Effects of a Wildfire on Selected Physical, Chemical and Biochemical Soil Properties in a Pinus massoniana Forest in South China
Abstract
:1. Introduction
2. Material and Methods
2.1. Site
2.2. Methods
2.3. Statistical Analyses
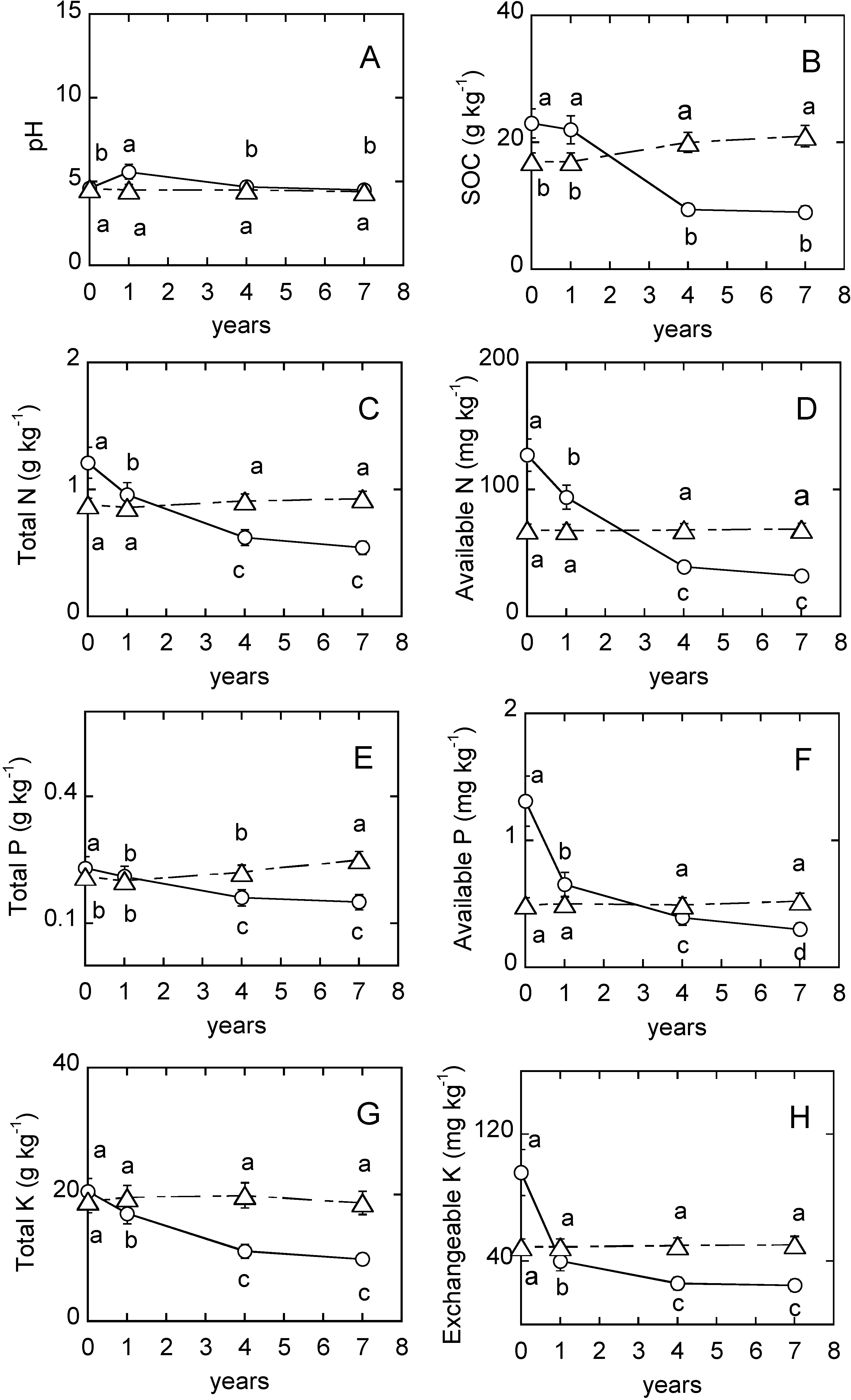
3. Results
| Variable | Treatment effect | Time effect | Treatment × time | |||
|---|---|---|---|---|---|---|
| F-value | p-value | F-value | p-value | F-value | p-value | |
| Bulk density | 82.06 | <0.0001 | 14.75 | <0.0001 | 29.14 | <0.0001 |
| Capillary porosity | 15.59 | 0.0012 | 5.8 | 0.0070 | 6.21 | 0.0053 |
| Noncapillary porosity | 44.94 | <0.0001 | 7.58 | 0.0020 | 7.06 | 0.0031 |
| Capillary moisture | 141.35 | <0.0001 | 28.91 | <0.0001 | 23.32 | <0.0001 |
| pH | 77.96 | <0.0001 | 44.56 | <0.0001 | 37.68 | <0.0001 |
| Organic carbon | 122.74 | <0.0001 | 125.20 | <0.0001 | 395.35 | <0.0001 |
| Total N | 11053.0 | <0.0001 | 31.82 | <0.0001 | 54.31 | <0.0001 |
| Available N | 50.46 | <0.0001 | 1127.31 | <0.0001 | 1145.71 | <0.0001 |
| Total P | 27.65 | <0.0001 | 3.90 | 0.0289 | 25.71 | <0.0001 |
| Available P | 272.44 | <0.0001 | 521.10 | <0.0001 | 558.74 | <0.0001 |
| Total K | 809.47 | <0.0001 | 224.74 | <0.0001 | 224.04 | <0.0001 |
