Comparative Drought Responses of Quercus ilex L. and Pinus sylvestris L. in a Montane Forest Undergoing a Vegetation Shift
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling Scheme
| Variable | Q. ilex Pure | Mixed | P. sylvestris Pure |
|---|---|---|---|
| Stand level | |||
| Stem density (stems·ha−1) | |||
| P. sylvestris | 65 (66% def.) | 257 (41% def.) | 428 |
| Q. ilex | 5262 | 2913 | 285 |
| Other | 87 | 242 | 326 |
| TOTAL | 5414 | 3412 | 1039 |
| DBH (cm) | |||
| P. sylvestris | 23.60 ± 7.66 | 27.70 ± 3.08 | 32.30 ± 1.38 |
| Q. ilex | 8.76 ± 0.30 | 8.40 ± 0.40 | 5.89 ± 0.85 |
| Basal area (m2·ha−1) | |||
| P. sylvestris | 3.44 (96% def.) | 23.79 (52% def.) | 41.75 |
| Q. ilex | 40.63 | 24.86 | 0.99 |
| Other | 0.48 | 2.9 | 1.65 |
| TOTAL | 44.55 | 51.55 | 44.39 |
| Leaf area index (m2·m−2) | |||
| P. sylvestris | nm | 0.58 | 0.91 |
| Q. ilex | 4.59 | 2.69 | nm |
| TOTAL | 4.59 | 3.27 | 1.02 |
| Measured trees | |||
| AL:AS (m2·cm−2) | |||
| P. sylvestris | nm | 0.076 ± 0.008 | 0.067 ± 0.004 |
| Q. ilex | 0.167 ± 0.001 | 0.139 ± 0.008 | nm |
| DBH (cm) | |||
| P. sylvestris | nm | 38.60 ± 1.81 | 39.90 ± 0.89 |
| Q. ilex | 12.61 ± 1.03 | 16.21 ± 1.58 | nm |
| Height (m) | |||
| P. sylvestris | nm | 14.24 ± 0.78 | 18.3 ± 0.62 |
| Q. ilex | ~5 | ~5 | nm |
| Species | Valley | Stand type | Ψ | Gs | KS–L | NSC | PLC |
|---|---|---|---|---|---|---|---|
| Pinus sylvestris | Tillar | Mixed | 8 [2010–2012] 1 | 11 [2010–2013] 1 | 8 [2010–2012] 1 | 10 [2012] 2 | 8 [2010–2012] 4 |
| Quercus ilex | Tillar | Mixed | 5 [2010–2011] 4 | 10 [2010–2013] 4 | 5 [2010–2011] 4 | 5 [2010–2011] 4 | |
| Pinus sylvestris | Tillar | Pure | 4 [2010–2011] 4 | 10 [2010–2011] 4 | 4 [2010–2011] 4 | 10 [2012] 2 | 4 [2010–2011] 4 |
| Quercus ilex | Tillar | Pure | 4 [2010–2011] 4 | 10 [2010–2011] 4 | 4 [2010–2011] 4 | 4 [2010–2011] 4 | |
| Quercus ilex | Torners | Pure | 19 [2012] 3 |
2.3. Water Potential Measurements
2.4. Sap Flow and Canopy Stomatal Conductance
2.5. Whole-Tree Leaf-Specific Hydraulic Conductance
2.6. Percentage Loss of Hydraulic Conductivity
2.7. Non-Structural Carbohydrates
2.8. Data Analysis
3. Results
3.1. Leaf Water Potential
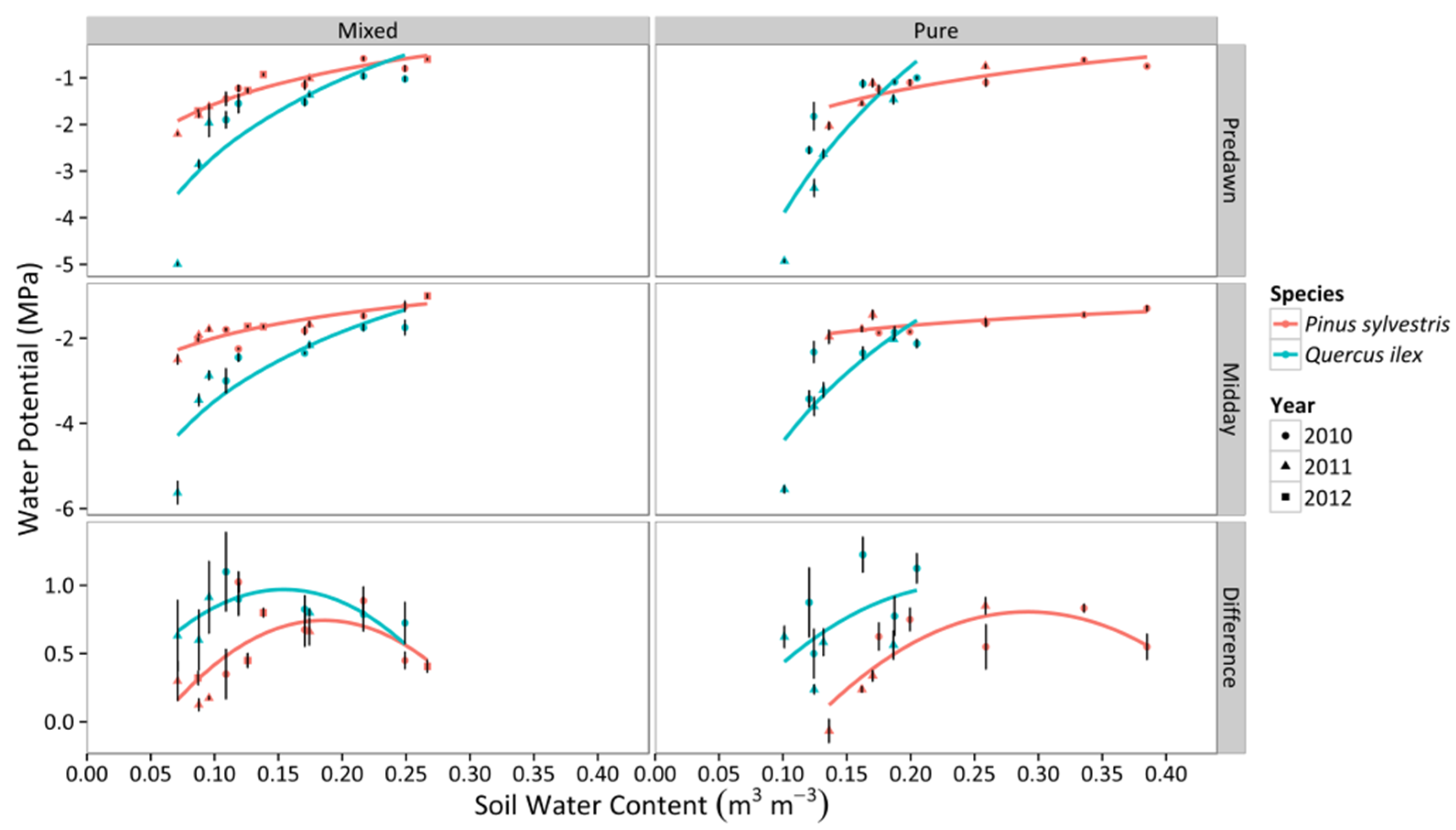
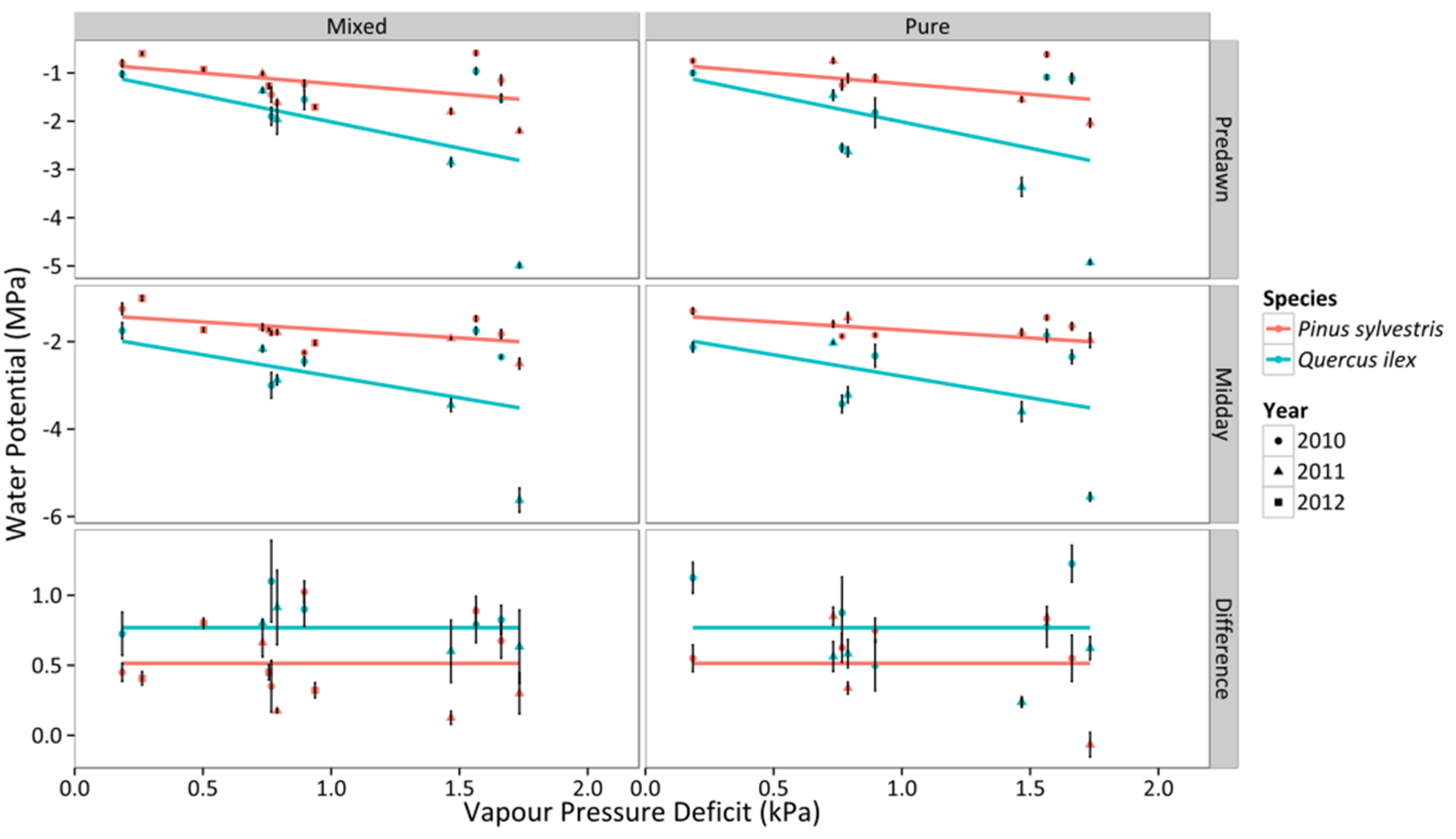
3.2. Canopy Stomatal Conductance
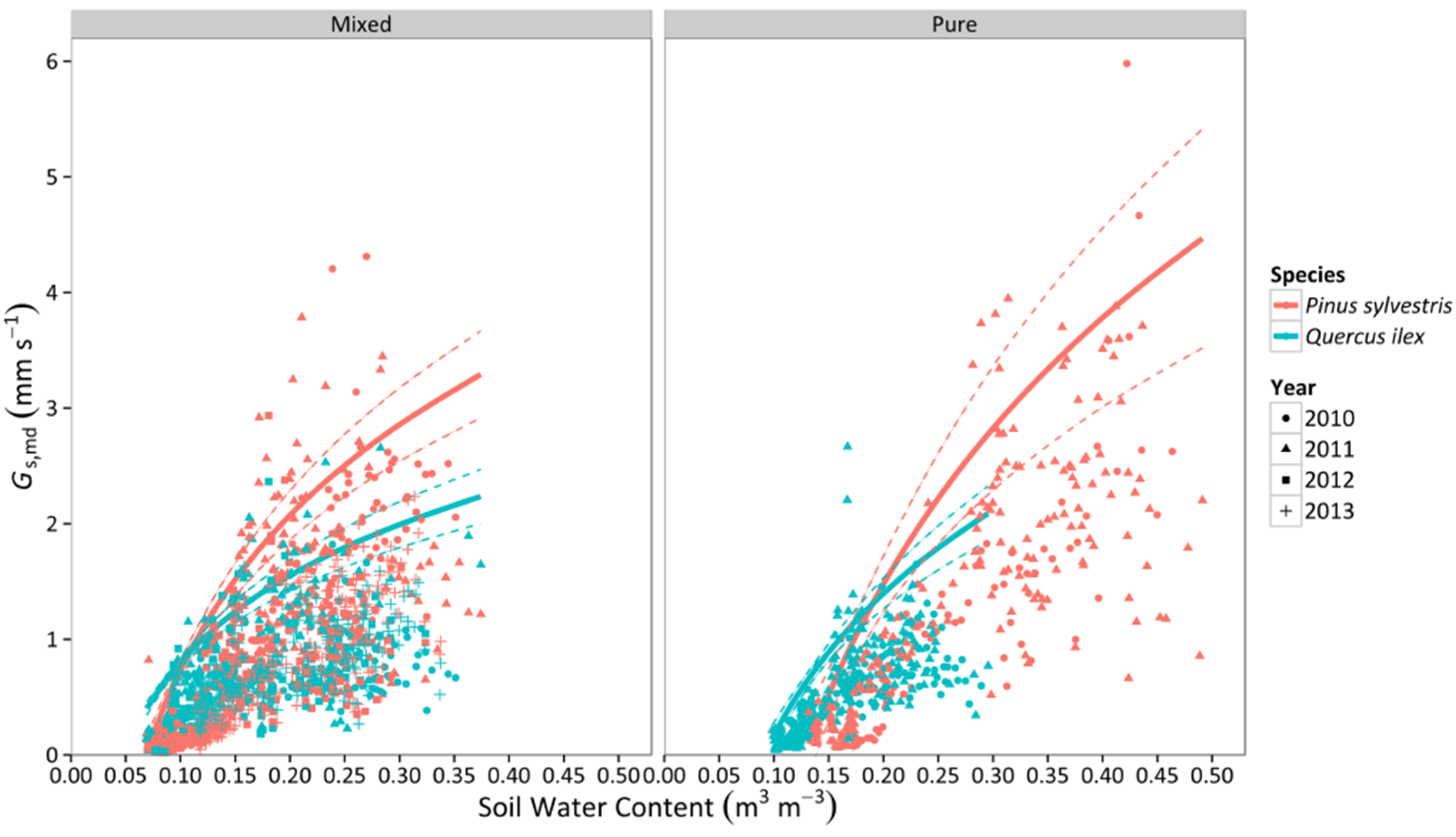
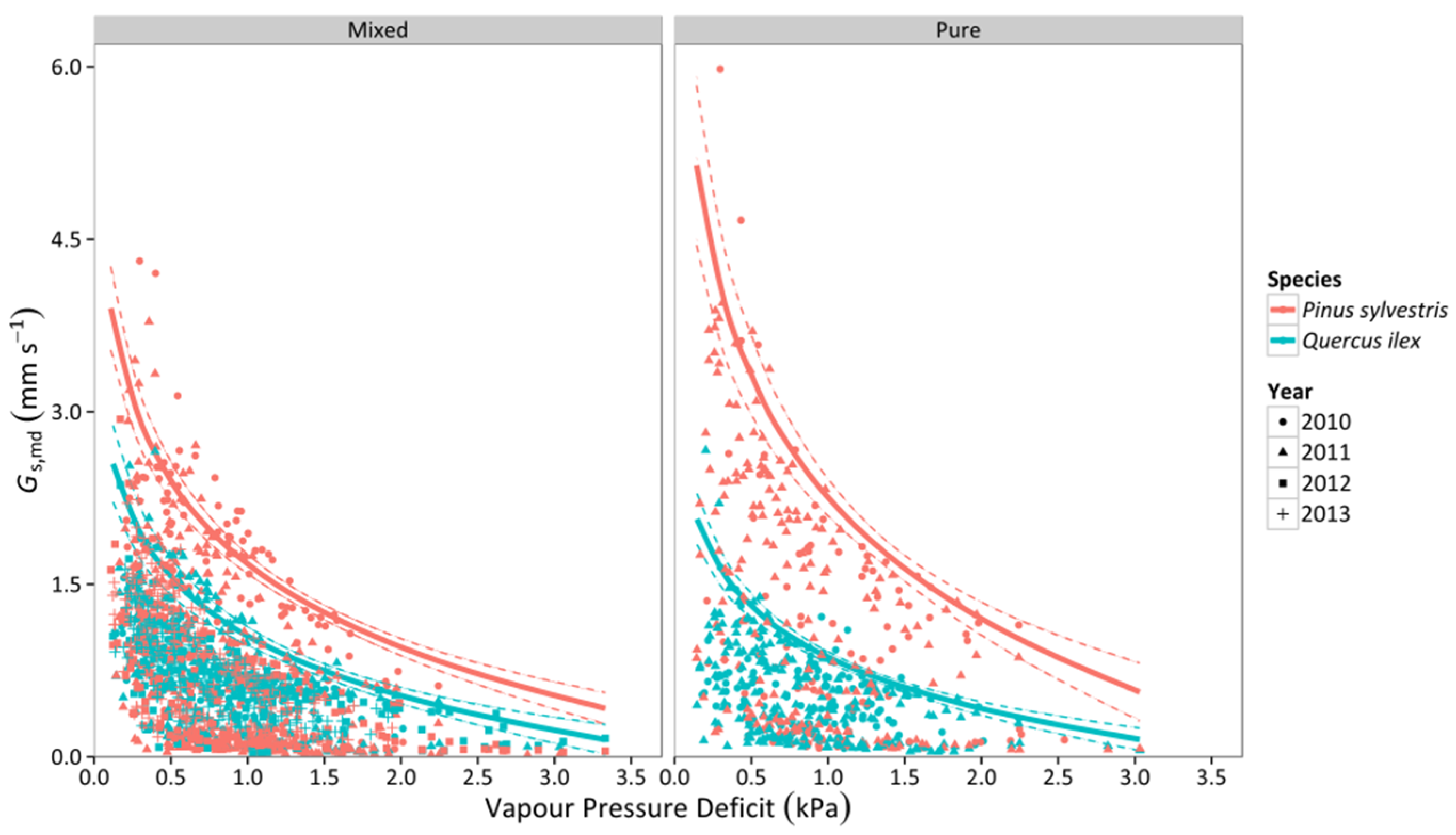
3.3. Whole-Tree Leaf-Specific Conductance
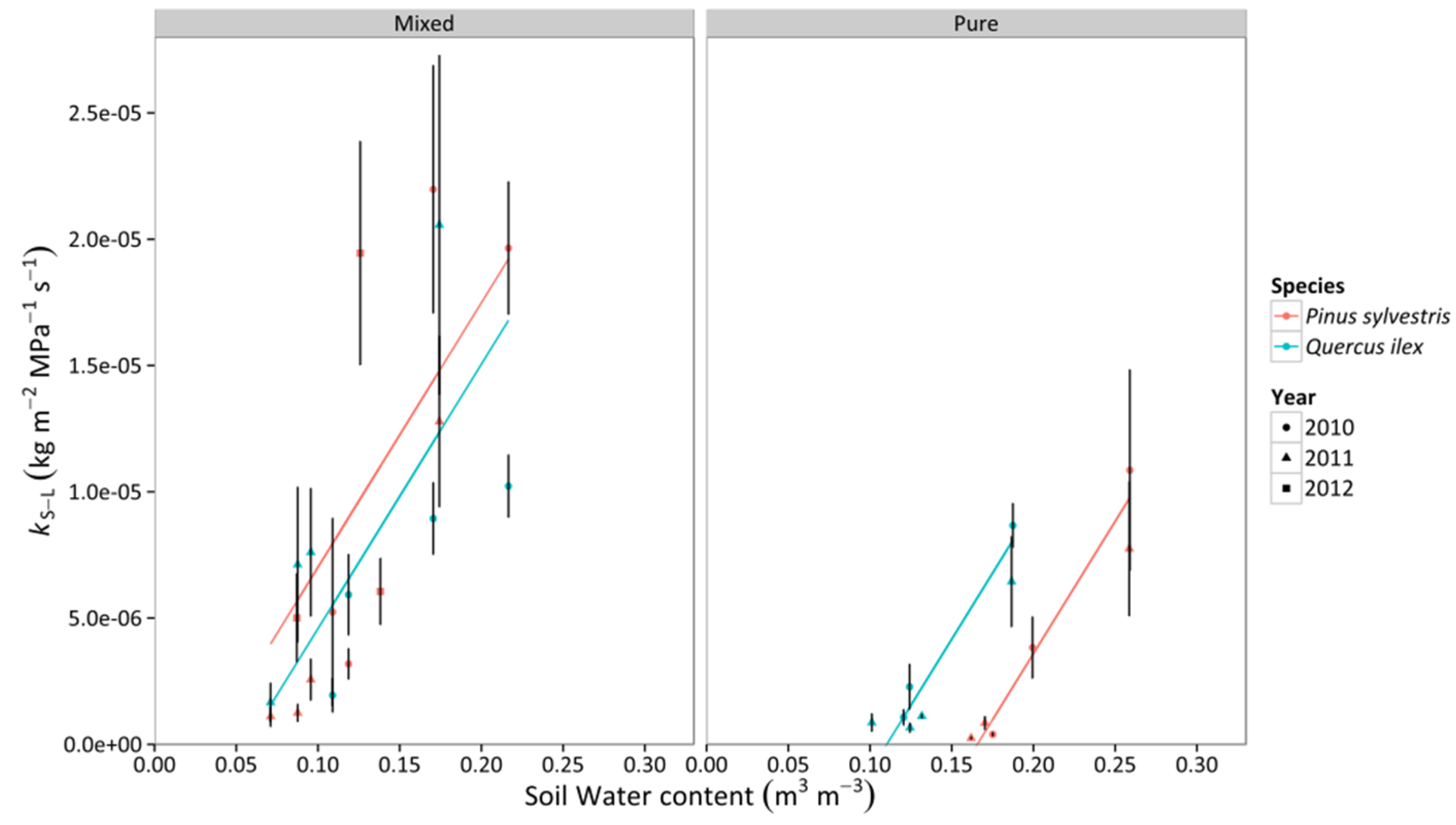
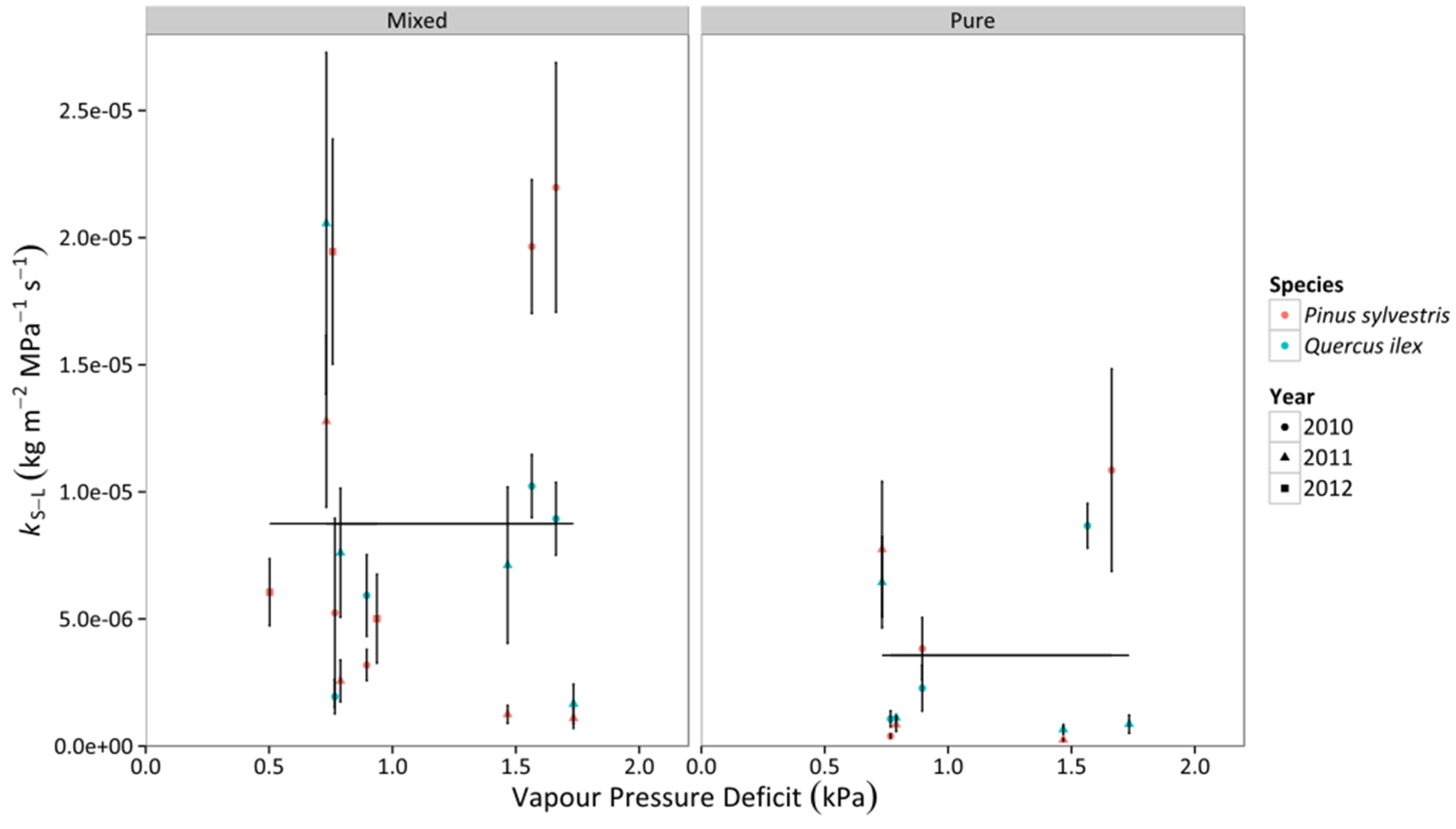
3.4. Percentage Loss of Hydraulic Conductivity

