Applications of the FIV Model to Study HIV Pathogenesis
Abstract
:1. Feline Immunodeficiency Virus
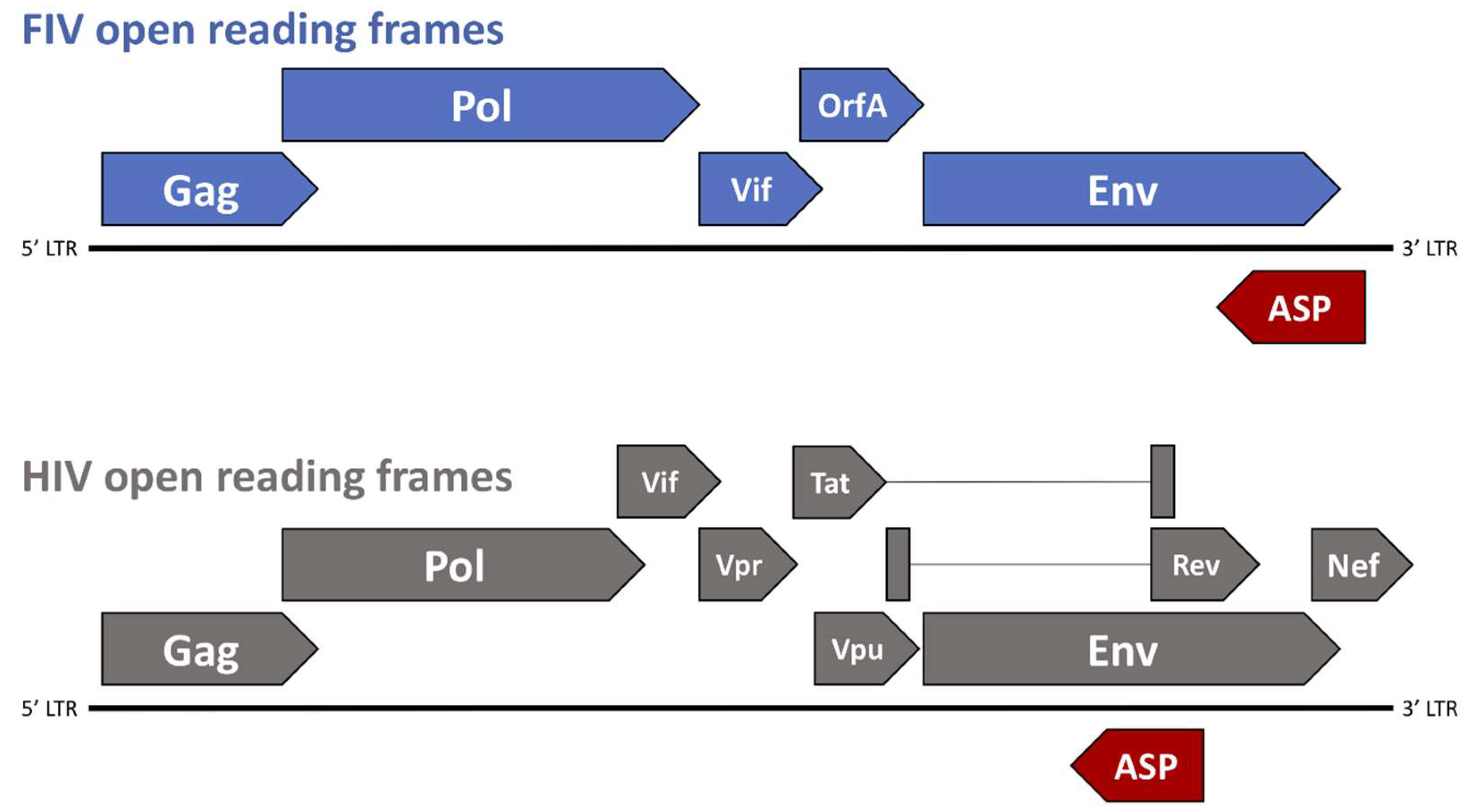
2. FIV as a Molecular Analogue to HIV
3. FIV as a Model to Study HIV Pathogenesis
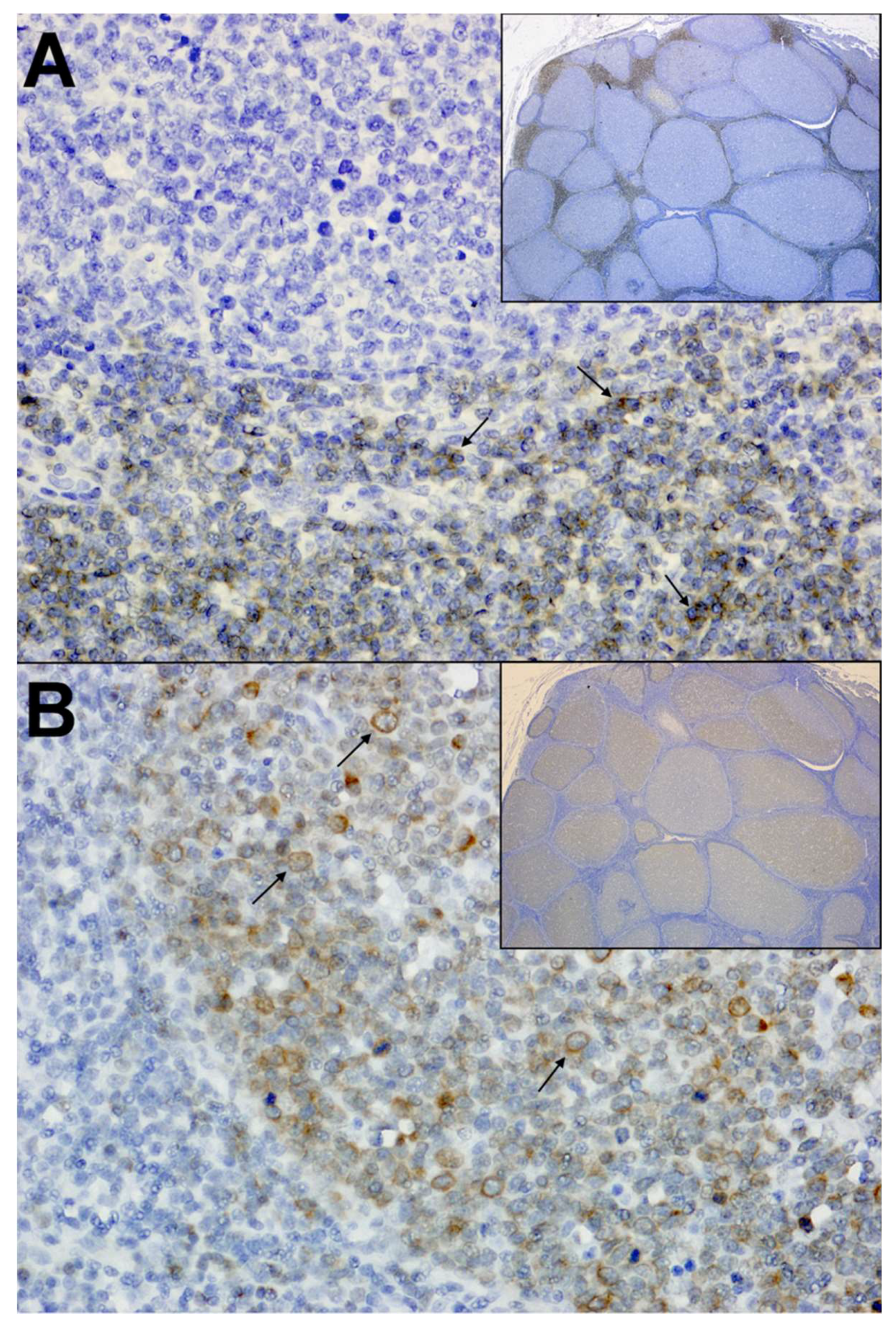
3.1. Immune Dysfunction
3.2. Neurologic Dysfunction
3.3. Vaccine Development
3.4. HIV-Induced Oral Disease
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Siebelink, K.H.; Chu, I.-H.; Rimmelzwaan, G.F.; Weijer, K.; van Herwijnen, R.; Knell, P.; Egberink, H.F.; Bosch, M.L.; Osterhaus, A.D. Feline immunodeficiency virus (FIV) infection in the cat as a model for HIV infection in man: Fiv-induced impairment of immune function. AIDS Res. Hum. Retrovir. 1990, 6, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Dean, G.A.; Himathongkham, S.; Sparger, E.E. Differential cell tropism of feline immunodeficiency virus molecular clones in vivo. J. Virol. 1999, 73, 2596–2603. [Google Scholar] [PubMed]
- English, R.V.; Johnson, C.M.; Gebhard, D.H.; Tompkins, M.B. In vivo lymphocyte tropism of feline immunodeficiency virus. J. Virol. 1993, 67, 5175–5186. [Google Scholar] [PubMed]
- Pedersen, N.; Yamamoto, J.K.; Ishida, T.; Hansen, H. Feline immunodeficiency virus infection. Vet. Immunol. Immunopathol. 1989, 21, 111–129. [Google Scholar] [CrossRef]
- Torten, M.; Franchini, M.; Barlough, J.E.; George, J.W.; Mozes, E.; Lutz, H.; Pedersen, N.C. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J. Virol. 1991, 65, 2225–2230. [Google Scholar] [PubMed]
- Hosie, M.J.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Lutz, H.; Marsilio, F. Feline immunodeficiency: Abcd guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Magden, E.; Miller, C.; MacMillan, M.; Bielefeldt-Ohmann, H.; Avery, A.; Quackenbush, S.L.; VandeWoude, S. Acute virulent infection with feline immunodeficiency virus (FIV) results in lymphomagenesis via an indirect mechanism. Virology 2013, 436, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C.; Ho, E.W.; Brown, M.L.; Yamamoto, J.K. Isolation of a t-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 1987, 235, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.H.; Lin, Y.-C.; Fink, E.; Grant, C.K. Feline immunodeficiency virus (FIV) as a model for study of lentivirus infections: Parallels with HIV. Curr. HIV Res. 2010, 8, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Burkhard, M.; Dean, G.A. Transmission and immunopathogenesis of fiv in cats as a model for HIV. Curr. HIV Res. 2003, 1, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Bęczkowski, P.M.; Litster, A.; Lin, T.L.; Mellor, D.J.; Willett, B.J.; Hosie, M.J. Contrasting clinical outcomes in two cohorts of cats naturally infected with feline immunodeficiency virus (FIV). Vet. Microbiol. 2015, 176, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Colitz, C.M. Feline uveitis: Diagnosis and treatment. Clin. Tech. Small Anim. Pract. 2005, 20, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Dow, S.W.; Poss, M.L.; Hoover, E.A. Feline immunodeficiency virus: A neurotropic lentivirus. J. Acquir. Immune Defic. Syndr. 1990, 3, 658–668. [Google Scholar] [PubMed]
- Fletcher, N.F.; Meeker, R.B.; Hudson, L.C.; Callanan, J.J. The neuropathogenesis of feline immunodeficiency virus infection: Barriers to overcome. Vet. J. 2011, 188, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Hopper, C.; Sparkes, A.; Gruffydd-Jones, T.; Crispin, S.; Muir, P.; Harbour, D.; Stokes, C. Clinical and laboratory findings in cats infected with feline immunodeficiency virus. Vet. Rec. 1989, 125, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Lappin, M. Opportunistic infections associated with retroviral infections in cats. Semin. Vet. Med. Surg. Small Anim. 1995, 10, 244–250. [Google Scholar] [PubMed]
- Meeker, R.B.; Hudson, L. Feline immunodeficiency virus neuropathogenesis: A model for HIV-induced cns inflammation and neurodegeneration. Vet. Sci. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Bielefeldt-Ohmann, H.; MacMillan, M.; Huitron-Resendiz, S.; Henriksen, S.; Elder, J.; VandeWoude, S. Strain-specific viral distribution and neuropathology of feline immunodeficiency virus. Vet. Immunol. Immunopathol. 2011, 143, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Boegler, K.; Carver, S.; MacMillan, M.; Bielefeldt-Ohmann, H.; VandeWoude, S. Pathogenesis of oral fiv infection. PLoS ONE 2017, 12, e0185138. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, A.P.; Franti, C.E.; Madewell, B.R.; Pedersen, N.C. Chronic oral infections of cats and their relationship to persistent oral carriage of feline calici-, immunodeficiency, or leukemia viruses. Vet. Immunol. Immunopathol. 1991, 29, 1–14. [Google Scholar] [CrossRef]
- Yamamoto, J.; Hansen, H.; Ho, E.; Morishita, T.; Okuda, T.; Sawa, T.; Nakamura, R.; Pedersen, N. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental united states and canada and possible mode of transmission. J. Am. Vet. Med. Assoc. 1989, 194, 213–220. [Google Scholar] [PubMed]
- Pedersen, N. Feline immunodeficiency virus infection. In Animal Models in AIDS: International TNO Meeting, Maastricht, The Netherlands, 23–26 October 1989; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1990; pp. 165–183. [Google Scholar]
- Del Fierro, G.; Meers, J.; Thomas, J.; Chadwick, B.; Park, H.; Robinson, W. Quantification of lymphadenopathy in experimentally induced feline immunodeficiency virus infection in domestic cats. Vet. Immunol. Immunopathol. 1995, 46, 3–12. [Google Scholar] [CrossRef]
- Bendinelli, M.; Pistello, M.; Lombardi, S.; Poli, A.; Garzelli, C.; Matteucci, D.; Ceccherini-Nelli, L.; Malvaldi, G.; Tozzini, F. Feline immunodeficiency virus: An interesting model for aids studies and an important cat pathogen. Clin. Microbiol. Rev. 1995, 8, 87–112. [Google Scholar] [PubMed]
- Lecollinet, S.; Richardson, J. Vaccination against the feline immunodeficiency virus: The road not taken. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 167–190. [Google Scholar] [CrossRef] [PubMed]
- Taniwaki, S.A.; Figueiredo, A.S.; Araujo, J.P., Jr. Virus–host interaction in feline immunodeficiency virus (FIV) infection. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Beatty, J.A.; Willett, B.J.; Gault, E.A.; Jarrett, O. A longitudinal study of feline immunodeficiency virus-specific cytotoxic t lymphocytes in experimentally infected cats, using antigen-specific induction. J. Virol. 1996, 70, 6199–6206. [Google Scholar] [PubMed]
- Guiot, A.-L.; Rigal, D.; Chappuis, G. Spontaneous programmed cell death (pcd) process of lymphocytes of fiv-infected cats: Cellular targets and modulation. Vet. Immunol. Immunopathol. 1997, 58, 93–106. [Google Scholar] [CrossRef]
- Hughes, M.; Ball, N.; Love, D.; Canfield, P.; Wigney, D.; Dawson, D.; Davis, P.; Malik, R. Disseminated mycobacterium genavense infection in a fiv-positive cat. J. Feline Med. Surg. 1999, 1, 23–29. [Google Scholar] [CrossRef]
- Poli, A.; Tozon, N.; Guidi, G.; Pistello, M. Renal alterations in feline immunodeficiency virus (FIV)-infected cats: A natural model of lentivirus-induced renal disease changes. Viruses 2012, 4, 1372–1389. [Google Scholar] [CrossRef] [PubMed]
- Diehl, L.J.; Mathiason-Dubard, C.K.; O’Neil, L.L.; Obert, L.A.; Hoover, E.A. Induction of accelerated feline immunodeficiency virus disease by acute-phase virus passage. J. Virol. 1995, 69, 6149–6157. [Google Scholar] [PubMed]
- Kornya, M.R.; Little, S.E.; Scherk, M.A.; Sears, W.C.; Bienzle, D. Association between oral health status and retrovirus test results in cats. J. Am. Vet. Med. Assoc. 2014, 245, 916–922. [Google Scholar] [CrossRef] [PubMed]
