Dengue Virus Induces the Release of sCD40L and Changes in Levels of Membranal CD42b and CD40L Molecules in Human Platelets
Abstract
1. Introduction
2. Material and Methods
2.1. DENV-2 Stock Preparation and Titration
2.2. Obtention of Platelets from the Plasma of Healthy Donors
2.3. DENV-2 Stimulation Assays of Platelets
2.4. Cytometric Analysis of Molecules in Platelets
2.5. Evaluation of sCD40L Levels in Platelet Supernatants by ELISA Test
2.6. Multi-Array for Cytokine and Chemokine Quantification
2.7. Analysis of Platelets by Confocal Transmission Microscopy
2.8. Statistical Analysis
3. Results
3.1. Cytometric Analysis for Identification of Platelets
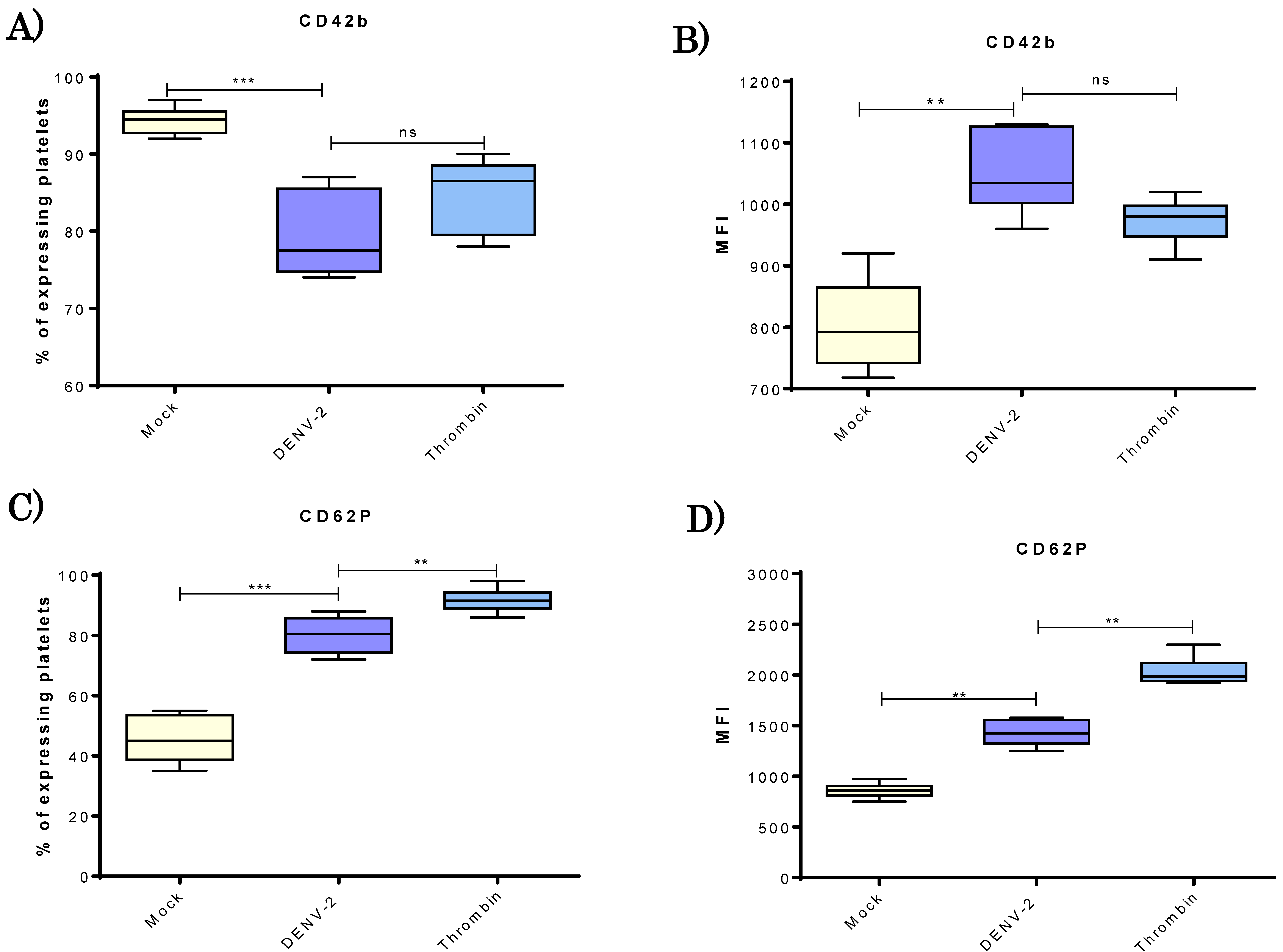
3.2. DENV-2 Induced Density of CD42b and CD62P Adhesion Molecules in Human Platelets
3.3. DENV-2-Exposed Platelets Released Higher Levels of GM-CSF, IL-6, IL-8, IL-10 and TNF-α.
3.4. DENV-2 Induced a Higher Display of MHC Class I Molecules in Platelets
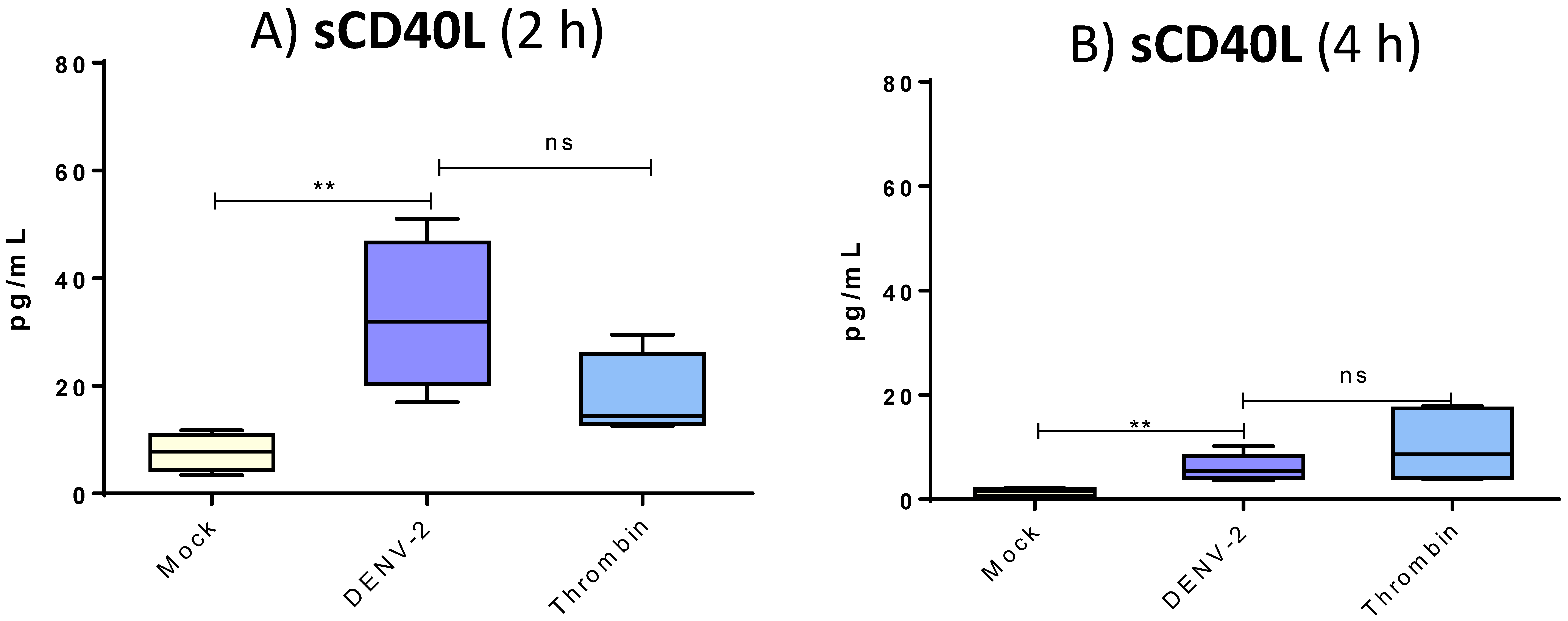
3.5. DENV-2 Induced Membranal Display of CD40L and Release of Its Soluble Form in Platelets
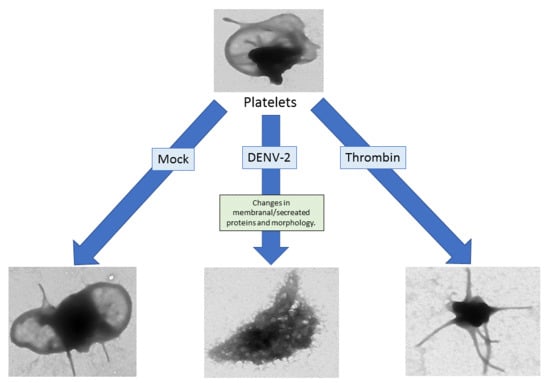
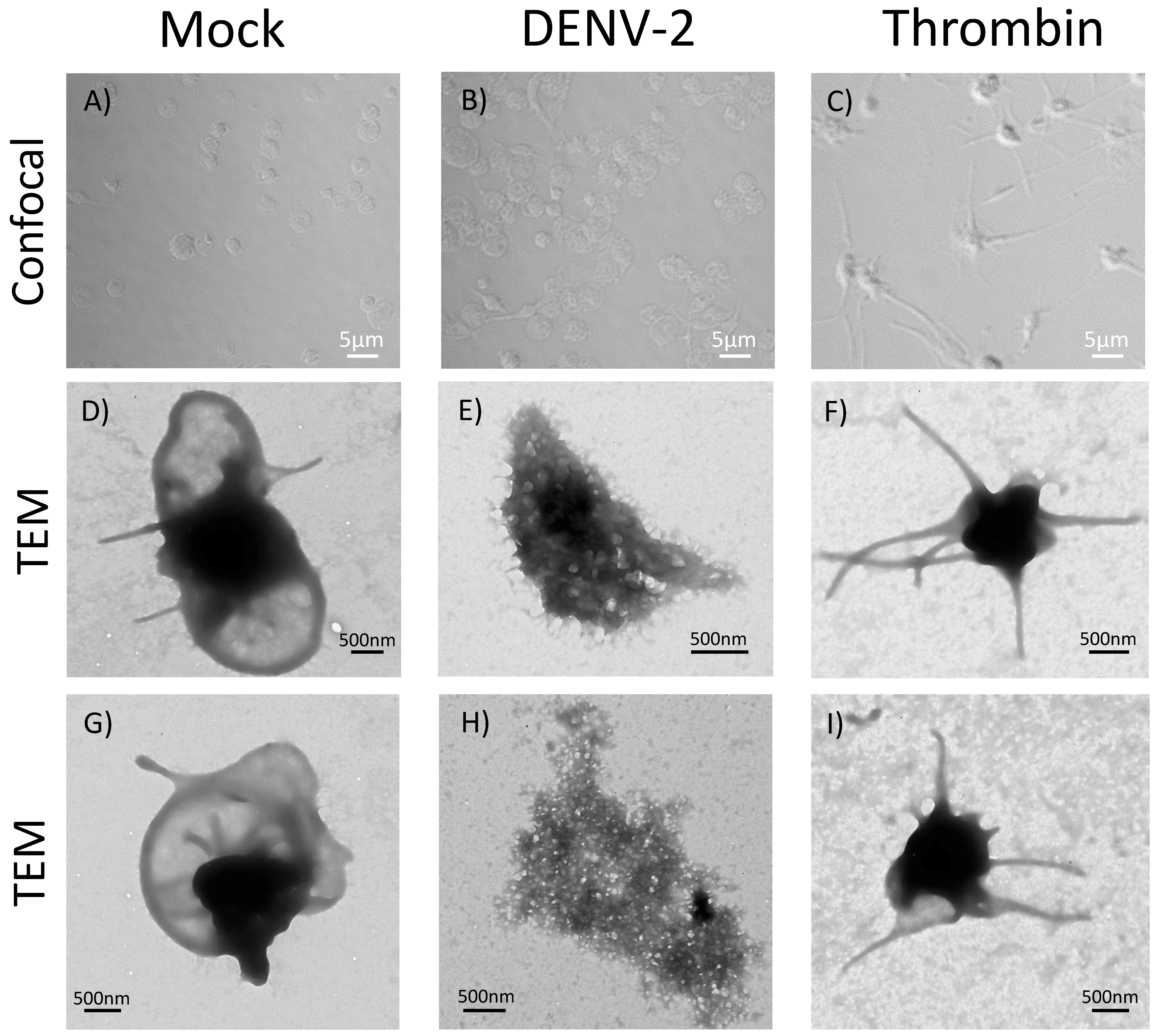
3.6. DENV-2-Induced Significant Morphological Changes in Human Platelets
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boulaftali, Y.; Hess, P.R.; Kahn, M.L.; Bergmeier, W. Platelet immunoreceptor tyrosine-based activation motif (ITAM) signaling and vascular integrity. Circ. Res. 2014, 114, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, S.; Tolley, N.D.; Dixon, D.A.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A.; Weyrich, A.S. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J. Cell Biol. 2001, 154, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Herter, J.M.; Rossaint, J.; Zarbock, A. Platelets in inflammation and immunity. J. Thromb. Haemost. 2014, 12, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
