Production of Bacteriophages by Listeria Cells Entrapped in Organic Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria, Phages, and Media
2.2. Alginate Gels and Cell Immobilisation by Entrapment
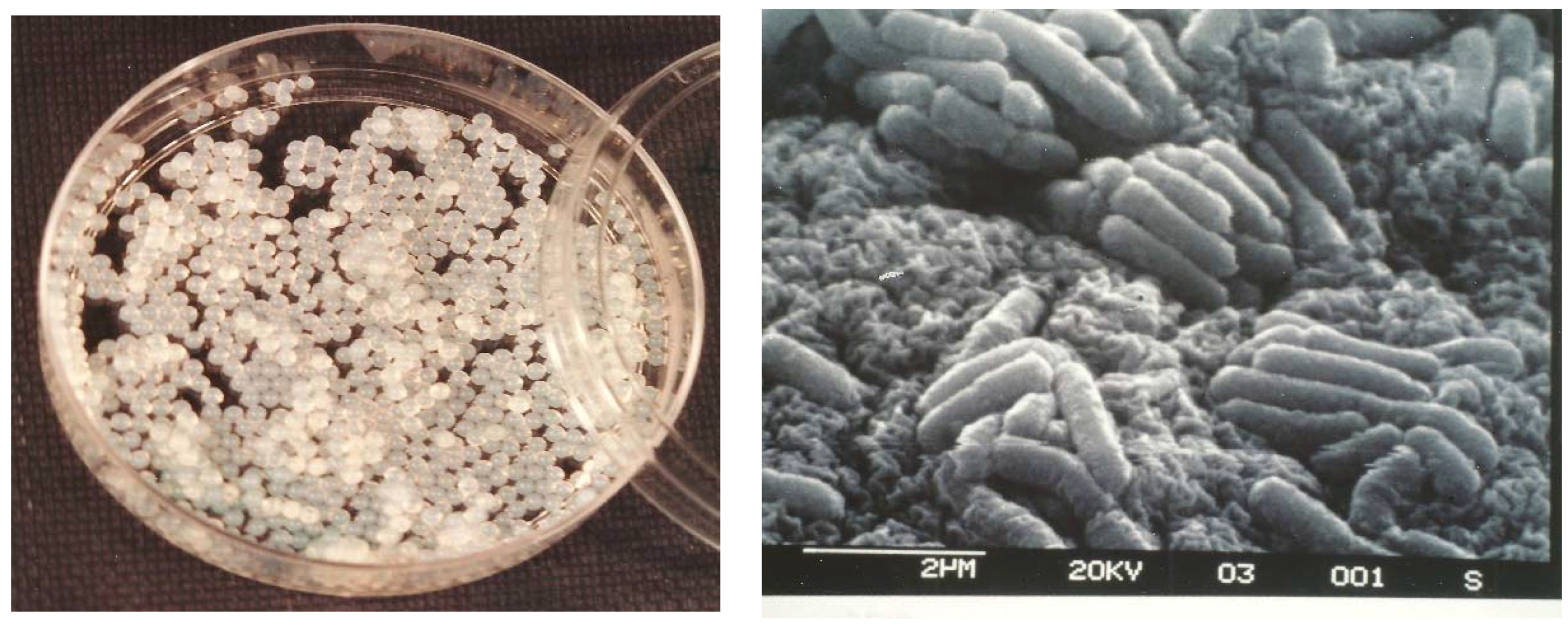
2.3. Morphology of Cells Immobilised in Beads
2.4. Phage Adsorption
2.5. Biomass Concentration
2.6. Phage Production
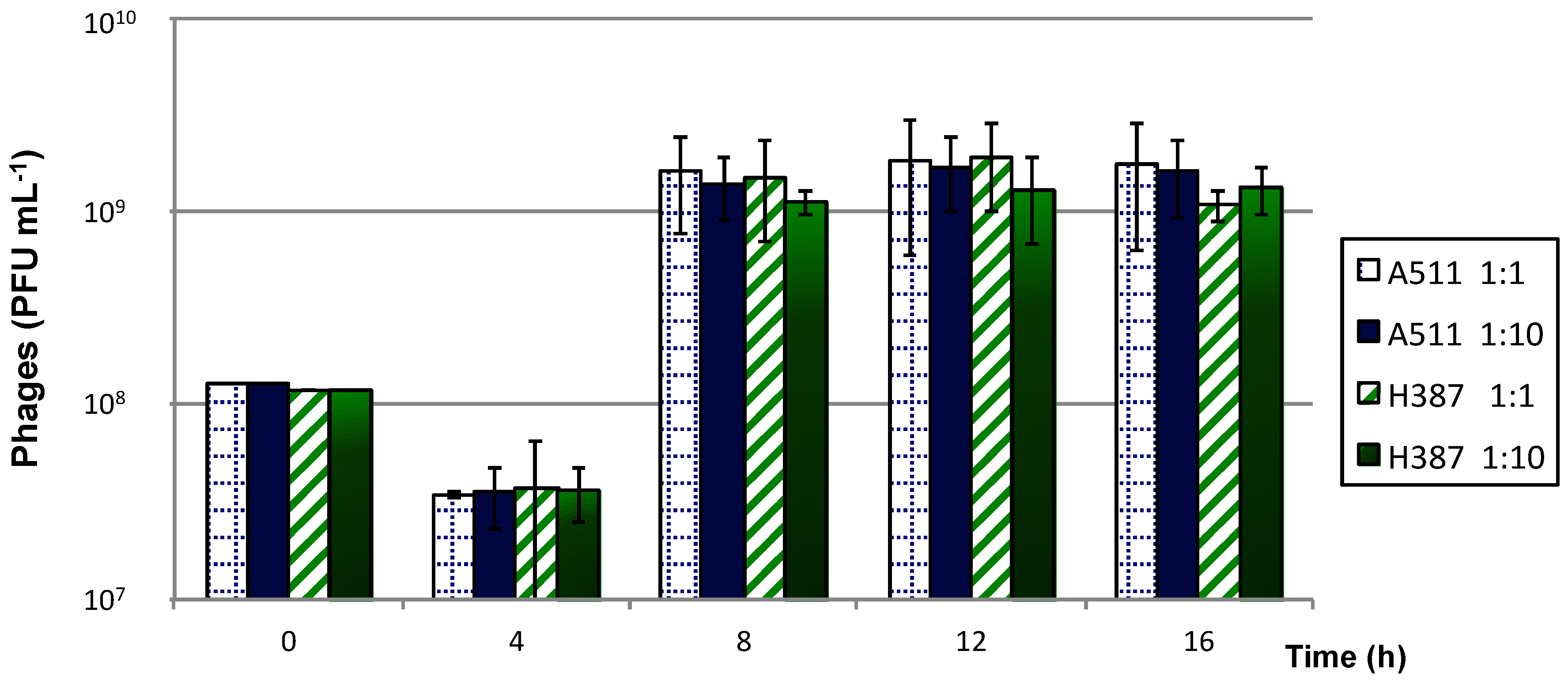
2.6.1. Free Cells
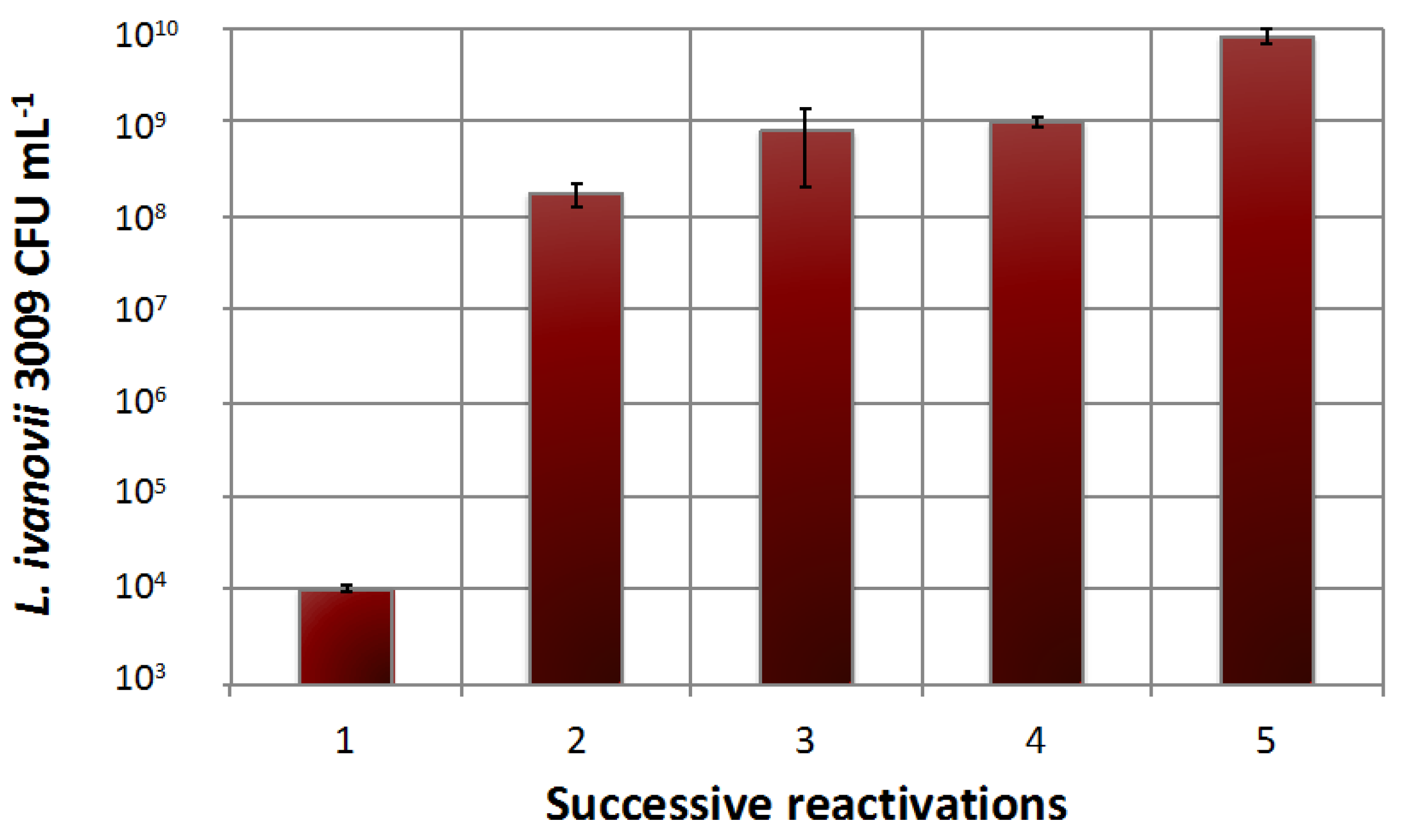
2.6.2. Immobilised Cells Used for Single and Successive Phage Propagations
2.7. Statistical Analysis
3. Results and Discussion
3.1. Morphological Observations
3.2. Phage Adsorption
3.3. Biomass Concentration
3.4. Phage Production
3.4.1. Free Cells
3.4.2. Immobilised Cells Used for Single and Successive Phage Propagations
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Frank, F.J.; Koffe, A.R. Surface-adherent growth of Listeria monocytogenes is associated with increased resistance to surfactant sanitizers and heat. J. Food Protect. 1989, 53, 550–554. [Google Scholar] [CrossRef]
- Directorate-General for Health and Consumers. Assessment of the Antibiotic Resistance Effects of Biocides, 28th Plenary; Commission Européenne, Scientific Committee on Emerging and Newly Identified Health Risks: Bruxelles, Belgium, 2009. [Google Scholar]
- Pearce, H.; Messager, S.; Maillard, J.-Y. Effect of biocides commonly used in the hospital environment on the transfer of antibiotic-resistance genes in Staphylococcus aureus. J. Hosp. Infect. 1999, 43, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and bacterial plant diseases. Front. Microbiol. 2017, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Stalin, N.; Srinivasan, P. Efficacy of potential phage cocktails against Vibrio harveyi and closely related Vibrio species isolated from shrimp aquaculture environment in the south east coast of India. Vet. Microbiol. 2017, 207, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Kim, Y.-T.; Ryu, S.; Lee, J.-H. Biocontrol and rapid detection of food-borne pathogens using bacteriophages and endolysins. Front. Microbiol. 2016, 7, 474. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T. Phage therapy of pulmonary infections. Bacteriophage 2015, 5, e1020260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagens, S.; Loessner, M.J. Phages of Listeria offer novel tools for diagnostics and biocontrol. Front. Microbiol. 2014, 5, 159. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Loessner, M.J.; Busse, M. Bacteriophage typing of Listeria species. Appl. Environ. Microbiol. 1990, 56, 1912–1918. [Google Scholar] [PubMed]
- Guenther, S.; Huwyler, D.; Richard, S.; Loessner, M.J. Virulent bacteriophages for biocontrol of Listeria monocytogenes in ready-to-eat foods. Appl. Environ. Microbiol. 2009, 75, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, J.; Dorscht, J.; Lurz, R.; Bielmann, R.; Wieland, M.; Zimmer, M.; Calendar, R.; Loessner, M.J. The terminally redundant, non-permuted genome of Listeria bacteriophage A511: A model for the SPO1-like myoviruses of Gram-positive bacteria. J. Bacteriol. 2008, 190, 5753–5765. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.N.G.; Figueiredo, A.C.L.; Miranda, F.A.; de Castro Almeida, R.C. Control of Listeria monocytogenes growth in soft cheeses by bacteriophage P100. Braz. J. Microbiol. 2014, 45, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Chaitiemwong, N.; Hazeleger, W.C.; Beumer, R.R. Inactivation of Listeria monocytogenes by disinfectants and bacteriophages in suspension and stainless-steel carrier tests. J. Food Protect. 2014, 77, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Chibeu, A.; Agius, L.; Gao, A.; Sabour, P.M.; Kropinski, A.M.; Balamurugan, S. Efficacy of bacteriophage LISTEX™P100 combined with chemical antimicrobials in reducing Listeria monocytogenes in cooked turkey and roast beef. Int. J. Food Microbiol. 2013, 167, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Miguéis, S.; Saraiva, C.; Esteves, A. Efficacy of LISTEX P100 at different concentrations for reduction of Listeria monocytogenes inoculated in sashimi. J. Food Protect. 2017, 12, 2094–2098. [Google Scholar] [CrossRef] [PubMed]
- Greer, G.G. Homologous bacteriophage control of Pseudomonas growth on beef spoilage. J. Food Protect. 1986, 49, 104–109. [Google Scholar] [CrossRef]