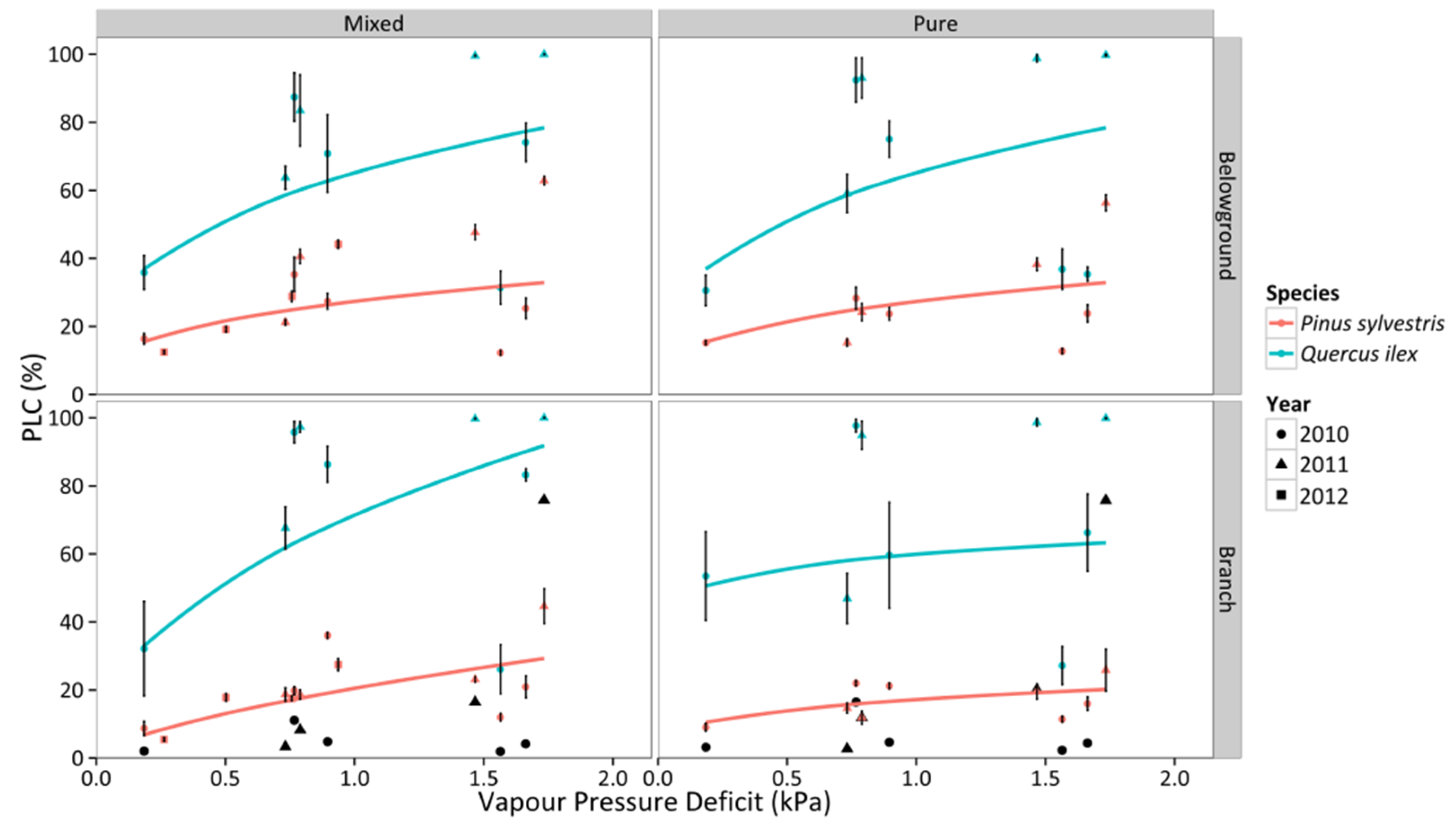
3.5. Non-Structural Carbohydrates and Starch
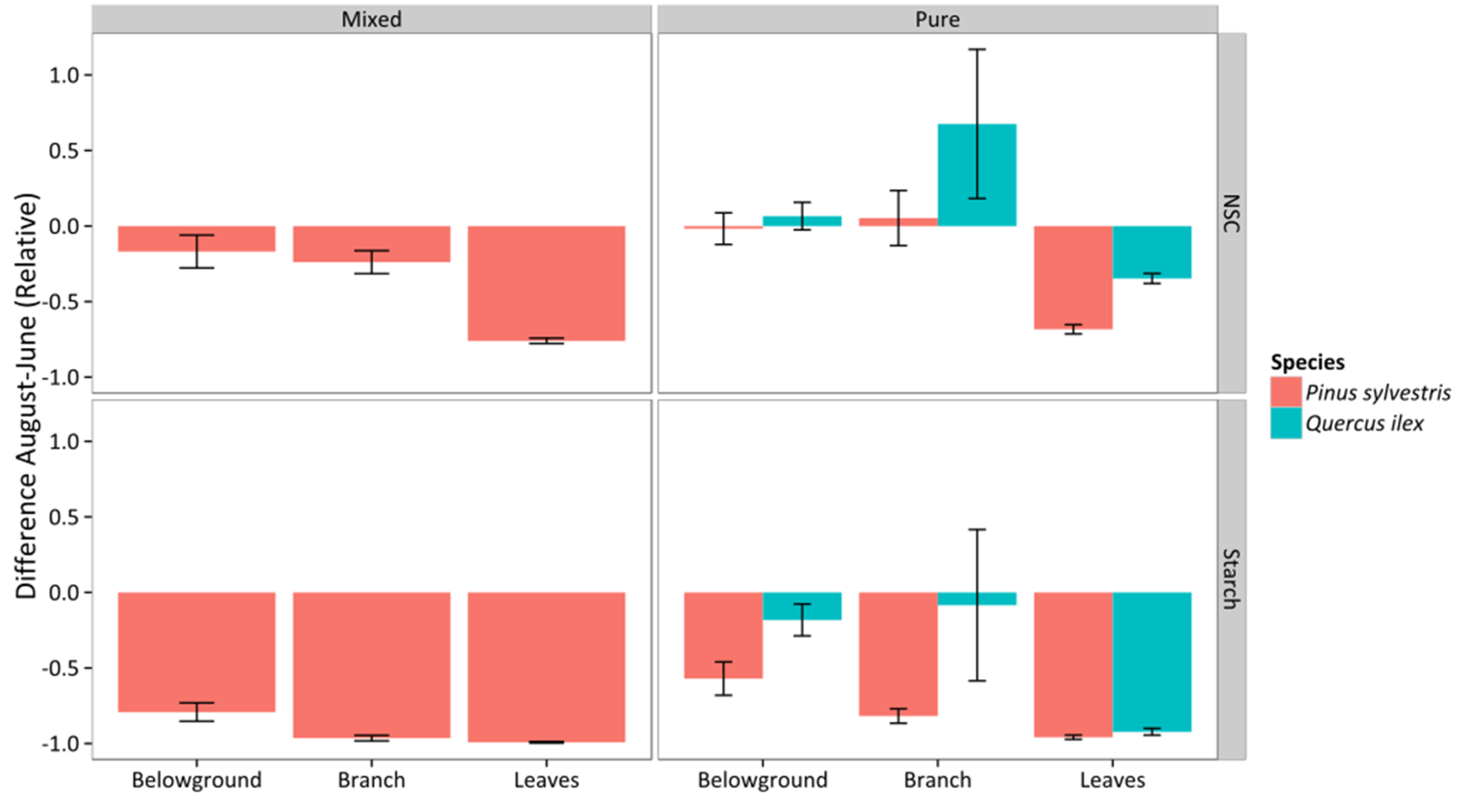
4. Discussion
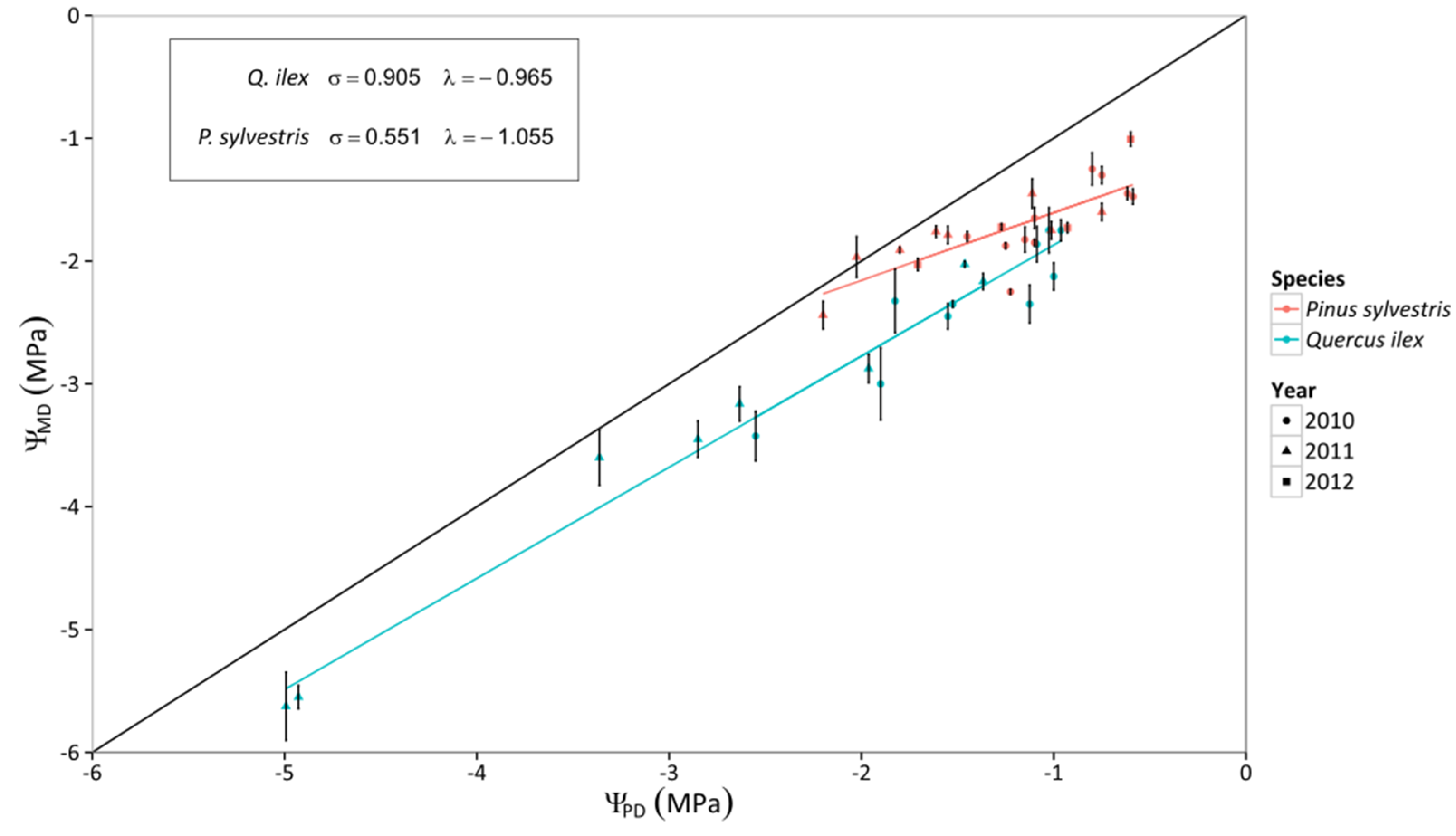
4.1. Contrasting Hydraulic Strategies in P. sylvestris and Q. ilex
4.2. Implications for the Mechanisms of Drought-Induced Mortality in the Two Study Species
4.3. Comparison of Mixed vs. Pure Stands and Implications for Vegetation Dynamics under Climate Change
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Williams, A.P.; Allen, C.D.; Macalady, A.K.; Griffin, D.; Woodhouse, C.A.; Meko, D.M.; Swetnam, T.W.; Rauscher, S.A.; Seager, R.; Grissino-Mayer, H.D. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Chang. 2012, 3, 292–297. [Google Scholar] [CrossRef]
- Dore, M.H.I. Climate change and changes in global precipitation patterns: What do we know? Environ. Int. 2005, 31, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Grulke, N.E.; Minnich, R.A.; Paine, T.D.; Seybold, S.J.; Chavez, D.J.; Fenn, M.E.; Riggan, P.J.; Dunn, A. Air pollution increases forest susceptibility to wildfires: A case study in the San Bernardino Mountains in southern California. In Wildland Fires and Air Pollution; Bytnerowicz, A., Arbaugh, M.J., Riebau, A.R., Andersen, C., Eds.; Elsevier Publishers: The Hague, The Netherlands, 2008; Volume 8, pp. 365–403. [Google Scholar]
- Linares, J.C.; Camarero, J.J.; Carreira, J.A. Interacting effects of climate and forest-cover changes on mortality and growth of the southernmost European fir forests. Glob. Ecol. Biogeogr. 2009, 18, 485–497. [Google Scholar] [CrossRef]
- Jian, Z.; Shongming, H.; Fangliang, H. Half-century evidence from western Canada shows forest dynamics are primarly driven by competition followed by climate. Proc. Natl. Acad. Sci. 2015, 12, 4009–4014. [Google Scholar]
- Jones, H.G. Stomatal control of photosynthesis and transpiration. J. Exp. Bot. 1998, 49, 387–398. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modelling isohydric and anisohydric behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; Yepez, E.A. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Poyatos, R.; Aguadé, D.; Retana, J.; Mencuccini, M. A new look at water transport regulation in plants. New Phytol. 2014, 204, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Domec, J.-C.; Johnson, D.M. Does homeostasis or disturbance of homeostasis in minimum leaf water potential explain the isohydric versus anisohydric behavior of Vitis vinifera L. Cultivars? Tree Physiol. 2012, 32, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; McAdam, S.A.M.; Jordan, G.J.; Martins, S.C.V. Conifer species adapt to low-rainfall climates by following one of two divergent pathways. Proc. Natl. Acad. Sci. 2014, 111, 14489–14493. [Google Scholar] [CrossRef] [PubMed]