- De Rozières, S.; Mathiason, C.K.; Rolston, M.R.; Chatterji, U.; Hoover, E.A.; Elder, J.H. Characterization of a highly pathogenic molecular clone of feline immunodeficiency virus clade C. J. Virol. 2004, 78, 8971–8982. [Google Scholar] [CrossRef] [PubMed]
- Weese, S.J.; Nichols, J.; Jalali, M.; Litster, A. The oral and conjunctival microbiotas in cats with and without feline immunodeficiency virus infection. Vet. Res. 2015, 46, 21. [Google Scholar] [CrossRef] [PubMed]
- Mancianti, F.; Giannelli, C.; Bendinelli, M.; Poli, A. Mycological findings in feline immunodeficiency virus-infected cats. J. Med. Vet. Mycol. 1992, 30, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G. Leishmaniosis of companion animals in europe: An update. Vet. Parasitol. 2015, 208, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, A.; Hopper, C.; Millard, W.; Gruffydd-Jones, T.; Harbour, D. Feline immunodeficiency virus infection clinicopathologic findings in 90 naturally occurring cases. J. Vet. Intern. Med. 1993, 7, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K. Feline immunodeficiency virus infection: An overview. Vet. J. 1998, 155, 123–137. [Google Scholar] [CrossRef]
- Callanan, J.; Jones, B.; Irvine, J.; Willett, B.; McCandlish, I.; Jarrett, O. Histologic classification and immunophenotype of lymphosarcomas in cats with naturally and experimentally acquired feline immunodeficiency virus infections. Vet. Pathol. 1996, 33, 264–272. [Google Scholar] [CrossRef] [PubMed]
- English, R.; Nelson, P.; Johnson, C.M.; Nasisse, M.; Tompkins, W.A.; Tompkins, M.B. Development of clinical disease in cats experimentally infected with feline immunodeficiency virus. J. Infect. Dis. 1994, 170, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Gabor, L.; Love, D.; Malik, R.; Canfield, P. Feline immunodeficiency virus status of australian cats with lymphosarcoma. Aust. Vet. J. 2001, 79, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Beatty, J. Viral causes of feline lymphoma: Retroviruses and beyond. Vet. J. 2014, 201, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Cho, K.-W.; Nishigaki, K.; Momoi, Y.; Nishimura, Y.; Mizuno, T.; Goto, Y.; Watari, T.; Tsujimoto, H.; Hasegawa, A. Molecular characteristics of malignant lymphomas in cats naturally infected with feline immunodeficiency virus. Vet. Immunol. Immunopathol. 1997, 57, 153–167. [Google Scholar] [CrossRef]
- Shiramizu, B.; Herndier, B.G.; McGrath, M.S. Identification of a common clonal human immunodeficiency virus integration site in human immunodeficiency virus-associated lymphomas. Cancer Res. 1994, 54, 2069–2072. [Google Scholar] [PubMed]
- Beatty, J.; Lawrence, C.; Callanan, J.; Grant, C.; Gault, E.; Neil, J.; Jarrett, O. Feline immunodeficiency virus (FIV)-associated lymphoma: A potential role for immune dysfunction in tumourigenesis. Vet. Immunol. Immunopathol. 1998, 65, 309–322. [Google Scholar] [CrossRef]
- Yamamoto, H.; Umemura, T.; Inoshima, Y.; Nakamura, M.; Adachi, I.; Miyazawa, T.; Mikami, T. Immunological and histological disorders in cats experimentally infected with feline immunodeficiency virus subtype b (TM2 strain). Vet. Microbiol. 1997, 57, 313–324. [Google Scholar] [CrossRef]
- Poli, A.; Abramo, F.; Taccini, E.; Guidi, G.; Barsotti, E.; Bendinelli, M.; Malvaldi, G. Renal involvement in feline immunodeficiency virus infection: A clinicopathological study. Nephron 1993, 64, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Takemura, N.; Sako, T.; Koyama, H.; Motoyoshi, S.; Inada, Y. Serum concentration of circulating immune complexes in cats infected with feline immunodeficiency virus detected by immune adherence hemagglutination method. J. Vet. Med. Sci. 1997, 59, 395–396. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Falcone, M.; Bigalli, L.; Massi, C.; Hofmann-Lehmann, R.; Lombardi, S.; Bendinelli, M.; Lutz, H. Circulating immune complexes and analysis of renal immune deposits in feline immunodeficiency virus-infected cats. Clin. Exp. Immunol. 1995, 101, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Burdo, T.H.; Miller, A.D. Animal models of HIV peripheral neuropathy. Future Virol. 2014, 9, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Podell, M.; March, P.A.; Buck, W.R.; Mathes, L.E. The feline model of neuroaids: Understanding the progression towards aids dementia. J. Psychopharmacol. 2000, 14, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Antony, J.; Liu, S.; Martinez, J.A.; Giuliani, F.; Zochodne, D.; Power, C. CD8+ lymphocyte-mediated injury of dorsal root ganglion neurons during lentivirus infection: CD154-dependent cell contact neurotoxicity. J. Neurosci. 2006, 26, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- Power, C.; Buist, R.; Johnston, J.; Del Bigio, M.; Ni, W.; Dawood, M.; Peeling, J. Neurovirulence in feline immunodeficiency virus-infected neonatal cats is viral strain specific and dependent on systemic immune suppression. J. Virol. 1998, 72, 9109–9115. [Google Scholar] [PubMed]
- Abramo, F.; BO, S.; Canese, M.G.; Poli, A. Regional distribution of lesions in the central nervous system of cats infected with feline immunodeficiency virus. AIDS Res. Hum. Retrovir. 1995, 11, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Steigerwald, E.S.; Sarter, M.; March, P.; Podell, M. Effects of feline immunodeficiency virus on cognition and behavioral function in cats. J. Acquir. Immune Defic. Syndr. 1999, 20, 411–419. [Google Scholar] [CrossRef]
- Maingat, F.; Vivithanaporn, P.; Zhu, Y.; Taylor, A.; Baker, G.; Pearson, K.; Power, C. Neurobehavioral performance in feline immunodeficiency virus infection: Integrated analysis of viral burden, neuroinflammation, and neuronal injury in cortex. J. Neurosci. 2009, 29, 8429–8437. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.; Prospero-Garcia, O.; Wheeler, D.; Wagaman, P.; Lerner, D.; Fox, H.; Whalen, L.; Bloom, F.; Elder, J.; Henriksen, S. Neurologic dysfunctions caused by a molecular clone of feline immunodeficiency virus, FIV-PPR. J. Neurovirol. 1996, 2, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Phipps, A.J.; Hayes, K.A.; Buck, W.R.; Podell, M.; Mathes, L.E. Neurophysiologic and immunologic abnormalities associated with feline immunodeficiency virus molecular clone FIV-PPR DNA inoculation. J. Acquir. Immune Defic. Syndr. 1999 2000, 23, 8–16. [Google Scholar] [CrossRef]
- Sparger, E.E. Fiv as a model for HIV: An overview. In In Vivo Models of HIV Disease and Control; Springer: Berlin, Germany, 2006; pp. 149–237. [Google Scholar]
- Kenyon, J.C.; Lever, A.M. The molecular biology of feline immunodeficiency virus (FIV). Viruses 2011, 3, 2192–2213. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.; Dombrowski, J.; O’Connor, T.; Montelaro, R.C.; Tonelli, Q.; Lawrence, K.; Seymour, C.; Goodness, J.; Pedersen, N.C.; Andersen, P.R. Biochemical and immunological characterization of the major structural proteins of feline immunodeficiency virus. J Gen. Virol. 1990, 71, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.-Y.; Fink, E.; Hong, Y.; Wang, C.; Grant, C.K.; Elder, J.H. Fine definition of the cxcr4-binding region on the v3 loop of feline immunodeficiency virus surface glycoprotein. PLoS ONE 2010, 5, e10689. [Google Scholar] [CrossRef] [PubMed]
- Egberink, H.F.; Ederveen, J.; Montelaro, R.C.; Pedersen, N.C.; Horzinek, M.C.; Koolen, M.J. Intracellular proteins of feline immunodeficiency virus and their antigenic relationship with equine infectious anaemia virus proteins. J. Gen. Virol. 1990, 71, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.; Schnölzer, M.; Hasselkus-Light, C.; Henson, M.; Lerner, D.; Phillips, T.; Wagaman, P.; Kent, S. Identification of proteolytic processing sites within the gag and pol polyproteins of feline immunodeficiency virus. J. Virol. 1993, 67, 1869–1876. [Google Scholar] [PubMed]