- Ganegama Arachchi, G.J.; Cridge, A.G.; Dias-Wanigasekera, B.M.; Cruz, C.D.; McIntyre, L.; Liu, R.; Flint, S.H.; Mutukumira, A.N. Effectiveness of phages in the decontamination of Listeria monocytogenes adhered to clean stainless steel, stainless steel coated with fish protein, and as a biofilm. J. Ind. Microbiol. Biotechnol. 2013, 10, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.A.; Chandry, P.S.; Kaur, M.; Kocharunchitt, C.; Bowman, J.P.; Fox, E.M. Novel biocontrol methods for Listeria monocytogenes biofilms in food production facilities. Front. Microbiol. 2018, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Ackermann, H.-W.; Pandian, S.; Picard, G.; Goulet, J. Biological inactivation of adhering Listeria monocytogenes by listeriaphages and a quaternary ammonium compound. Appl. Environ. Microbiol. 1993, 59, 2914–2917. [Google Scholar] [PubMed]
- Lee, S.; Kim, M.G.; Lee, H.S.; Heo, S.; Kwon, M.; Kim, G. Isolation and characterization of Listeria phages for control of growth of Listeria monocytogenes in milk. Korean J. Food Sci. Anim. Resour. 2017, 37, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Agboluaje, M.; Sauvageau, D. Bacteriophage production in bioreactors. Methods Mol. Biol. 2018, 1692, 173–193. [Google Scholar]
- Sargeant, K.; Yeo, K.G.; Lethbridge, J.H.; Shooter, K.V. Production of bacteriophage T7. Appl. Microbiol. 1968, 16, 1483–1488. [Google Scholar] [PubMed]
- Chen, B.Y.; Lim, H.C. Bioreactor studies on temperature induction of the Qmutant of bacteriophage λ in Escherichia coli. J. Biotechnol. 1996, 51, 1–20. [Google Scholar] [CrossRef]
- Sauvageau, D.; Cooper, D.G. Two-stage, self-cycling process for the production of bacteriophages. Microb. Cell Fact. 2010, 9, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Los, M.; Wegrzyn, G.; Neubauer, P. A role for bacteriophage T4 rI gene function in the control of phage development during pseudolysogeny and in slow growing host cells. Res. Microbiol. 2003, 154, 547–552. [Google Scholar] [CrossRef]