- Gollan, T.; Turner, N.C.; Schulze, E.D. The responses of stomata and leaf gas exchange to vapour pressure deficits and soil water content. Oecologia 1985, 65, 356–362. [Google Scholar] [CrossRef]
- Hartmann, H.; Ziegler, W.; Kolle, O.; Trumbore, S. Thirst beats hunger-declining hydration during drought prevents carbon starvation in Norway spruce saplings. New Phytol. 2013, 200, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Bréda, N.; Cochard, H.; Dreyer, E.; Granier, A. Water transfer in a mature oak stand (Quercus petraea): Seasonal evolution and effects of a severe drought. Can. J. For. Res. 1993, 23, 1136–1143. [Google Scholar] [CrossRef]
- Eamus, D.; Boulain, N.; Cleverly, J.; Breshears, D.D. Global change-type drought-induced tree mortality: Vapor pressure deficit is more important than temperature per se in causing decline in tree health. Ecol. Evol. 2013, 3, 2711–2729. [Google Scholar] [CrossRef] [PubMed]
- Quero, J.L.; Sterck, F.J.; Martínez-Vilalta, J.; Villar, R. Water-use strategies of six co-existing Mediterranean woody species during a summer drought. Oecologia 2011, 166, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Breshears, D.D. Drought-induced shift of a forest-woodland ecotone: Rapid landscape response to climate variation. Proc. Natl. Acad. Sci. 1998, 95, 14839–14842. [Google Scholar] [CrossRef] [PubMed]
- Galiano, L.; Martínez-Vilalta, J.; Lloret, F. Drought-induced multifactor decline of Scots pine in the Pyrenees and potential vegetation change by the expansion of co-occurring Oak species. Ecosystems 2010, 13, 978–991. [Google Scholar] [CrossRef]
- Lloret, F.; Escudero, A.; Iriondo, J.M.; Martínez-Vilalta, J.; Valladares, F. Extreme climatic events and vegetation: The role of stabilizing processes. Glob. Chang. Biol 2012, 18, 797–805. [Google Scholar] [CrossRef]
- Filella, I.; Llusià, J.; Piñol, J.; Peñuelas, J. Leaf gas exchange and fluorescence of Phillyrea latifolia, Pistacia lentiscus and Quercus ilex saplings in severe drought and high temperature conditions. Environ. Exp. Bot. 1998, 39, 213–220. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Berry, J.A.; Smith, D.D.; Sperry, J.S.; Anderegg, L.D.L.; Field, C.B. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc. Natl. Acad. Sci. 2012, 109, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, M.; Gómez-Aparicio, L.; Quero, J.L.; Valladares, F. Non-linear effects of drought under shade: Reconciling physiological and ecological models in plant communities. Oecologia 2012, 169, 293–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poyatos, R.; Martínez-Vilalta, J.; Čermák, J.; Ceulemans, R.; Granier, A.; Irvine, J.; Köstner, B.; Lagergren, F.; Meiresonne, L.; Nadezhdina, N.; Zimmermann, R.; Llorens, P.; Mencuccini, M. Plasticity in hydraulic architecture of Scots pine across Eurasia. Oecologia 2007, 153, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Martin-StPaul, N.K.; Limousin, J.-M.; Vogt-Schilb, H.; Rodríguez-Calcerrada, J.; Rambal, S.; Longepierre, D.; Misson, L. The temporal response to drought in a Mediterranean evergreen tree: Comparing a regional precipitation gradient and a throughfall exclusion experiment. Glob. Chang. Biol. 2013, 19, 2413–2426. [Google Scholar] [CrossRef] [PubMed]
- Poyatos, R.; Aguadé, D.; Galiano, L.; Mencuccini, M.; Martínez-Vilalta, J. Drought-induced defoliation and long periods of near-zero gas exchange play a key role in accentuating metabolic decline of Scots pine. New Phytol. 2013, 200, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Irvine, J.; Perks, M.P.; Magnani, F.; Grace, J. The response of Pinus sylvestris to drought: Stomatal control of transpiration and hydraulic conductance. Tree Physiol. 1998, 18, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Aguadé, D.; Poyatos, R.; Gómez, M.; Oliva, J.; Martínez-Vilalta, J. The role of defoliation and root rot pathogen infection in driving the mode of drought-related physiological decline in Scots pine (Pinus sylvestris L.). Tree Physiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Galiano, L.; Martínez-Vilalta, J.; Lloret, F. Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 yr after a drought episode. New Phytol. 2011, 190, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Barbeta, A.; Ogaya, R.; Peñuelas, J. Comparative study of diurnal and nocturnal sap flow of Quercus ilex and Phillyrea latifolia in a Mediterranean Holm oak forest in Prades (Catalonia, NE Spain). Trees-Struct. Funct. 2012, 26, 1651–1659. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Mangirón, M.; Ogaya, R.; Sauret, M.; Serrano, L.; Peñuelas, J.; Piñol, J. Sap flow of three co-occurring Mediterranean woody species under varying atmospheric and soil water conditions. Tree Physiol. 