| Available K | 51.14 | <0.0001 | 1381.81 | <0.0001 | 1442.90 | <0.0001 |
| Urease | 26513.6 | <0.0001 | 667.09 | <0.0001 | 9.63 | 0.0007 |
| Acid phosphatase | 20344.6 | <0.0001 | 182.03 | <0.0001 | 257.04 | <0.0001 |
| Catalase | 2264.07 | <0.0001 | 361.95 | <0.0001 | 77.03 | <0.0001 |
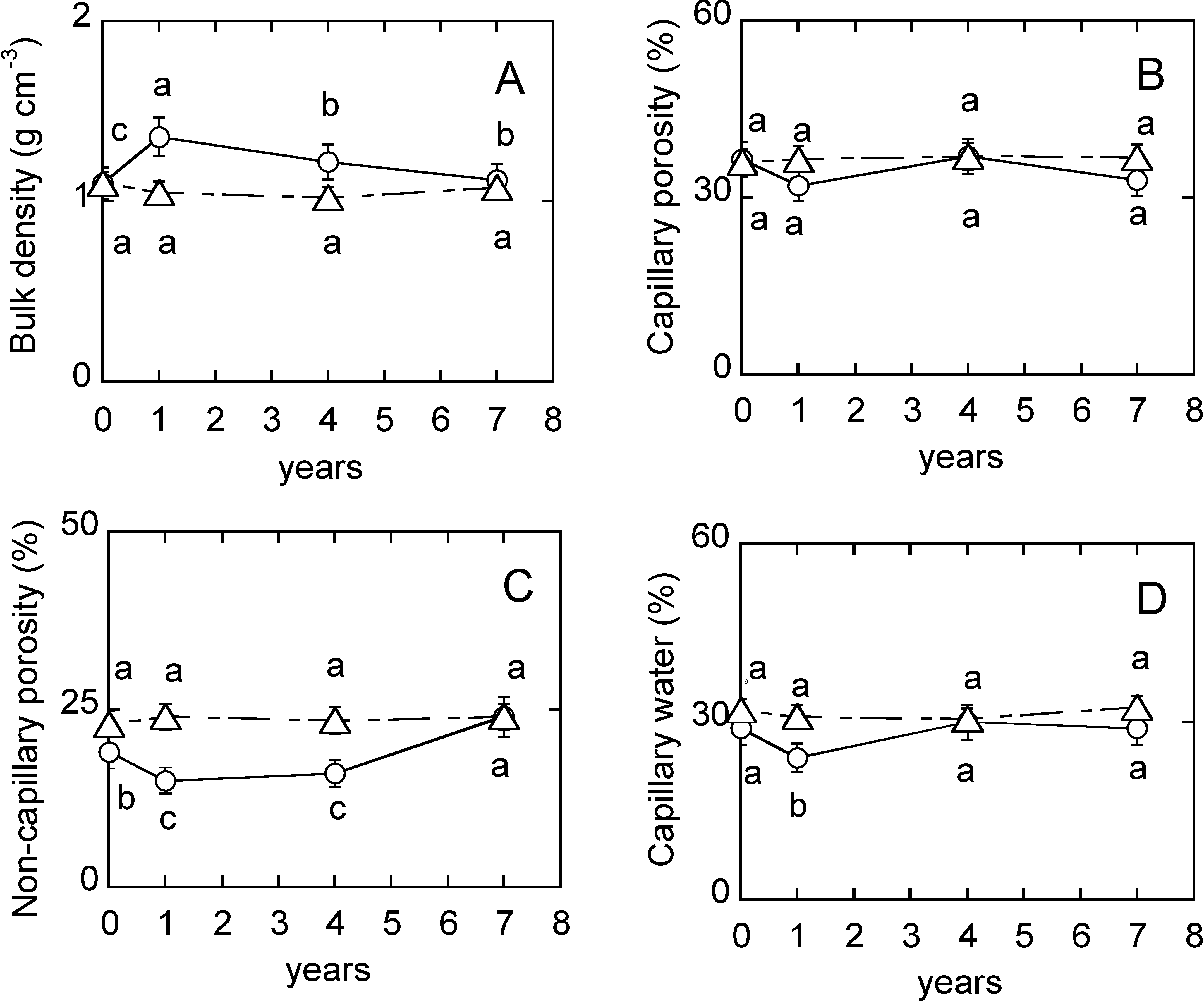
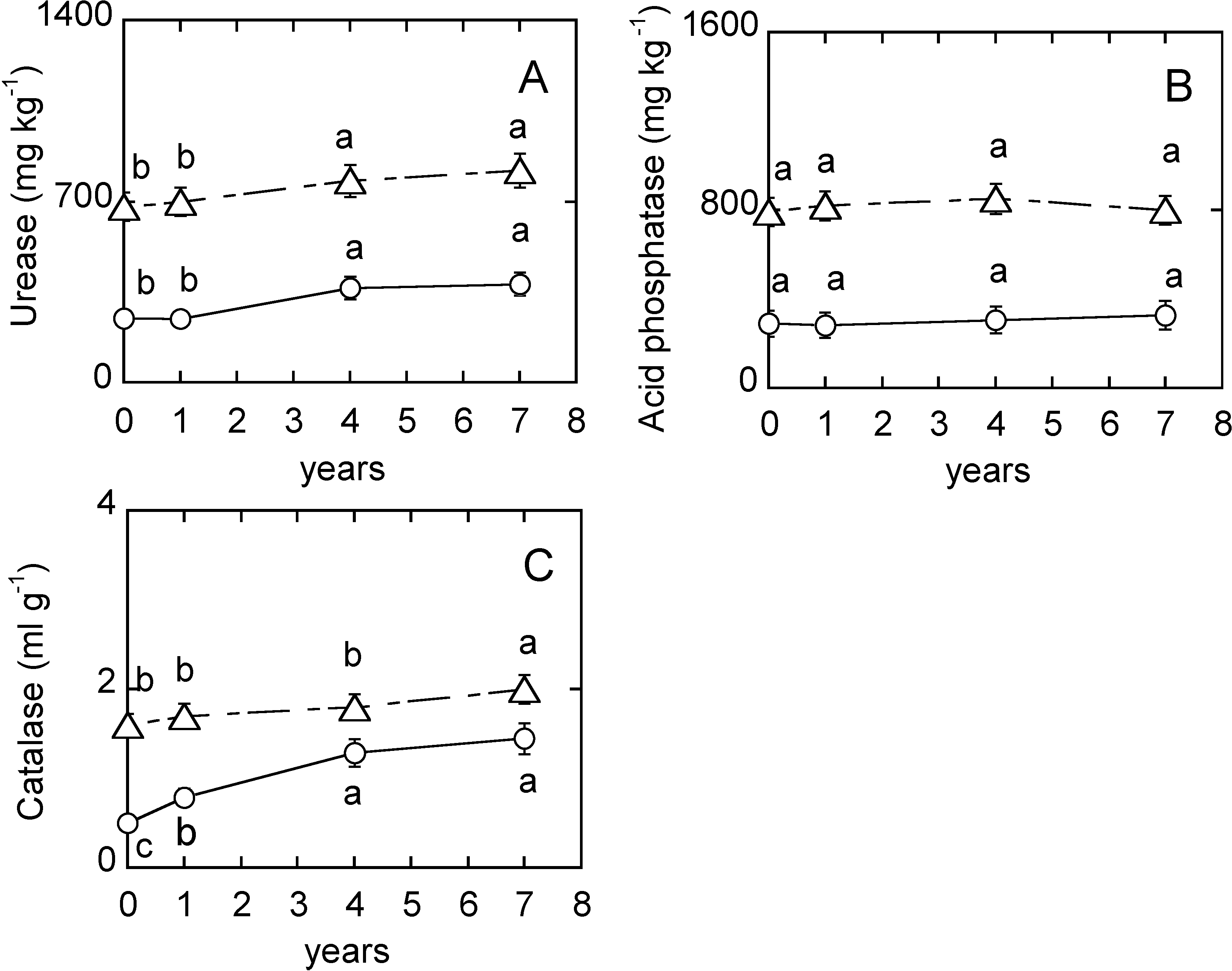
4. Discussion
4.1. Soil Physical Properties
4.2. Soil pH
4.3. Soil Organic Carbon
4.4. Soil Nutrients
4.5. Soil Enzymes
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gray, D.M.; Dighton, J. Nutrient utilization by pine seedlings and soil microbes in oligotrophic pine barrens forest soils subjected to prescribed fire treatment. Soil Biol. Biochem. 2009, 41, 1957–1965. [Google Scholar] [CrossRef]
- Dezzeo, N.; Chacón, N. Carbon and nutrients loss in aboveground biomass along a fire induced forest-savanna gradient in the Gran Sabana, southern Venezuela. For. Ecol. Manag. 2005, 209, 343–352. [Google Scholar]
- Viegas, D.X. Forest fires in Portugal in 2005—an overview. Int. For. Fire News 2006, 34, 22–30. [Google Scholar]
- Castro, A.C.M.; Carvalho, J.P.; Meixedo, J.P. A qualitative description of soil parameters variation due to a prescribed fire in Portuguese northwestern forests using Fuzzy Boolean Nets—The case study of Cabreira mountain. Geoderma 2012, 191, 89–96. [Google Scholar]