- Park, S.H.; Park, T.H. Analysis of two-stage continuous operation of Escherichia coli containing bacteriophage λ vector. Bioprocess Eng. 2000, 23, 187–190. [Google Scholar] [CrossRef]
- Chen, X.A.; Cen, P.L. A novel three-stage process for continuous production of penicillin G acylase by a temperature-sensitive expression system of Bacillus subtilis phage phi105. Chem. Biochem. Q. 2005, 19, 367–372. [Google Scholar]
- Oh, J.S.; Cho, D.; Park, T.H. Two-stage continuous operation of recombinant Escherichia coli using the bacteriophage λ Q-vector. Bioprocess Biosyst. Eng. 2005, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ziv, N.; Brandt, N.J.; Gresham, D. The use of chemostats in microbial systems biology. J. Vis. Exp. 2013, 80, 50168. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Levin, B.R.; Stewart, F.M. A complex community in a simple habitat: An experimental study with bacteria and phage. Ecology 1977, 58, 369–378. [Google Scholar] [CrossRef]
- Schwienhorst, A.; Lindemann, B.F.; Eigen, M. Growth kinetics of a bacteriophage in continuous culture. Biotechnol. Bioeng. 1996, 50, 217–221. [Google Scholar] [CrossRef]
- Nabergoj, D.; Kuzmić, N.; Drakslar, B.; Podgornik, A. Effect of dilution rate on productivity of continuous bacteriophage production in cellstat. Appl. Microbiol. Biotechnol. 2018, 102, 3649–3661. [Google Scholar] [CrossRef] [PubMed]
- Diviès, C.; Prévost, H.; Cavin, J.F. Les bactéries immobilisées dans l’industrie laitière. Process. Mag. 1989, 1041, 28–33. [Google Scholar]
- Gacesa, P. Alginates. Carbohydr. Polym. 1988, 53, 161–182. [Google Scholar] [CrossRef]
- Ackermann, H.-W.; Dubow, M.S. Phage multiplication. In Viruses of Prokaryotes; CRC Press, Inc.: Boca Raton, FL, USA, 1987; Volume 1, pp. 49–85. [Google Scholar]
- Mahony, J.; Tremblay, D.M.; Labrie, S.J.; Moineau, S.; van Sinderen, D. Investigating the requirement for calcium during lactococcal phage infection. Int. J. Food Microbiol. 2015, 201, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Gosmann, B.; Rehm, H.J. Influence of growth behaviour and physiology of alginate-entrapped microorganisms on the oxygen consumption. Appl. Microbiol. Biotechnol. 1988, 29, 554–559. [Google Scholar] [CrossRef]