2003, 23, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Galiano, L.; Martínez-Vilalta, J.; Sabaté, S.; Lloret, F. Determinants of drought effects on crown condition and their relationship with depletion of carbon reserves in a Mediterranean holm oak forest. Tree Physiol. 2012, 32, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; Piñol, J. Drought-induced mortality and hydraulic architecture in pine populations of the NE Iberian Peninsula. For. Ecol. Manag. 2002, 161, 247–256. [Google Scholar] [CrossRef]
- Ogaya, R.; Barbeta, A.; Başnou, C.; Peñuelas, J. Satellite data as indicators of tree biomass growth and forest dieback in a Mediterranean Holm oak forest. Ann. For. Sci. 2015, 72, 135–144. [Google Scholar] [CrossRef]
- Vilà-Cabrera, A.; Martínez-Vilalta, J.; Galiano, L.; Retana, J. Patterns of forest decline and regeneration across Scots pine populations. Ecosystems 2013, 16, 323–335. [Google Scholar] [CrossRef]
- Poyatos, R. CREAF, Cerdanyola del Vallès, Spain. Unpublished work. 2015. [Google Scholar]
- Ogaya, R.; Peñuelas, J. Phenological patterns of Quercus ilex, Phillyrea latifolia, and Arbutus unedo growing under a field experimental drought. Ecoscience 2004, 11, 263–270. [Google Scholar]
- Hereş, A.M.; Martínez-Vilalta, J.; López, B.C. Growth patterns in relation to drought-induced mortality at two Scots pine (Pinus sylvestris L.) sites in NE Iberian peninsula. Trees-Struct. Func. 2012, 26, 621–630. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J. Universitat Autònoma de Barcelona. Personal communication, 2014. [Google Scholar]
- Rosas, T.; Galiano, L.; Ogaya, R.; Peñuelas, J.; Martínez-Vilalta, J. Dynamics of non-structural carbohydrates in three Mediterranean woody species following long-term experimental drought. Front. Plant Sci. 2013, 4, 400. [Google Scholar] [CrossRef] [PubMed]
- Ogaya Inurrigarra, R. Plant Ecophysiological Responses to a Field Experimental Drought in the Prades Holm Oak Forest; Universitat Autònoma de Barcelona: Cerdanyola del Vallès, Spain, 2003. [Google Scholar]
- Phillips, N.; Oren, R. A comparison of daily representations of canopy conductance based on two conditional time-averaging methods and the dependence of daily conductance on environmental factors. Ann. For. Sci. 1998, 55, 217–235. [Google Scholar] [CrossRef]