- Von der Helm, K. Retroviral proteases: Structure, function and inhibition-from a non-anticipated viral enzyme to the target of a most promising HIV therapy. Biol. Chem. Hoppe Seyler 1996, 377, 765–774. [Google Scholar]
- Gadsden, M.H.; McIntosh, E.; Game, J.C.; Wilson, P.J.; Haynes, R. Dutp pyrophosphatase is an essential enzyme in saccharomyces cerevisiae. EMBO J. 1993, 12, 4425–4431. [Google Scholar] [PubMed]
- Khan, E.; Mack, J.P.; Katz, R.A.; Kulkosky, J.; Skalka, A.M. Retroviral integrase domains: DNA binding and the recognition of ltr sequences. Nucleic Acids Res. 1991, 19, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Vink, C.; van der Linden, K.H.; Plasterk, R. Activities of the feline immunodeficiency virus integrase protein produced in escherichia coli. J. Virol. 1994, 68, 1468–1474. [Google Scholar] [PubMed]
- North, T.W.; Cronn, R.C.; Remington, K.M.; Tandberg, R.T.; Judd, R.C. Characterization of reverse transcriptase from feline immunodeficiency virus. J. Biol. Chem. 1990, 265, 5121–5128. [Google Scholar] [PubMed]
- Foley, B.T.; Leitner, T.K.; Apetrei, C.; Hahn, B.; Mizrachi, I.; Mullins, J.; Rambaut, A.; Wolinsky, S.; Korber, B.T.M. HIV Sequence Compendium 2015; Los Alamos National Lab.(LANL): Los Alamos, NM, USA, 2015. [Google Scholar]
- De Parseval, A.; Elder, J.H. Binding of recombinant feline immunodeficiency virus surface glycoprotein to feline cells: Role of cxcr4, cell-surface heparans, and an unidentified non-cxcr4 receptor. J. Virol. 2001, 75, 4528–4539. [Google Scholar] [CrossRef] [PubMed]
- De Parseval, A.; Ngo, S.; Sun, P.; Elder, J.H. Factors that increase the effective concentration of cxcr4 dictate feline immunodeficiency virus tropism and kinetics of replication. J. Virol. 2004, 78, 9132–9143. [Google Scholar] [CrossRef] [PubMed]
- De Parseval, A.; Chatterji, U.; Sun, P.; Elder, J.H. Feline immunodeficiency virus targets activated cd4+ t cells by using CD134 as a binding receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 13044–13049. [Google Scholar] [CrossRef] [PubMed]
- De Parseval, A.; Grant, C.K.; Sastry, K.J.; Elder, J.H. Sequential CD134-CXCR4 interactions in feline immunodeficiency virus (FIV): Soluble CD134 activates FIV Env for CXCR4-dependent entry and reveals a cryptic neutralization epitope. J. Virol. 2006, 80, 3088–3091. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, M.; White, R.L.; de Parseval, A.; Sastry, K.J.; Morris, G.; Grant, C.K.; Elder, J.H. Mapping of the cxcr4 binding site within variable region 3 of the feline immunodeficiency virus surface glycoprotein. J. Virol. 2008, 82, 9134–9142. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Fuller, F.J.; Tompkins, W.A. Mechanism of feline immunodeficiency virus envelope glycoprotein-mediated fusion. Virology 2004, 321, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Dean, G.A.; Reubel, G.H.; Moore, P.F.; Pedersen, N.C. Proviral burden and infection kinetics of feline immunodeficiency virus in lymphocyte subsets of blood and lymph node. J. Virol. 1996, 70, 5165–5169. [Google Scholar] [PubMed]
- Willett, B.J.; Hosie, M.J. Chemokine receptors and co-stimulatory molecules: Unravelling feline immunodeficiency virus infection. Vet. Immunol. Immunopathol. 2008, 123, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hosie, M.J.; Pajek, D.; Samman, A.; Willett, B.J. Feline immunodeficiency virus (FIV) neutralization: A review. Viruses 2011, 3, 1870–1890. [Google Scholar] [CrossRef] [PubMed]
- Willett, B.J.; Hosie, M.J. The virus–receptor interaction in the replication of feline immunodeficiency virus (FIV). Curr. Opin. in Virol. 2013, 3, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.A.; Murphy, P.M.; Farber, J.M. Chemokine receptors as HIV-1 coreceptors: Roles in viral entry, tropism, and disease. Ann. Rev. Immunol. 1999, 17, 657–700. [Google Scholar] [CrossRef] [PubMed]
- Doms, R.W. Chemokine receptors and HIV entry. AIDS 2001, 15, S34–S35. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.H.; Sundstrom, M.; de Rozieres, S.; de Parseval, A.; Grant, C.K.; Lin, Y.-C. Molecular mechanisms of fiv infection. Vet. Immunol. Immunopathol. 2008, 123, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Mosier, D.E. How HIV changes its tropism: Evolution and adaptation? Curr. Opin. HIV AIDS 2009, 4, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Peters, P.J.; Duenas-Decamp, M.J.; Sullivan, W.M.; Brown, R.; Ankghuambom, C.; Luzuriaga, K.; Robinson, J.; Burton, D.R.; Bell, J.; Simmonds, P. Variation in HIV-1 R5 macrophage-tropism correlates with sensitivity to reagents that block envelope: CD4 interactions but not with sensitivity to other entry inhibitors. Retrovirology 2008, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Clapham, P.R.; McKnight, A. HIV-1 receptors and cell tropism. Br. Med. Bull. 2001, 58, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Regoes, R.R.; Bonhoeffer, S. The HIV coreceptor switch: A population dynamical perspective. Trends Microbiol. 2005, 13, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, K.; Mikami, T. Molecular biology of the feline immunodeficiency virus auxiliary genes. J. Gen. Virol. 1996, 77, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- LaRue, R.S.; Lengyel, J.; Jónsson, S.R.; Andrésdóttir, V.; Harris, R.S. Lentiviral vif degrades the apobec3z3/apobec3h protein of its mammalian host and is capable of cross-species activity. J. Virol. 2010, 84, 8193–8201. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, M.; Chatterji, U.; Schaffer, L.; de Rozières, S.; Elder, J.H. Feline immunodeficiency virus orfa alters gene expression of splicing factors and proteasome-ubiquitination proteins. Virology 2008, 371, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Gemeniano, M.C.; Sawai, E.T.; Leutenegger, C.M.; Sparger, E.E. Feline immunodeficiency virus orf-a is required for virus particle formation and virus infectivity. J. Virol. 2003, 77, 8819–8830. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.; De Parseval, A.; Lerner, D.; Neil, J.; Thompson, F.; Elder, J. Influence of ORF2 on host cell tropism of feline immunodeficiency virus. Virology 1996, 215, 10–16. [Google Scholar] [CrossRef] [PubMed]
- De Parseval, A.; Elder, J.H. Demonstration that Orf2 encodes the feline immunodeficiency virus transactivating (TAT) protein and characterization of a unique gene product with partial rev activity. J. Virol. 1999, 73, 608–617. [Google Scholar] [PubMed]
- Gemeniano, M.C.; Sawai, E.T.; Sparger, E.E. Feline immunodeficiency virus Orf-A localizes to the nucleus and induces cell cycle arrest. Virology 2004, 325, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Fink, E.; Hu, Q.-Y.; Kiosses, W.B.; Elder, J.H. Orfa downregulates feline immunodeficiency virus primary receptor cd134 on the host cell surface and is important in viral infection. J. Virol. 2010, 84, 7225–7232. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.H. Human immunodeficiency virus may encode a novel protein on the genomic DNA plus strand. Science 1988, 239, 1420–1422. [Google Scholar] [CrossRef] [PubMed]
- Briquet, S.; Vaquero, C. Immunolocalization studies of an antisense protein in HIV-1-infected cells and viral particles. Virology 2002, 292, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Bukrinsky, M.I.; Etkin, A.F. Plus strand of the HIV provirus DNA is expressed at early stages of infection. AIDS Res. Hum. Retrovir. 1990, 6, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi-Ishihara, M.; Yamagishi, M.; Hara, T.; Matsuda, Y.; Takahashi, R.; Miyake, A.; Nakano, K.; Yamochi, T.; Ishida, T.; Watanabe, T. HIV-1-encoded antisense rna suppresses viral replication for a prolonged period. Retrovirology 2012, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Landry, S.; Halin, M.; Lefort, S.; Audet, B.; Vaquero, C.; Mesnard, J.M.; Barbeau, B. Detection, characterization and regulation of antisense transcripts in HIV-1. Retrovirology 2007, 4, 71. [Google Scholar] [CrossRef] [PubMed]