- Chapman, L.M.; Aggrey, A.A.; Field, D.J.; Srivastava, K.; Ture, S.; Yui, K.; Topham, D.J.; Baldwin, W.M., 3rd; Morrell, C.N. Platelets present antigen in the context of MHC class I. J. Immunol. 2012, 189, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Assinger, A. Platelets and infection—An emerging role of platelets in viral infection. Front. Immunol. 2014, 5, 649. [Google Scholar] [CrossRef] [PubMed]
- Chabert, A.; Hamzeh-Cognasse, H.; Pozzetto, B.; Cognasse, F.; Schattner, M.; Gomez, R.M.; Garraud, O. Human platelets and their capacity of binding viruses: Meaning and challenges? BMC Immunol. 2015, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Hottz Eugenio, D.; Bozza Fernando, A.; Bozza Patrícia, T. Platelets in immune response to virus and immunopathology of viral infections. Front. Med. 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Vieira-de-Abreu, A.; Campbell, R.A.; Weyrich, A.S.; Zimmerman, G.A. Platelets: Versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin. Immunopathol. 2012, 34, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Cognasse, F.; Nguyen, K.A.; Damien, P.; McNicol, A.; Pozzetto, B.; Hamzeh-Cognasse, H.; Garraud, O. The Inflammatory Role of Platelets via Their TLRs and Siglec Receptors. Front. Immunol. 2015, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Fujita, S.; Nakanishi, T.; Yokoi, T.; Shimamoto, K.; Miyamoto, R.; Ito, T. Platelet-derived microparticles cause CD154-dependent activation of dendritic cells. Platelets 2012, 23, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Sprague, D.L.; Elzey, B.D.; Crist, S.A.; Waldschmidt, T.J.; Jensen, R.J.; Ratliff, T.L. Platelet-mediated modulation of adaptive immunity: Unique delivery of CD154 signal by platelet-derived membrane vesicles. Blood 2008, 111, 5028–5036. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, R.; Benson, M.J.; de Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed]