- Irvine, J.; Law, B.E.; Kurpius, M.R.; Anthoni, P.M.; Moore, D.; Schwarz, P.A. Age-related changes in ecosystem structure and function and effects on water and carbon exchange in Ponderosa pine. Tree Physiol. 2004, 24, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Pammenter, N.W.; van Der Willigen, C. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiol. 1998, 18, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; Prat, E.; Oliveras, I.; Piñol, J. Xylem hydraulic properties of roots and stems of nine woody species from a Holm oak forest in NE Spain. Oecologia 2002, 133, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Martin-StPaul, N.K.; Longepierre, D.; Huc, R.; Delzon, S.; Burlett, R.; Joffre, R.; Rambal, S.; Cochard, H. How reliable are methods to assess xylem vulnerability to cavitation? The issue of ‘open vessel’ artifact in oaks. Tree Physiol. 2014, 34, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Hoch, G.; Popp, M.; Körner, C. Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos 2002, 98, 361–374. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Cochard, H.; Mencuccini, M.; Sterck, F.; Herrero, A.; Korhonen, J.F.J.; Llorens, P.; Nikinmaa, E.; Nolè, A.; Poyatos, R.; Ripullone, F.; Sass-Klaassen, U.; Zweifel, R. Hydraulic adjustment of Scots pine across Europe. New Phytol. 2009, 184, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, R.; Steppe, K.; Sterck, F.J. Stomatal regulation by microclimate and tree water relations: Interpreting ecophysiological field data with a hydraulic plant model. J. Exp. Bot. 2007, 58, 2113–2131. [Google Scholar] [CrossRef] [PubMed]
- Baquedano, F.J.; Castillo, F.J. Drought tolerance in the Mediterranean species Quercus coccifera, Quercus ilex, Pinus halepensis, and Juniperus phoenicea. Photosynthetica 2007, 45, 229–238. [Google Scholar] [CrossRef]
- Duursma, R.A.; Kolari, P.; Peramaki, M.; Nikinmaa, E.; Hari, P.; Delzon, S.; Loustau, D.; Ilvesniemi, H.; Pumpanen, J.; Makela, A. Predicting the decline in daily maximum transpiration rate of two pine stands during drought based on constant minimum leaf water potential and plant hydraulic conductance. Tree Physiol. 2008, 28, 265. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.M.; Meinzer, F.C.; Brooks, J.R.; Domec, J.C. Vertical stratification of soil water storage and release dynamics in pacific northwest coniferous forests. Agric. For. Meteorol. 2005, 130, 39–58. [Google Scholar] [CrossRef]
- Canadell, J.; Jackson, R.B.; Ehleringer, J.B.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. Maximum rooting depth of vegetation types at the global scale. Oecologia 1996, 108, 583–595. [Google Scholar] [CrossRef]