- Neill, C.; Patterson, W.A., III; Crary, D.W., Jr. Responses of soil carbon, nitrogen and cations to the frequency and seasonality of prescribed burning in a Cape Cod oak-pine forest. For. Ecol. Manag. 2007, 250, 234–243. [Google Scholar] [CrossRef]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliott, P.F. Fire effects on belowground sustainability: A review and synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- DeBano, L.F.; Neary, D.G.; Ffolliott, D.F. Fire’s Effects on Ecosystems; John Wiley and Sons, Inc.: New York, NY, USA, 1998; p. 612. [Google Scholar]
- Michalzik, B.; Martin, S. Effects of experimental duff fires on C, N and P fluxes into the mineral soil at a coniferous and broadleaf forest site. Geoderma 2013, 197–198, 169–176. [Google Scholar]
- Arocena, J.M.; Opio, C. Prescribed fire-induced changes in properties of subboreal forest soils. Geoderma 2003, 113, 1–16. [Google Scholar] [CrossRef]
- Wells, C.G.; Campbell, R.E.; DeBano, L.F.; Lewis, C.E.; Fedriksen, R.L.; Franklin, E.C.; Froelich, R.C.; Dunn, P.H. Effects of fire on soil: A state-of-knowledge review. In USDA Forest Service General Technical Report WO-7; USDA Forest Service: Washington, DC, USA, 1979. [Google Scholar]