- Kotowicz, K.; Dixon, G.L.; Klein, N.J.; Peters, M.J.; Callard, R.E. Biological function of CD40 on human endothelial cells: Costimulation with CD40 ligand and interleukin-4 selectively induces expression of vascular cell adhesion molecule-1 and P-selectin resulting in preferential adhesion of lymphocytes. Immunology 2000, 100, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Q.; Wei, R.L.; Cheng, J.W.; Cai, J.P.; Li, Y. The expression of intercellular adhesion molecule-1 induced by CD40-CD40L ligand signaling in orbital fibroblasts in patients with Graves’ ophthalmopathy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4652–4660. [Google Scholar] [CrossRef] [PubMed]
- Hammwohner, M.; Ittenson, A.; Dierkes, J.; Bukowska, A.; Klein, H.U.; Lendeckel, U.; Goette, A. Platelet expression of CD40/CD40 ligand and its relation to inflammatory markers and adhesion molecules in patients with atrial fibrillation. Exp. Biol. Med. 2007, 232, 581–589. [Google Scholar]
- Hottz, E.D.; Oliveira, M.F.; Nunes, P.C.; Nogueira, R.M.; Valls-de-Souza, R.; Da Poian, A.T.; Weyrich, A.S.; Zimmerman, G.A.; Bozza, P.T.; Bozza, F.A. Dengue induces platelet activation, mitochondrial dysfunction and cell death through mechanisms that involve DC-SIGN and caspases. J. Thromb. Haemost. 2013, 11, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Lopes, J.F.; Freitas, C.; Valls-de-Souza, R.; Oliveira, M.F.; Bozza, M.T.; Da Poian, A.T.; Weyrich, A.S.; Zimmerman, G.A.; Bozza, F.A.; et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood 2013, 122, 3405–3414. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Medeiros-de-Moraes, I.M.; Vieira-de-Abreu, A.; de Assis, E.F.; Vals-de-Souza, R.; Castro-Faria-Neto, H.C.; Weyrich, A.S.; Zimmerman, G.A.; Bozza, F.A.; Bozza, P.T. Platelet activation and apoptosis modulate monocyte inflammatory responses in dengue. J. Immunol. 2014, 193, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Zapata, J.C.; Cox, D.; Salvato, M.S. The role of platelets in the pathogenesis of viral hemorrhagic fevers. PLoS Negl. Trop. Dis. 2014, 8, e2858. [Google Scholar] [CrossRef] [PubMed]
- Hotzz, E.; Tolley, N.D.; Zimmerman, G.A.; Weyrich, A.S.; Bozza, F.A. Platelets in dengue infection. Hematology 2011, 8, e33–e38. [Google Scholar] [CrossRef]
- Hathirat, P.; Isarangkura, P.; Srichaikul, T.; Suvatte, V.; Mitrakul, C. Abnormal hemostasis in dengue hemorrhagic fever. Southeast Asian J. Trop. Med. Public Health 1993, 24 (Suppl. 1), 80–85. [Google Scholar] [PubMed]
- Alonzo, M.T.; Lacuesta, T.L.; Dimaano, E.M.; Kurosu, T.; Suarez, L.A.; Mapua, C.A.; Akeda, Y.; Matias, R.R.; Kuter, D.J.; Nagata, S.; et al. Platelet apoptosis and apoptotic platelet clearance by macrophages in secondary dengue virus infections. J. Infect. Dis. 2012, 205, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- John, D.V.; Lin, Y.S.; Perng, G.C. Biomarkers of severe dengue disease—A review. J. Biomed. Sci. 2015, 22, 83. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.; Cardosa, M.J.; Guzman, M.G. Of cascades and perfect storms: The immunopathogenesis of dengue haemorrhagic fever-dengue shock syndrome (DHF/DSS). Immunol. Cell Biol. 2007, 85, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Garraud, O.; Hamzeh-Cognasse, H.; Cognasse, F. Platelets and cytokines: How and why? Transfus. Clin. Biol. 2012, 19, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.-H.; Sim, E.-H.; Goh, R.-Y.; Park, J.-I.; Han, J.-Y. Platelet activation: The mechanisms and potential biomarkers. BioMed Res. Int. 2016, 2016, 9060143. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Mick, E.; Mikhalev, E.; Benjamin, E.J.; Tanriverdi, K.; Freedman, J.E. Sex differences in platelet toll-like receptors and their association with cardiovascular risk factors. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Kar, M.; Singla, M.; Chandele, A.; Kabra, S.K.; Lodha, R.; Medigeshi, G.R. Dengue Virus Entry and Replication Does Not Lead to Productive Infection in Platelets. Open Forum Infect. Dis. 2017, 4, ofx051. [Google Scholar] [CrossRef] [PubMed]
- Noisakran, S.; Onlammon, N.; Songprakhon, P.; Hsiao, H.M.; Chokephaibulkit, K.; Perng, G.C. Cell in dengue virus infection in vivo. Adv. Virol. 2010, 2010, 164878. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Nandi, D.; Batra, H.; Singhal, R.; Annarapu, G.K.; Bhattacharyya, S.; Seth, T.; Dar, L.; Medigeshi, G.R.; Vrati, S.; et al. Platelet activation determines the severity of thrombocitopenia in dengue infection. Sci. Rep. 2017, 7, 41697. [Google Scholar] [CrossRef] [PubMed]
- Trugilho, M.R.O.; Hottz, E.D.; Brunoro, G.V.F.; Teixeira-Ferreira, A.; Carvalho, P.C.; Salazar, G.A.; Zimmerman, G.A.; Bozza, F.A.; Bozza, P.T.; Perales, J. Platelet proteome reveals novel pathways of platelet activation and platelet-mediated immunoregulation in dengue. PLoS Pathog. 2017, 13, e1006385. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.J.; Clemetson, J.M. Platelet GPIb complex as a target for anti-thrombotic drug development. Thromb. Haemost. 2008, 99, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Bozza, F.A.; Cruz, O.G.; Zagne, S.M.; Azeredo, E.L.; Nogueira, R.M.; Assis, E.F.; Bozza, P.T.; Kubelka, C.F. Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect. Dis. 2008, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Henn, V.; Slupsky, J.R.; Grafe, M.; Anagnostopoulos, I.; Forster, R.; Muller-Berghaus, G.; Kroczek, R.A. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998, 391, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Tramontini Gomes de Sousa Cardozo, F.; Baimukanova, G.; Lanteri, M.C.; Keating, S.M.; Moraes Ferreira, F.; Heitman, J.; Pannuti, C.S.; Pati, S.; Romano, C.M.; Cerdeira Sabino, E. Serum from dengue virus-infected patients with and without plasma leakage differentially affects endothelial cells barrier function in vitro. PLoS ONE 2017, 12, e0178820. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Avellaneda, D.; Mosso-Pani, M.A.; Sánchez-Torres, L.E.; Castro-Mussot, M.E.; Corona-de la Peña, N.A.; Salazar, M.I. Dengue Virus Induces the Release of sCD40L and Changes in Levels of Membranal CD42b and CD40L Molecules in Human Platelets. Viruses 2018, 10, 357. https://doi.org/10.3390/v10070357
Núñez-Avellaneda D, Mosso-Pani MA, Sánchez-Torres LE, Castro-Mussot ME, Corona-de la Peña NA, Salazar MI. Dengue Virus Induces the Release of sCD40L and Changes in Levels of Membranal CD42b and CD40L Molecules in Human Platelets. Viruses. 2018; 10(7):357. https://doi.org/10.3390/v10070357
Chicago/Turabian StyleNúñez-Avellaneda, Daniel, Manuel Alejandro Mosso-Pani, Luvia E. Sánchez-Torres, María Eugenia Castro-Mussot, Norma Angélica Corona-de la Peña, and Ma. Isabel Salazar. 2018. "Dengue Virus Induces the Release of sCD40L and Changes in Levels of Membranal CD42b and CD40L Molecules in Human Platelets" Viruses 10, no. 7: 357. https://doi.org/10.3390/v10070357
APA StyleNúñez-Avellaneda, D., Mosso-Pani, M. A., Sánchez-Torres, L. E., Castro-Mussot, M. E., Corona-de la Peña, N. A., & Salazar, M. I. (2018). Dengue Virus Induces the Release of sCD40L and Changes in Levels of Membranal CD42b and CD40L Molecules in Human Platelets. Viruses, 10(7), 357. https://doi.org/10.3390/v10070357