- Laverdure, S.; Gross, A.; Arpin-Andre, C.; Clerc, I.; Beaumelle, B.; Barbeau, B.; Mesnard, J.M. HIV-1 antisense transcription is preferentially activated in primary monocyte-derived cells. J. Virol. 2012, 86, 13785–13789. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, L.B.; Ambrus, J.L., Jr.; Krawczyk, K.A.; Sharma, S.; Brooks, S.; Hsiao, C.B.; Schwartz, S.A. Human immunodeficiency virus-type 1 LTR DNA contains an intrinsic gene producing antisense RNA and protein products. Retrovirology 2006, 3, 80. [Google Scholar] [CrossRef] [PubMed]
- Michael, N.L.; Vahey, M.T.; d’Arcy, L.; Ehrenberg, P.K.; Mosca, J.D.; Rappaport, J.; Redfield, R.R. Negative-strand rna transcripts are produced in human immunodeficiency virus type 1-infected cells and patients by a novel promoter downregulated by tat. J. Virol. 1994, 68, 979–987. [Google Scholar] [PubMed]
- Torresilla, C.; Do Carmo, S.; Larocque, É.; Douceron, E.; Mesnard, J.-M.; Mahieux, R.; Barbeau, B. The antisense protein of HTLV-2 positively modulates HIV-1 replication. Retrovirology 2014, 11, P118. [Google Scholar] [CrossRef]
- Torresilla, C.; Larocque, E.; Landry, S.; Halin, M.; Coulombe, Y.; Masson, J.Y.; Mesnard, J.M.; Barbeau, B. Detection of the HIV-1 minus-strand-encoded antisense protein and its association with autophagy. J. Virol. 2013, 87, 5089–5105. [Google Scholar] [CrossRef] [PubMed]
- Vanhee-Brossollet, C.; Thoreau, H.; Serpente, N.; D’Auriol, L.; Levy, J.P.; Vaquero, C. A natural antisense rna derived from the HIV-1 env gene encodes a protein which is recognized by circulating antibodies of HIV+ individuals. Virology 1995, 206, 196–202. [Google Scholar] [CrossRef]
- Cassan, E.; Arigon-Chifolleau, A.M.; Mesnard, J.M.; Gross, A.; Gascuel, O. Concomitant emergence of the antisense protein gene of HIV-1 and of the pandemic. Proc. Natl. Acad. Sci. USA 2016, 113, 11537–11542. [Google Scholar] [CrossRef] [PubMed]
- Briquet, S.; Richardson, J.; Vanhee-Brossollet, C.; Vaquero, C. Natural antisense transcripts are detected in different cell lines and tissues of cats infected with feline immunodeficiency virus. Gene 2001, 267, 157–164. [Google Scholar] [CrossRef]
- Durkin, K.; Rosewick, N.; Artesi, M.; Hahaut, V.; Griebel, P.; Arsic, N.; Burny, A.; Georges, M.; Van den Broeke, A. Characterization of novel bovine leukemia virus (BLV) antisense transcripts by deep sequencing reveals constitutive expression in tumors and transcriptional interaction with viral micrornas. Retrovirology 2016, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- McCune, J.M. The dynamics of CD4+ t-cell depletion in HIV disease. Nature 2001, 410, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Obert, L.A.; Hoover, E.A. Early pathogenesis of transmucosal feline immunodeficiency virus infection. J. Virol. 2002, 76, 6311–6322. [Google Scholar] [CrossRef] [PubMed]
- Obert, L.; Hoover, E. Relationship of lymphoid lesions to disease course in mucosal feline immunodeficiency virus type c infection. Vet. Pathol. 2000, 37, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.C.; Dean, G.A.; Pedersen, N.C.; Moore, P.F. Immunopathologic changes in the thymus during the acute stage of experimentally induced feline immunodeficiency virus infection in juvenile cats. J. Virol. 1997, 71, 8632–8641. [Google Scholar] [PubMed]
- Serrano-Villar, S.; Sainz, T.; Lee, S.A.; Hunt, P.W.; Sinclair, E.; Shacklett, B.L.; Ferre, A.L.; Hayes, T.L.; Somsouk, M.; Hsue, P.Y. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-aids morbidity and mortality. PLoS Pathog. 2014, 10, e1004078. [Google Scholar] [CrossRef] [PubMed]
- Loughran, T.J. Clonal diseases of large granular lymphocytes [see comments]. Blood 1993, 82, 1–14. [Google Scholar] [PubMed]
- Phillips, J.; Lanier, L. Lectin-dependent and anti-CD3 induced cytotoxicity are preferentially mediated by peripheral blood cytotoxic T lymphocytes expressing Leu-7 antigen. J. Immunol. 1986, 136, 1579–1585. [Google Scholar] [PubMed]
- Schmidt, R.E.; Murray, C.; Daley, J.F.; Schlossman, S.; Ritz, J. A subset of natural killer cells in peripheral blood displays a mature T cell phenotype. J. Exp. Med. 1986, 164, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Sprague, W.S.; Apetrei, C.; Avery, A.C.; Peskind, R.L.; Vandewoude, S. Large granular lymphocytes are universally increased in human, macaque, and feline lentiviral infection. Vet. Immunol. Immunopathol. 2015, 167, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Alekshun, T.J.; Sokol, L. Diseases of large granular lymphocytes. Cancer Control 2007, 14, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Boveri, E.; Riboni, R.; Antico, P.; Malacrida, A.; Pastorini, A. CD3+ T large granular lymphocyte leukaemia in a HIV+, HCV+, HBV+ patient. Virchows Arch. 2009, 454, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.R.; Cavenagh, J.D.; Milne, T.; Howe, D.; Wilkes, S.J.; Sinnott, P.; Forster, G.E.; Helbert, M. Benign monoclonal expansion of cd8+ lymphocytes in HIV infection. J. Clin. Pathol. 2000, 53, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Sprague, W.; TerWee, J.; VandeWoude, S. Temporal association of large granular lymphocytosis, neutropenia, proviral load, and fasl mrna in cats with acute feline immunodeficiency virus infection. Vet. Immunol. Immunopathol. 2010, 134, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Fogle, J.E.; Mexas, A.M.; Tompkins, W.A.; Tompkins, M.B. CD4+ CD25+ T regulatory cells inhibit CD8+ IFN-γ production during acute and chronic fiv infection utilizing a membrane TGF-β-dependent mechanism. AIDS Res. Hum. Retrovir. 2010, 26, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Kinter, A.L.; Horak, R.; Sion, M.; Riggin, L.; McNally, J.; Lin, Y.; Jackson, R.; O’Shea, A.; Roby, G.; Kovacs, C. CD25+ regulatory T cells isolated from HIV-infected individuals suppress the cytolytic and nonlytic antiviral activity of HIV-specific CD8+ t cells in vitro. AIDS Res. Hum. Retrovir. 2007, 23, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.M.; Fogle, J.E.; Tompkins, M.B. Infection with feline immunodeficiency virus directly activates CD4+ CD25+ T regulatory cells. J. Virol. 2013, 87, 9373–9378. [Google Scholar] [CrossRef] [PubMed]
- Petito, C.K. Human immunodeficiency virus type 1 compartmentalization in the central nervous system. J. Neurovirol. 2004, 10, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Zenger, E.; Tiffany-Castiglioni, E.; Collisson, E.W. Cellular mechanisms of feline immunodeficiency virus (FIV)-induced neuropathogenesis. Front. Biosci. 1997, 2, d527–d537. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, N.; Bexiga, M.; Brayden, D.; Brankin, B.; Willett, B.; Hosie, M.; Jacque, J.M.; Callanan, J. Lymphocyte migration through the blood–brain barrier (BBB) in feline immunodeficiency virus infection is significantly influenced by the pre-existence of virus and tumour necrosis factor (TNF)-α within the central nervous system (CNS): Studies using an in vitro feline bbb model. Neuropathol. Appl. Neurobiol. 2009, 35, 592–602. [Google Scholar] [PubMed]
- Hudson, L.; Bragg, D.; Tompkins, M.; Meeker, R. Astrocytes and microglia differentially regulate trafficking of lymphocyte subsets across brain endothelial cells. Brain Res. 2005, 1058, 148–160. [Google Scholar] [CrossRef] [PubMed]