- Cachon, R.; Catté, M.; Nommé, R.; Prévost, H.; Diviès, C. Kinetic behaviour of Lactococcus lactis ssp. lactis bv. diacetylactis immobilized in calcium alginate gel beads. Process Biochem. 1995, 6, 503–510. [Google Scholar]
- Prévost, H.; Diviès, C.; Rousseau, E. Continuous yoghurt production with Lactobacillus bulgaricus and Streptococcus thermophilus entrapped in Ca-alginate. Biotechnol. Lett. 1985, 4, 247–252. [Google Scholar] [CrossRef]
- Audet, P.; Paquin, C.; Lacroix, C. Immobilized growing lactic acid bacteria with κ-carrageenan—Locust bean gum gel. Appl. Microbiol. Biotechnol. 1988, 1, 11–18. [Google Scholar] [CrossRef]
- Steenson, L.R.; Klaenhammer, T.R.; Swaisgood, H.E. Calcium alginate-immobilized cultures of lactic streptococci are protected from bacteriophages. J. Dairy Sci. 1987, 70, 1121–1127. [Google Scholar] [CrossRef]
- Champagne, C.P.; Morin, N.; Couture, R.; Gagnon, C.; Jelen, P.; Lacroix, C. The potential of immobilized cell technology to produce freeze-dried, phage-protected cultures of Lactococcus lactis. Food Res. Intern. 1992, 25, 419–427. [Google Scholar] [CrossRef]
- Champagne, C.P.; Moineau, S.; Lafleur, S.; Savard, T. The effect of bacteriophages on the acidification of a vegetable juice medium by microencapsulated Lactobacillus plantarum. Food Microbiol. 2017, 63, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Matsumura, M.; Veliky, I.A. Diffusion characteristics of substrates in Ca alginate gel beads. Biotechnol. Bioeng. 1984, 1, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Zink, R.; Loessner, M.J. Classification of virulent and temperate bacteriophages of Listeria spp. on the basis of morphology and protein analysis. Appl. Environ. Microbiol. 1992, 58, 296–302. [Google Scholar] [PubMed]
- Ackermann, H.-W.; Dubow, M.S. Description and identification of new phages. In Viruses of Prokaryotes; CRC Press, Inc.: Boca Raton, FL, USA, 1987; Volume 1, pp. 103–142. [Google Scholar]
- Ortel, S.; Ackermann, H.-A. Morphologie von Neuen Listeriaphagen. Zentralbl. Bakteriol. Mikrobiol. Hyg. Ser. A Med. Microbiol. Infect. Dis. Virol. Parasitol. 1985, 260, 423–427. [Google Scholar]