- Kolb, T.E.; Stone, J.E. Differences in leaf gas exchange and water relations among species and tree sizes in an Arizona pine-oak forest. Tree Physiol. 2000, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Donovan, L.A.; Richards, J.H.; Linton, M.J. Magnitude and mechanisms of disequilibrium between predawn plant and soil water potentials. Ecology 2003, 84, 463–470. [Google Scholar] [CrossRef]
- Plaut, J.A.; Yepez, E.A.; Hill, J.; Pangle, R.; Sperry, J.S.; Pockman, W.T.; McDowell, N.G. Hydraulic limits preceding mortality in a piñon-juniper woodland under experimental drought. Plant, Cell & Environ. 2012, 35, 1601–1617. [Google Scholar]
- Nardini, A.; Gullo, M.A.L.; Trifilò, P.; Salleo, S. The challenge of the Mediterranean climate to plant hydraulics: Responses and adaptations. Environ. Exp. Bot. 2014, 103, 68–79. [Google Scholar] [CrossRef]
- Cochard, H.; Badel, E.; Herbette, S.; Delzon, S.; Choat, B.; Jansen, S. Methods for measuring plant vulnerability to cavitation: A critical review. J. Exp. Bot. 2013. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ruiz, J.M.; Cochard, H.; Mayr, S.; Beikircher, B.; Diaz-Espejo, A.; Rodriguez-Dominguez, C.M.; Badel, E.; Fernández, J.E. Vulnerability to cavitation in Olea europaea current-year shoots: Further evidence of an open-vessel artifact associated with centrifuge and air-injection techniques. Physiol. Plant. 2014, 152, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Urli, M.; Lamy, J.-B.; Sin, F.; Burlett, R.; Delzon, S.; Porté, A.J. The high vulnerability of Quercus robur to drought at its southern margin paves the way for Quercus ilex. Plant Ecol. 2015, 216, 177–187. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sancho-Knapik, D.; Barrón, E.; Camarero, J.J.; Vilagrosa, A.; Gil-Pelegrín, E. Morphological and physiological divergences within Quercus ilex support the existence of different ecotypes depending on climatic dryness. Ann. Bot. 2014, 114, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Salmon, Y.; Torres-Ruiz, J.M.; Poyatos, R.; Martínez-Vilalta, J.; Meir, P.; Cochard, H.; Mencuccini, M. Balancing the risks of hydraulic failure and carbon starvation: A twig scale analysis in declining scots pine. Plant, Cell & Environ. 2015. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Woodruff, D.R.; Marias, D.E.; McCulloh, K.A.; Sevanto, S. Dynamics of leaf water relations components in co-occurring iso- and anisohydric conifer species. Plant, Cell & Environ. 2014, 37, 2577–2586. [Google Scholar]