- González-Scarano, F.; Martín-García, J. The neuropathogenesis of aids. Nat. Rev. Immunol. 2005, 5, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, R.; Schwinn, A.; Narayan, O.; Zink, C.; Kreth, H.; Roggendorf, W.; Dörries, R.; Schwender, S.; Imrich, H.; Ter Meulen, V. Human immunodeficiency virus infection in microglia: Correlation between cells infected in the brain and cells cultured from infectious brain tissue. Ann. Neurol. 1992, 31, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Maeda, K.; Tohya, Y.; Furuya, T.; Miyazawa, T.; Horimoto, T.; Norimine, J.; Kai, C.; Mikami, T. Replicative difference in early-passage feline brain cells among feline immunodeficiency virus isolates. Arch. Virol. 1992, 125, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Abramo, F.; Iorio, C.D.; Cantile, C.; Carli, M.A.; Pollera, C.; Vago, L.; Tosoni, A.; Costanzi, G. Neuropathology in cats experimentally infected wit feline immunodeficiency virus: A morphological, immunocytochemical and morphometric study. J. Neurovirol. 1997, 3, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Meeker, R.B.; Poulton, W.; Feng, W.-H.; Hudson, L.; Longo, F.M. Suppression of immunodeficiency virus-associated neural damage by the P75 neurotrophin receptor ligand, LM11A-31, in an in vitro feline model. J. Neuroimmune Pharmacol. 2012, 7, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Hudson, L.C.; Tompkins, M.B.; Vahlenkamp, T.W.; Meeker, R.B. Compartmentalization and evolution of feline immunodeficiency virus between the central nervous system and periphery following intracerebroventricular or systemic inoculation. J. Neurovirol. 2006, 12, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.M.; Hoke, A.; Zhu, Y.; Johnston, J.B.; van Marle, G.; Silva, C.; Zochodne, D.W.; Power, C. Peripheral neuropathy in lentivirus infection: Evidence of inflammation and axonal injury. Aids 2004, 18, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Burdo, T.H.; Lackner, A.; Williams, K.C. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol. Rev. 2013, 254, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Burdo, T.H. Monocyte mobilization, activation markers, and unique macrophage populations in the brain: Observations from siv infected monkeys are informative with regard to pathogenic mechanisms of HIV infection in humans. J. Neuroimmune Pharmacol. 2012, 7, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Lackner, A.; Mallard, J. Non-human primate models of siv infection and cns neuropathology. Curr. Opin. Virol. 2016, 19, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.C.; Hickey, W.F. Central nervous system damage, monocytes and macrophages, and neurological disorders in aids. Ann. Rev. Neurosci. 2002, 25, 537–562. [Google Scholar] [CrossRef] [PubMed]
- Laast, V.A.; Pardo, C.A.; Tarwater, P.M.; Queen, S.E.; Reinhart, T.A.; Ghosh, M.; Adams, R.J.; Zink, M.C.; Mankowski, J.L. Pathogenesis of simian immunodeficiency virus-induced alterations in macaque trigeminal ganglia. J. Neuropathol. Exp. Neurol. 2007, 66, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Laast, V.A.; Shim, B.; Johanek, L.M.; Dorsey, J.L.; Hauer, P.E.; Tarwater, P.M.; Adams, R.J.; Pardo, C.A.; McArthur, J.C.; Ringkamp, M. Macrophage-mediated dorsal root ganglion damage precedes altered nerve conduction in siv-infected macaques. Am. J. Pathol. 2011, 179, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, H.C.; Chen, W.; Borzan, J.; Mankowski, J.L.; Höke, A. Mitochondrial dysfunction in distal axons contributes to human immunodeficiency virus sensory neuropathy. Ann Neurol. 2011, 69, 100–110. [Google Scholar] [CrossRef] [PubMed]
- VandeWoude, S.; Apetrei, C. Going wild: Lessons from naturally occurring T-lymphotropic lentiviruses. Clin. Microbiol. Rev. 2006, 19, 728–762. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.V.; Lin, K.-C.; Mansfield, K.; Wachtman, L.M. Specific pathogen-free status alters immunophenotype in rhesus macaques: Implications for the study of simian immunodeficiency virus. AIDS Res. Hum. Retrovir. 2011, 27, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Denton, P.W.; Garcia, J.V. Humanized mouse models of HIV infection. AIDS Rev. 2011, 13, 135–148. [Google Scholar] [PubMed]
- Zheng, J.; Ghorpade, A.; Niemann, D.; Cotter, R.L.; Thylin, M.R.; Epstein, L.; Swartz, J.M.; Shepard, R.B.; Liu, X.; Nukuna, A.; et al. Lymphotropic virions affect chemokine receptor-mediated neural signaling and apoptosis: Implications for human immunodeficiency virus type 1-associated dementia. J. Virol. 1999, 73, 8256–8267. [Google Scholar] [PubMed]
- Hesselgesser, J.; Taub, D.; Baskar, P.; Greenberg, M.; Hoxie, J.; Kolson, D.L.; Horuk, R. Neuronal apoptosis induced by HIV-1 GP120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr. Biol. CB 1998, 8, 595–598. [Google Scholar] [CrossRef]
- Zheng, J.; Thylin, M.R.; Ghorpade, A.; Xiong, H.; Persidsky, Y.; Cotter, R.; Niemann, D.; Che, M.; Zeng, Y.C.; Gelbard, H.A.; et al. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J. Neuroimmunol. 1999, 98, 185–200. [Google Scholar] [CrossRef]
- Meucci, O.; Fatatis, A.; Simen, A.A.; Bushell, T.J.; Gray, P.W.; Miller, R.J. Chemokines regulate hippocampal neuronal signaling and GP120 neurotoxicity. Proc. Natl. Acad. Sci. USA 1998, 95, 14500–14505. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A.; Sucher, N.J.; Kaiser, P.K.; Dreyer, E.B. Synergistic effects of HIV coat protein and nmda receptor-mediated neurotoxicity. Neuron 1991, 7, 111–118. [Google Scholar] [CrossRef]
- Haughey, N.J.; Mattson, M.P. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins tat and GP120. J. Acquir. Immune Defic. Syndr. 2002, 31 (Suppl. 2), S55–S61. [Google Scholar] [CrossRef] [PubMed]
- Ballester, L.Y.; Capo-Velez, C.M.; Garcia-Beltran, W.F.; Ramos, F.M.; Vazquez-Rosa, E.; Rios, R.; Mercado, J.R.; Melendez, R.I.; Lasalde-Dominicci, J.A. Up-regulation of the neuronal nicotinic receptor alpha7 by HIV glycoprotein 120: Potential implications for HIV-associated neurocognitive disorder. J. Biol. Chem. 2012, 287, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Bredt, D.S.; Snyder, S.H. Nitric oxide mediates glutamate-linked enhancement of CGMP levels in the cerebellum. Proc. Natl. Acad. Sci. USA 1989, 86, 9030–9033. [Google Scholar] [CrossRef] [PubMed]
- Garthwaite, J.; Garthwaite, G.; Palmer, R.M.; Moncada, S. Nmda receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur. J. Pharmacol. 1989, 172, 413–416. [Google Scholar] [CrossRef]
- Kornau, H.C.; Seeburg, P.H.; Kennedy, M.B. Interaction of ion channels and receptors with PDZ domain proteins. Curr. Opin. Neurobiol. 1997, 7, 368–373. [Google Scholar] [CrossRef]
- Christopherson, K.S.; Hillier, B.J.; Lim, W.A.; Bredt, D.S. PSD-95 assembles a ternary complex with the N-methyl-d-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J. Biol. Chem. 1999, 274, 27467–27473. [Google Scholar] [CrossRef] [PubMed]
- Rameau, G.A.; Tukey, D.S.; Garcin-Hosfield, E.D.; Titcombe, R.F.; Misra, C.; Khatri, L.; Getzoff, E.D.; Ziff, E.B. Biphasic coupling of neuronal nitric oxide synthase phosphorylation to the nmda receptor regulates ampa receptor trafficking and neuronal cell death. J. Neurosci. 2007, 27, 3445–3455. [Google Scholar] [CrossRef] [PubMed]
- Bredt, D.S. Nitric oxide signaling specificity—The heart of the problem. J. Cell Sci. 2003, 116, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bragg, D.C.; Meeker, R.B.; Duff, B.A.; English, R.V.; Tompkins, M.B. Neurotoxicity of FIV and FIV envelope protein in feline cortical cultures. Brain Res. 1999, 816, 431–437. [Google Scholar] [CrossRef]
- Brenneman, D.E.; Westbrook, G.L.; Fitzgerald, S.P.; Ennist, D.L.; Elkins, K.L.; Ruff, M.R.; Pert, C.B. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature 1988, 335, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.K.; Pu, R.; Sato, E.; Hohdatsu, T. Feline immunodeficiency virus pathogenesis and development of a dual-subtype feline-immunodeficiency-virus vaccine. AIDS 2007, 21, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Uhl, E.; Heaton-Jones, T.; Pu, R.; Yamamoto, J. Fiv vaccine development and its importance to veterinary and human medicine: A review: FIV vaccine 2002 update and review. Vet. Immunol. Immunopathol. 2002, 90, 113–132. [Google Scholar] [CrossRef]