- Adams, M.H. Methods of study of bacterial viruses. In Bacteriophages; Interscience Publishers, Inc.: New York, NY, USA, 1959; pp. 443–457. [Google Scholar]
- Boyaval, P.; Lebrun, A.; Goulet, J. Étude de l’immobilisation de Lactobacillus helveticus dans des billes d'alginate de calcium. Le Lait 1985, 65, 185–199. [Google Scholar] [CrossRef]
- Börner, R.A.; Aliaga, M.T.A.; Mattiasson, B. Microcultivation of anaerobic bacteria single cells entrapped in alginate microbeads. Biotechnol. Lett. 2013, 35, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, H.; Matsumura, M.; Tanaka, H. Oxygen diffusivity in gel beads containing viable cells. Biotechnol. Bioeng. 1989, 34, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-C.; Huang, C.-T. Effects of the growth of Trichosporon cutaneum in calcium alginate gel beads upon bead structure and oxygen transfer characteristics. Enzym. Microb. Technol. 1988, 10, 284–292. [Google Scholar] [CrossRef]
- Ogbonna, J.C.; Amano, Y.; Nakamura, K. Elucidation of optimum conditions for immobilization of viable cells by using calcium alginate. J. Ferment. Bioeng. 1989, 67, 92–96. [Google Scholar] [CrossRef]
- Klumpp, J.; Loessner, M.J. Listeria phages: Genomes, evolution, and application. Bacteriophage 2013, 3, e26861. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.-W. Bacteriophage taxonomy. Microbiol. Aust. 2011, 32, 90–94. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, B.; Philippe, C.; Loessner, M.J.; Goulet, J.; Moineau, S. Production of Bacteriophages by Listeria Cells Entrapped in Organic Polymers. Viruses 2018, 10, 324. https://doi.org/10.3390/v10060324
Roy B, Philippe C, Loessner MJ, Goulet J, Moineau S. Production of Bacteriophages by Listeria Cells Entrapped in Organic Polymers. Viruses. 2018; 10(6):324. https://doi.org/10.3390/v10060324
Chicago/Turabian StyleRoy, Brigitte, Cécile Philippe, Martin J. Loessner, Jacques Goulet, and Sylvain Moineau. 2018. "Production of Bacteriophages by Listeria Cells Entrapped in Organic Polymers" Viruses 10, no. 6: 324. https://doi.org/10.3390/v10060324




