Primed for Discovery: Atomic-Resolution Cryo-EM Structure of a Reovirus Entry Intermediate
Abstract
:1. Introduction
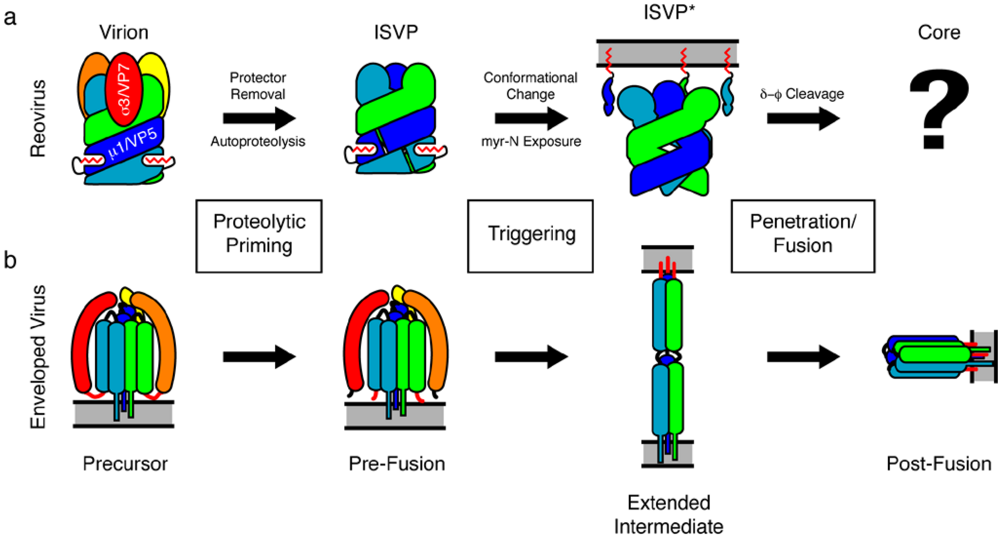
2. The Mechanism of Reovirus Membrane Penetration
3. Achieving High-Resolution Cryo-EM Virus Structures
4. Conclusions
Acknowledgments
References
- Zhang, X.; Jin, L.; Fang, Q.; Hui, W.H.; Zhou, Z.H. 3.3 A cryo-EM structure of a nonenveloped virus reveals a priming mechanism for cell entry . Cell 2010, 141, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Liemann, S.; Chandran, K.; Baker, T.S.; Nibert, M.L.; Harrison, S.C. Structure of the reovirus membrane-penetration protein, Mu1, in a complex with is protector protein, Sigma3. Cell 2002, 108, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Fang, Q.; Shah, S.; Atanasov, I.C.; Zhou, Z.H. Subnanometer-resolution structures of the grass carp reovirus core and virion. J. Mol. Biol. 2008, 382, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.H.; Deussing, J.; Peters, C.; Dermody, T.S. Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 2002, 277, 24609–24617. [Google Scholar] [CrossRef] [PubMed]
- Nibert, M.L.; Odegard, A.L.; Agosto, M.A.; Chandran, K.; Schiff, L.A. Putative autocleavage of reovirus mu1 protein in concert with outer-capsid disassembly and activation for membrane permeabilization. J. Mol. Biol. 2005, 345, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Odegard, A.L.; Chandran, K.; Zhang, X.; Parker, J.S.; Baker, T.S.; Nibert, M.L. Putative autocleavage of outer capsid protein micro1, allowing release of myristoylated peptide micro1N during particle uncoating, is critical for cell entry by reovirus. J. Virol. 2004, 78, 8732–8745. [Google Scholar] [CrossRef] [PubMed]
- Chandran, K.; Parker, J.S.; Ehrlich, M.; Kirchhausen, T.; Nibert, M.L. The delta region of outer-capsid protein micro 1 undergoes conformational change and release from reovirus particles during cell entry. J. Virol. 2003, 77, 13361–13375. [Google Scholar] [CrossRef] [PubMed]
- Ivanovic, T.; Agosto, M.A.; Zhang, L.; Chandran, K.; Harrison, S.C.; Nibert, M.L. Peptides released from reovirus outer capsid form membrane pores that recruit virus particles. EMBO J. 2008, 27, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Chandran, K.; Farsetta, D.L.; Nibert, M.L. Strategy for nonenveloped virus entry: a hydrophobic conformer of the reovirus membrane penetration protein micro 1 mediates membrane disruption. J. Virol. 2002, 76, 9920–9933. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Settembre, E.C.; Aoki, S.T.; Zhang, X.; Bellamy, A.R.; Dormitzer, P.R.; Harrison, S.C.; Grigorieff, N. Molecular interactions in rotavirus assembly and uncoating seen by high-resolution cryo-EM. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 10644–10648. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.T.; Settembre, E.C.; Trask, S.D.; Greenberg, H.B.; Harrison, S.C.; Dormitzer, P.R. Structure of rotavirus outer-layer protein VP7 bound with a neutralizing Fab. Science 2009, 324, 1444–1447. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Garcea, R.L.; Grigorieff, N.; Harrison, S.C. Subunit interactions in bovine papillomavirus. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 6298–6303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chipman, P.R.; Corver, J.; Johnson, P.R.; Zhang, Y.; Mukhopadhyay, S.; Baker, T.S.; Strauss, J.H.; Rossmann, M.G.; Kuhn, R.J. Visualization of membrane protein domains by cryo-electron microscopy of dengue virus. Nat. Struct. Biol. 2003, 10, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Grigorieff, N. SIGNATURE: a single-particle selection system for molecular electron microscopy. J. Struct. Biol. 2007, 157, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Grigorieff, N. FREALIGN: high-resolution refinement of single particle structures. J. Struct. Biol. 2007, 157, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Agosto, M.A.; Ivanovic, T.; Nibert, M.L. Mammalian reovirus, a nonfusogenic nonenveloped virus, forms size-selective pores in a model membrane. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 16496–16501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Agosto, M.A.; Ivanovic, T.; King, D.S.; Nibert, M.L.; Harrison, S.C. Requirements for the formation of membrane pores by the reovirus myristoylated micro1N peptide. J. Virol. 2009, 83, 7004–7014. [Google Scholar] [CrossRef] [PubMed]
- Lucic, V.; Forster, F.; Baumeister, W. Structural studies by electron tomography: from cells to molecules. Annu. Rev. Biochem. 2005, 74, 833–865. [Google Scholar] [CrossRef] [PubMed]
- Forster, F.; Medalia, O.; Zauberman, N.; Baumeister, W.; Fass, D. Retrovirus envelope protein complex structure in situ studied by cryo-electron tomography. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 4729–4734. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Share and Cite
Trask, S.D.; Guglielmi, K.M.; Patton, J.T. Primed for Discovery: Atomic-Resolution Cryo-EM Structure of a Reovirus Entry Intermediate. Viruses 2010, 2, 1340-1346. https://doi.org/10.3390/v2061340
Trask SD, Guglielmi KM, Patton JT. Primed for Discovery: Atomic-Resolution Cryo-EM Structure of a Reovirus Entry Intermediate. Viruses. 2010; 2(6):1340-1346. https://doi.org/10.3390/v2061340
Chicago/Turabian StyleTrask, Shane D., Kristen M. Guglielmi, and John T. Patton. 2010. "Primed for Discovery: Atomic-Resolution Cryo-EM Structure of a Reovirus Entry Intermediate" Viruses 2, no. 6: 1340-1346. https://doi.org/10.3390/v2061340
APA StyleTrask, S. D., Guglielmi, K. M., & Patton, J. T. (2010). Primed for Discovery: Atomic-Resolution Cryo-EM Structure of a Reovirus Entry Intermediate. Viruses, 2(6), 1340-1346. https://doi.org/10.3390/v2061340