- Pu, R.; Coleman, J.; Coisman, J.; Sato, E.; Tanabe, T.; Arai, M.; Yamamoto, J.K. Dual-subtype FIV vaccine (Fel-O-Vax® FIV) protection against a heterologous subtype B FIV isolate. J. Feline Med. Surg. 2005, 7, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Bęczkowski, P.M.; Harris, M.; Techakriengkrai, N.; Beatty, J.A.; Willett, B.J.; Hosie, M.J. Neutralising antibody response in domestic cats immunised with a commercial feline immunodeficiency virus (FIV) vaccine. Vaccine 2015, 33, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Westman, M.; Malik, R.; Hall, E.; Harris, M.; Norris, J. The protective rate of the feline immunodeficiency virus vaccine: An australian field study. Vaccine 2016, 34, 4752–4758. [Google Scholar] [CrossRef] [PubMed]
- Dunham, S.P.; Bruce, J.; Klein, D.; Flynn, J.N.; Golder, M.C.; MacDonald, S.; Jarrett, O.; Neil, J.C. Prime-boost vaccination using DNA and whole inactivated virus vaccines provides limited protection against virulent feline immunodeficiency virus. Vaccine 2006, 24, 7095–7108. [Google Scholar] [CrossRef] [PubMed]
- Dunham, S.; Bruce, J.; MacKay, S.; Golder, M.; Jarrett, O.; Neil, J. Limited efficacy of an inactivated feline immunodeficiency virus vaccine. Vet. Rec. 2006, 158, 561–562. [Google Scholar] [CrossRef] [PubMed]
- Hosie, M.J.; Osborne, R.; Reid, G.; Neil, J.C.; Jarrett, O. Enhancement after feline immunodeficiency virus vaccination. Vet. Immunol. Immunopathol. 1992, 35, 191–197. [Google Scholar] [CrossRef]
- Lombardi, S.; Garzelli, C.; Pistello, M.; Massi, C.; Matteucci, D.; Baldinotti, F.; Cammarota, G.; Da Prato, L.; Bandecchi, P.; Tozzini, F. A neutralizing antibody-inducing peptide of the V3 domain of feline immunodeficiency virus envelope glycoprotein does not induce protective immunity. J. Virol. 1994, 68, 8374–8379. [Google Scholar] [PubMed]
- Siebelink, K.; Tijhaar, E.; Huisman, R.C.; Huisman, W.; De Ronde, A.; Darby, I.H.; Francis, M.J.; Rimmelzwaan, G.F.; Osterhaus, A. Enhancement of feline immunodeficiency virus infection after immunization with envelope glycoprotein subunit vaccines. J. Virol. 1995, 69, 3704–3711. [Google Scholar] [PubMed]
- Richardson, J.; Moraillon, A.; Baud, S.; Cuisinier, A.; Sonigo, P.; Pancino, G. Enhancement of feline immunodeficiency virus (FIV) infection after DNA vaccination with the fiv envelope. J. Virol. 1997, 71, 9640–9649. [Google Scholar] [PubMed]
- Karlas, J.A.; Siebelink, K.; Peer, M.A.V.; Huisman, W.; Cuisinier, A.M.; Rimmelzwaan, G.F.; Osterhaus, A. Vaccination with experimental feline immunodeficiency virus vaccines, based on autologous infected cells, elicits enhancement of homologous challenge infection. J Gen. Virol. 1999, 80, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Giannecchini, S.; Isola, P.; Sichi, O.; Matteucci, D.; Pistello, M.; Zaccaro, L.; Del Mauro, D.; Bendinelli, M. Aids vaccination studies using an ex vivo feline immunodeficiency virus model: Failure to protect and possible enhancement of challenge infection by four cell-based vaccines prepared with autologous lymphoblasts. J. Virol. 2002, 76, 6882–6892. [Google Scholar] [CrossRef] [PubMed]
- Lun, W.-H.; Takeda, A.; Nakamura, H.; Kano, M.; Mori, K.; Sata, T.; Nagai, Y.; Matano, T. Loss of virus-specific CD4+ T cells with increases in viral loads in the chronic phase after vaccine-based partial control of primary simian immunodeficiency virus replication in macaques. J. Gen. Virol. 2004, 85, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Mueller, Y.M.; Do, D.H.; Altork, S.R.; Artlett, C.M.; Gracely, E.J.; Katsetos, C.D.; Legido, A.; Villinger, F.; Altman, J.D.; Brown, C.R. Il-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger siv-specific CD8+ t cell responses. J. Immunol. 2008, 180, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.E.; Montefiori, D.; Mitchell, W. Antibody-dependent enhancement of human immunodeficiency virus type 1 infection. Lancet 1988, 331, 790–794. [Google Scholar] [CrossRef]
- Staprans, S.I.; Hamilton, B.L.; Follansbee, S.E.; Elbeik, T.; Barbosa, P.; Grant, R.M.; Feinberg, M.B. Activation of virus replication after vaccination of HIV-1-infected individuals. J. Exp. Med. 1995, 182, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Villinger, F.; Rowe, T.; Parekh, B.S.; Green, T.A.; Mayne, A.E.; Grimm, B.; McClure, H.M.; Lackner, A.A.; Dailey, P.J.; Ansari, A.A. Chronic immune stimulation accelerates siv-induced disease progression. J. Med. Primatol. 2001, 30, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Staprans, S.I.; Barry, A.P.; Silvestri, G.; Safrit, J.T.; Kozyr, N.; Sumpter, B.; Nguyen, H.; McClure, H.; Montefiori, D.; Cohen, J.I. Enhanced siv replication and accelerated progression to aids in macaques primed to mount a cd4 t cell response to the siv envelope protein. Proc. Natl. Acad. Sci. USA 2004, 101, 13026–13031. [Google Scholar] [CrossRef] [PubMed]
- Huisman, W.; Martina, B.; Rimmelzwaan, G.; Gruters, R.; Osterhaus, A. Vaccine-induced enhancement of viral infections. Vaccine 2009, 27, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Montefiori, D.C. Role of complement and FC receptors in the pathogenesis of HIV-1 infection. In Immunopathogenesis of HIV Infection; Springer: Berlin, Germany, 1997; pp. 119–138. [Google Scholar]
- Müller-Eberhard, H.J. Molecular organization and function of the complement system. Ann. Rev. Biochem. 1988, 57, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Willey, S.; Aasa-Chapman, M.M.; O’Farrell, S.; Pellegrino, P.; Williams, I.; Weiss, R.A.; Neil, S.J. Extensive complement-dependent enhancement of HIV-1 by autologous non-neutralising antibodies at early stages of infection. Retrovirology 2011, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Szabó, J.; Prohászka, Z.; Tóth, F.D.; Gyuris, Á.; Segesdi, J.; Bánhegyi, D.; Ujhelyi, E.; Minárovits, J.; Füst, G. Strong correlation between the complement-mediated antibody-dependent enhancement of HIV-1 infection and plasma viral load. AIDS 1999, 13, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.E.; Kawamura, T.; Lake, D.; Masuho, Y.; Mitchell, W.; Hersh, E.M. Antibodies to the primary immunodominant domain of human immunodeficiency virus type 1 (HIV-1) glycoprotein gp41 enhance HIV-1 infection in vitro. J. Virol. 1990, 64, 5301–5305. [Google Scholar] [PubMed]
- Robinson, W.; Gorny, M.; Xu, J.; Mitchell, W.; Zolla-Pazner, S. Two immunodominant domains of GP41 bind antibodies which enhance human immunodeficiency virus type 1 infection in vitro. J. Virol. 1991, 65, 4169–4176. [Google Scholar] [PubMed]
- Montefiori, D.C.; Robinson, W.E.; Mitchell, W.M. Antibody-independent, complement-mediated enhancement of HIV-1 infection by mannosidase i and ii inhibitors. Antivir. Res. 1989, 11, 137–146. [Google Scholar] [CrossRef]
- Boyer, V.; Desgranges, C.; Trabaud, M.; Fischer, E.; Kazatchkine, M. Complement mediates human immunodeficiency virus type 1 infection of a human t cell line in a CD4- and antibody-independent fashion. J. Exp. Med. 1991, 173, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Montefiori, D.C.; Stewart, K.; Ahearn, J.M.; Zhou, J. Complement-mediated binding of naturally glycosylated and glycosylation-modified human immunodeficiency virus type 1 to human CR2 (CD21). J. Virol. 1993, 67, 2699–2706. [Google Scholar] [PubMed]
