleiner, D.E.; Howell, C.D. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology 2006, 131, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Sarasin-Filipowicz, M.; Oakeley, E.J.; Duong, F.H.; Christen, V.; Terracciano, L.; Filipowicz, W.; Heim, M.H. Interferon signaling and treatment outcome in chronic hepatitis C. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 7034–7039. [Google Scholar] [CrossRef] [PubMed]
- He, X.S.; Ji, X.; Hale, M.B.; Cheung, R.; Ahmed, A.; Guo, Y.; Nolan, G.P.; Pfeffer, L.M.; Wright, T. L.; Risch, N.; Tibshirani, R.; Greenberg, H. B. Global transcriptional response to interferon is a determinant of HCV treatment outcome and is modified by race. Hepatology 2006, 44, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Klucher, K.M.; Freeman, J.A.; Hausman, D.F.; Fontana, D.; Williams, D.E. Interferon lambda as a potential new therapeutic for hepatitis C. Ann. N.Y. Acad. Sci. 2009, 1182, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.J.; Shiffman, M.L.; Zaman, A.; Yoffe, B.; de la Torre, A.; Flamm, S.; Gordon, S.C.; Marotta, P.; Vierling, J.M.; Carlos Lopez-Talavera, J.; Byrnes-Blake, K.; Fontana, D.; Freeman, J.; Gray, T.; Hausman, D.; Hunder, N.N.; Lawitz, E. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection . Hepatology 2010. [Google Scholar]
- Fried, M.W. Side effects of therapy of hepatitis C and their management . Hepatology 2002, 36, S237–S244. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.