- Limousin, J.M.; Rambal, S.; Ourcival, J.M.; Rocheteau, A.; Joffre, R.; Rodriguez Cortina, R. Long term transpiration change with rainfall decline in a Mediterranean Quercus ilex forest. Glob. Chang. Biol. 2009, 15, 2163–2175. [Google Scholar] [CrossRef]
- Pangle, R.E.; Limousin, J.-M.; Plaut, J.A.; Yepez, E.A.; Hudson, P.J.; Boutz, A.L.; Gehres, N.; Pockman, W.T.; McDowell, N.G. Prolonged experimental drought reduces plant hydraulic conductance and transpiration and increases mortality in a piñon-juniper woodland. Ecol. Evol. 2015, 5, 1618–1638. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.J.; O’Grady, A.P.; Tissue, D.T.; White, D.A.; Ottenschlaeger, M.L.; Pinkard, E.A. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytol. 2013, 197, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Urli, M.; Porté, A.J.; Cochard, H.; Guengant, Y.; Burlett, R.; Delzon, S. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol. 2013, 33, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Barbeta, A.; Mejía-Chang, M.; Ogaya, R.; Voltas, J.; Dawson, T.E.; Peñuelas, J. The combined effects of a long-term experimental drought and an extreme drought on the use of plant-water sources in a Mediterranean forest. Glob. Chang. Biolo. 2014, 21, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Ogaya, R.; Peñuelas, J. Tree growth, mortality, and above-ground biomass accumulation in a Holm oak forest under a five-year experimental field drought. Plant Ecol. 2007, 189, 291–299. [Google Scholar] [CrossRef]
- Rigling, A.; Bigler, C.; Eilmann, B.; Feldmeyer-Christe, E.; Gimmi, U.; Ginzler, C.; Graf, U.; Mayer, P.; Vacchiano, G.; Weber, P. Driving factors of a vegetation shift from scots pine to pubescent oak in dry alpine forests. Glob. Chang. Biol. 2013, 19, 229–240. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguadé, D.; Poyatos, R.; Rosas, T.; Martínez-Vilalta, J. Comparative Drought Responses of Quercus ilex L. and Pinus sylvestris L. in a Montane Forest Undergoing a Vegetation Shift. Forests 2015, 6, 2505-2529. https://doi.org/10.3390/f6082505
Aguadé D, Poyatos R, Rosas T, Martínez-Vilalta J. Comparative Drought Responses of Quercus ilex L. and Pinus sylvestris L. in a Montane Forest Undergoing a Vegetation Shift. Forests. 2015; 6(8):2505-2529. https://doi.org/10.3390/f6082505
Chicago/Turabian StyleAguadé, David, Rafael Poyatos, Teresa Rosas, and Jordi Martínez-Vilalta. 2015. "Comparative Drought Responses of Quercus ilex L. and Pinus sylvestris L. in a Montane Forest Undergoing a Vegetation Shift" Forests 6, no. 8: 2505-2529. https://doi.org/10.3390/f6082505