- Reisinger, E.C.; Vogetseder, W.; Berzow, D.; Köfler, D.; Bitterlich, G.; Lehr, H.A.; Wachter, H.; Dierich, M.P. Complement-mediated enhancement of HIV-1 infection of the monoblastoid cell line U937. AIDS 1990, 4, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Sölder, B.; Schulz, T.; Hengster, P.; Löwer, J.; Larcher, C.; Bitterlich, G.; Kurth, R.; Wachter, H.; Dierich, M. HIV and HIV-infected cells differentially activate the human complement system independent of antibody. Immunol. Lett. 1989, 22, 135–145. [Google Scholar] [CrossRef]
- Spear, G.T.; Jiang, H.; Sullivan, B.L.; Gewürz, H.; Landay, A.L.; Lint, T.F. Direct binding of complement component C1q to human immunodeficiency virus (HIV) and human T lymphotrophic virus-I (HTLV-I) coinfected cells. AIDS Res. Hum. Retrovir. 1991, 7, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Broche, S.; Baud, S.; Leste-Lasserre, T.; Féménia, F.; Levy, D.; Moraillon, A.; Pancino, G.; Sonigo, P. Lymphoid activation: A confounding factor in aids vaccine development? J. Gen. Virol. 2002, 83, 2515–2521. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.M.; Greenwell-Wild, T.; Peng, G.; Hale-Donze, H.; Orenstein, J.M. Co-infection with opportunistic pathogens promotes human immunodeficiency virus type 1 infection in macrophages. J. Infect. Dis. 1999, 179, S457–S460. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Orenstein, J.M. Immune stimulation and HIV-1 viral replication. J. Leukoc. Biol. 1997, 62, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Spouge, J.L.; Conley, S.R.; Tsai, W.-P.; Merges, M.J.; Nara, P.L. Human plasma enhances the infectivity of primary human immunodeficiency virus type 1 isolates in peripheral blood mononuclear cells and monocyte-derived macrophages. J. Virol. 1995, 69, 6054–6062. [Google Scholar] [PubMed]
- Thibault, S.; Tardif, M.R.; Barat, C.; Tremblay, M.J. TLR2 signaling renders quiescent naive and memory CD4+ T cells more susceptible to productive infection with X4 and R5 HIV-type 1. J. Immunol. 2007, 179, 4357–4366. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.D.; Kirchhoff, F.; Czajak, S.C.; Sehgal, P.K.; Desrosiers, R.C. Protective effects of a live attenuated siv vaccine with a deletion in the nef gene. Science 1992, 258, 1938–1941. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.T.; Silvestri, G. Non-human primate models in aids research. Curr. Opin. HIV AIDS 2013, 8, 255–261. [Google Scholar] [PubMed]
- Hessell, A.J.; Hangartner, L.; Hunter, M.; Havenith, C.E.; Beurskens, F.J.; Bakker, J.M.; Lanigan, C.M.; Landucci, G.; Forthal, D.N.; Parren, P.W. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 2007, 449, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Mascola, J.R.; Stiegler, G.; VanCott, T.C.; Katinger, H.; Carpenter, C.B.; Hanson, C.E.; Beary, H.; Hayes, D.; Frankel, S.S.; Birx, D.L. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 2000, 6, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Hatziioannou, T.; Evans, D.T. Animal models for HIV/AIDs research. Nat. Rev. Microbiol. 2012, 10, 852–867. [Google Scholar] [CrossRef] [PubMed]
- Gauduin, M.-C.; Parren, P.W.; Weir, R.; Barbas, C.F.; Burton, D.R.; Koup, R.A. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat. Med. 1997, 3, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.; Hanley, M.B.; Moreno, M.B.; Wieder, E.; McCune, J.M. Human immunodeficiency virus-1 infection interrupts thymopoiesis and multilineage hematopoiesis in vivo. Blood 1998, 91, 2672–2678. [Google Scholar] [PubMed]
- McCune, J.M.; Namikawa, R.; Shih, C.-C.; Rabin, L.; Kaneshima, H. Suppression of HIV infection in AZT-treated SCID-HU mice. Science 1990, 247, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Safrit, J.T.; Fung, M.S.; Andrews, C.A.; Braun, D.G.; Sun, W.N.; Chang, T.W.; Koup, R.A. Hu-PBL-SCID mice can be protected from HIV-1 infection by passive transfer of monoclonal antibody to the principal neutralizing determinant of envelope GP120. AIDS 1993, 7, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Mataftsi, M.; Skoura, L.; Sakellari, D. Hiv infection and periodontal diseases: An overview of the post-HAART era. Oral Dis. 2011, 17, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Coogan, M.M.; Greenspan, J.; Challacombe, S.J. Oral lesions in infection with human immunodeficiency virus. Bull. World Health Organ. 2005, 83, 700–706. [Google Scholar] [PubMed]
- Miziara, I.D.; Weber, R. Oral lesions as predictors of highly active antiretroviral therapy failure in brazilian HIV-infected children. J. Oral Pathol. Med. 2008, 37, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.F.; de Araújo Castro, G.F.B.; de Souza, I.P.R.; Pinheiro, M. Pediatric HIV-related oral manifestations: A five-year retrospective study. Braz. Oral Res. 2004, 18, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Vaseliu, N.; Carter, A.B.; Kline, N.E.; Kozinetz, C.; Cron, S.G.; Matusa, R.; Kline, M.W. Longitudinal study of the prevalence and prognostic implications of oral manifestations in romanian children infected with human immunodeficiency virus type 1. Pediatr. Infect. Dis. J. 2005, 24, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Klatt, N.R.; Chomont, N.; Douek, D.C.; Deeks, S.G. Immune activation and HIV persistence: Implications for curative approaches to HIV infection. Immunol. Rev. 2013, 254, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Klatt, N.R.; Funderburg, N.T.; Brenchley, J.M. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013, 21, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Mehandru, S.; Poles, M.A.; Tenner-Racz, K.; Horowitz, A.; Hurley, A.; Hogan, C.; Boden, D.; Racz, P.; Markowitz, M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 2004, 200, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J.M.; Price, D.A.; Douek, D.C. Hiv disease: Fallout from a mucosal catastrophe? Nat. Immunol. 2006, 7, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Baskin, G.; Murphey-Corb, M.; Watson, E.; Martin, L. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/delta. Vet. Pathol. Online 1988, 25, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Casteleyn, C.; Breugelmans, S.; Simoens, P.; Van den Broeck, W. The tonsils revisited: Review of the anatomical localization and histological characteristics of the tonsils of domestic and laboratory animals. Clin. Dev. Immunol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- McClure, H.; Anderson, D.; Fultz, P.; Ansari, A.; Lockwood, E.; Brodie, A. Spectrum of disease in macaque monkeys chronically infected with SIV/SMM. Vet. Immunol. Immunopathol. 1989, 21, 13–24. [Google Scholar] [CrossRef]
- Dos Santos, L.D.C.; Castro, G.F.; de Souza, I.P.R.; Oliveira, R.H.S. Oral manifestations related to immunosuppression degree in HIV-positive children. Braz. Dent. J. 2001, 12, 135–138. [Google Scholar]
- Sparkes, A.; Caney, S.M. Feline Medicine: Self-Assessment Color Review; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Willett, B.; Flynn, N.; Hosic, M. FIV infection of the domestic cat: An animal model for aids. Immunol. Today 1997, 18, 182–189. [Google Scholar] [CrossRef]
- Yamamoto, J.K.; Sparger, E.; Ho, E.W.; Andersen, P.R.; O’connor, T.; Mandell, C.; Lowenstine, L.; Munn, R.; Pedersen, N. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am. J. Vet. Res. 1988, 49, 1246–1258. [Google Scholar] [PubMed]
- Brady, L.; Walker, C.; Oxford, G.; Stewart, C.; Magnusson, I.; McArthur, W. Oral diseases, mycology and periodontal microbiology of HIV-1-infected women. Mol. Oral Microbiol. 1996, 11, 371–380. [Google Scholar] [CrossRef]
- Flaitz, C.M.; Hicks, M.J. Oral candidiasis in children with immune suppression: Clinical appearance and therapeutic considerations. ASDC J. Dent. Child. 1999, 66, 154, 161–164. [Google Scholar] [PubMed]
- Holt, S.C.; Ebersole, J.L. Porphyromonas gingivalis, treponema denticola, and tannerella forsythia: The ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontology 2000 2005, 38, 72–122. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Saxena, D.; Chen, Z.; Liu, G.; Abrams, W.R.; Phelan, J.A.; Norman, R.G.; Fisch, G.S.; Corby, P.M.; Dewhirst, F. HIV infection and microbial diversity in saliva. J. Clin. Microbiol. 2014, 52, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Love, D.; Johnson, J.; Moore, L. Bacteroides species from the oral cavity and oral-associated diseases of cats. Vet. Microbiol. 1989, 19, 275–281. [Google Scholar] [CrossRef]
- Murray, P.A.; Grassi, M.; Winkler, J.R. The microbiology of HIV-associated periodontal lesions. J. Clin. Periodontol. 1989, 16, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Nokta, M. Oral manifestations associated with HIV infection. Curr. HIV/AIDS Rep. 2008, 5, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.M.; Love, D.N. Associations amongst three feline porphyromonas species from the gingival margin of cats during periodontal health and disease. Vet. Microbiol. 1999, 65, 195–207. [Google Scholar] [CrossRef]
- Rams, T.E.; Andriolo, M., Jr.; Feik, D.; Abel, S.N.; McGivern, T.M.; Slots, J. Microbiological study of HIV-related periodontitis. J. Periodontol. 1991, 62, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Sims, T.; Moncla, B.; Page, R. Serum antibody response to antigens of oral gram-negative bacteria by cats with plasma cell gingivitis-pharyngitis. J. Dent. Res. 1990, 69, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Vahlenkamp, T.; Egberink, H.; Hartmann, K.; Witvrouw, M.; Pannecouque, C.; Casara, P.; Nave, J.; De Clercq, E. Antiretroviral activities of acyclic nucleoside phosphonates [9-(2-phosphonylmethoxyethyl) adenine, 9-(2-phosphonylmethoxyethyl) guanine, (R)-9-(2-phosphonylmethoxypropyl) adenine, and mdl 74,968] in cell cultures and murine sarcoma virus-infected newborn nmri mice. Antimicrob. Agents Chemother. 1997, 41, 611–616. [Google Scholar] [PubMed]
- Vahlenkamp, T.W.; De Ronde, A.; Balzarini, J.; Naesens, L.; De Clercq, E.; Van Eijk, M.; Horzinek, M.C.; Egberink, H.F. (R)-9-(2-phosphonylmethoxypropyl)-2, 6-diaminopurine is a potent inhibitor of feline immunodeficiency virus infection. Antimicrob. Agents Chemother. 1995, 39, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Remington, K.M.; Preston, B.D.; Schinazi, R.F.; North, T.W. A novel point mutation at position 156 of reverse transcriptase from feline immunodeficiency virus confers resistance to the combination of (−)-β-2′, 3′-dideoxy-3′-thiacytidine and 3′-azido-3′-deoxythymidine. J. Virol. 1998, 72, 2335–2340. [Google Scholar] [PubMed]
- Hartmann, K.; Wooding, A.; Bergmann, M. Efficacy of antiviral drugs against feline immunodeficiency virus. Vet. Sci. 2015, 2, 456–476. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.M.; McCrackin, M.A.; Schinazi, R.F.; Hill, P.B.; Vahlenkamp, T.W.; Tompkins, M.B.; Hartmann, K. Antiviral efficacy of nine nucleoside reverse transcriptase inhibitors against feline immunodeficiency virus in feline peripheral blood mononuclear cells. Am. J. Vet. Res. 2014, 75, 273–281. [Google Scholar] [CrossRef] [PubMed]
- De Rozieres, S.; Thompson, J.; Sundstrom, M.; Gruber, J.; Stump, D.S.; Aymeric, P.; VandeWoude, S.; Elder, J.H. Replication properties of clade A/C chimeric feline immunodeficiency viruses and evaluation of infection kinetics in the domestic cat. J. Virol. 2008, 82, 7953–7963. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Mackie, R.S.; Harrison, T.; Shariat, B.; Kind, T.; Kehl, T.; Löchelt, M.; Boucher, C.; VandeWoude, S. Targeted enrichment for pathogen detection and characterization in three felid species. J. Clin. Microbiol. 2017, 55, 1658–1670. [Google Scholar] [CrossRef] [PubMed]
- Paulson, J.N.; Pop, M.; Bravo, H. Metagenomeseq: Statistical analysis for sparse high-throughput sequencing. Bioconductor 2014, 1, 1–32. [Google Scholar]
- Paulson, J.N.; Pop, M.; Bravo, H. Metagenomeseq: Statistical analysis for sparse high-throughput sequencing. Bioconduct. Package 2013, 1, 1–35. [Google Scholar]
- Hunt, P.W.; Deeks, S.G.; Rodriguez, B.; Valdez, H.; Shade, S.B.; Abrams, D.I.; Kitahata, M.M.; Krone, M.; Neilands, T.B.; Brand, R.J. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. Aids 2003, 17, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Leggott, P.J. Oral manifestations of HIV infection in children. Oral Surg. Oral Med. Oral Pathol. 1992, 73, 187–192. [Google Scholar] [CrossRef]
- Heron, S.E.; Elahi, S. HIV infection and compromised mucosal immunity: Oral manifestations and systemic inflammation. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Girard, N.; Servet, E.; Biourge, V.; Hennet, P. Periodontal health status in a colony of 109 cats. J. Vet. Dent. 2009, 26, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.; Tutt, C. Periodontal disease in cats: Back to basics—With an eye on the future. J. Feline Med. Surg. 2015, 17, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Zahradnik, R.; Magnusson, I.; Walker, C.; McDonell, E.; Hillman, C.; Hillman, J. Preliminary assessment of safety and effectiveness in humans of probiora3™, a probiotic mouthwash. J. Appl. Microbiol. 2009, 107, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Seminario-Amez, M.; López-López, J.; Estrugo-Devesa, A.; Ayuso-Montero, R.; Jané-Salas, E. Probiotics and oral health: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e282–e288. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Durukan, A.; Ozcelik, O.; Pauwels, M.; Quirynen, M.; Haytac, M.C. Clinical and microbiological effects of lactobacillus reuteri probiotics in the treatment of chronic periodontitis: A randomized placebo-controlled study. J. Clin. Periodontol. 2013, 40, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Galilee, M.; Alian, A. The structure of FIV reverse transcriptase and its implications for non-nucleoside inhibitor resistance. PLoS Pathog. 2018, 14, e1006849. [Google Scholar] [CrossRef] [PubMed]
- Doménech, A.; Miró, G.; Collado, V.M.; Ballesteros, N.; Sanjosé, L.; Escolar, E.; Martin, S.; Gomez-Lucia, E. Use of recombinant interferon omega in feline retrovirosis: From theory to practice. Vet. Immunol. Immunopathol. 2011, 143, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Leal, R.O.; Gil, S.; Duarte, A.; McGahie, D.; Sepúlveda, N.; Niza, M.M.; Tavares, L. Evaluation of viremia, proviral load and cytokine profile in naturally feline immunodeficiency virus infected cats treated with two different protocols of recombinant feline interferon omega. Res. Vet. Sci. 2015, 99, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Mari, K.; Maynard, L.; Sanquer, A.; Lebreux, B.; Eun, H.M. Therapeutic effects of recombinant feline interferon-co on feline leukemia virus (FELV)-infected and felv/feline immunodeficiency virus (FIV)-coinfected symptomatic cats. J. Vet. Intern. Med. 2004, 18, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Künzi, M.S.; Pitha, P.M. Role of interferon-stimulated gene ISG-15 in the interferon-ω-mediated inhibition of human immunodeficiency virus replication. J. Interferon Cytokine Res. 1996, 16, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Adolf, G. Human interferon omega—A review. Mult. Scler. Houndmills Basingstoke Engl. 1995, 1, S44–S47. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, C.; Abdo, Z.; Ericsson, A.; Elder, J.; VandeWoude, S. Applications of the FIV Model to Study HIV Pathogenesis. Viruses 2018, 10, 206. https://doi.org/10.3390/v10040206
Miller C, Abdo Z, Ericsson A, Elder J, VandeWoude S. Applications of the FIV Model to Study HIV Pathogenesis. Viruses. 2018; 10(4):206. https://doi.org/10.3390/v10040206
Chicago/Turabian StyleMiller, Craig, Zaid Abdo, Aaron Ericsson, John Elder, and Sue VandeWoude. 2018. "Applications of the FIV Model to Study HIV Pathogenesis" Viruses 10, no. 4: 206. https://doi.org/10.3390/v10040206