- Scharenbroch, B.C.; Nix, B.; Jacobs, K.A.; Bowles, M.L. Two decades of low-severity prescribed fire increases soil nutrient availability in a Midwestern, USA oak (Quercus) forest. Geoderma 2012, 183–184, 80–91. [Google Scholar]
- Wanthongchai, K.; Bauhus, J.; Goldammer, J.G. Nutrient losses through prescribed burning of aboveground litter and understorey in dry dipterocarp forests of different fire history. Catena 2008, 74, 321–332. [Google Scholar]
- Ulery, A.L.; Graham, R.C. Forest fire effects soil color and texture. Soil Sci. Soc. Am. J. 1993, 57, 135–140. [Google Scholar] [CrossRef]
- Wüthrich, C.; Schaub, D.; Weber, M.; Marxer, P.; Conedera, M. Soil respiration and soil microbial biomass after fire in a sweet chestnut forest in southern Switzerland. Catena 2002, 48, 201–215. [Google Scholar] [CrossRef]
- Miesel, J.R.; Goebel, P.C.; Corace, R.G., III; Hix, D.M.; Kolka, R.; Palik, B.; Mladenoff, D. Fire Effects on soils in Lake States Forests: A compilation of published research to facilitate long-term investigations. Forests 2012, 3, 1034–1070. [Google Scholar]
- Hamman, S.T.; Burke, I.C.; Knapp, E.E. Soil nutrients and microbial activity after early and late season prescribed burns in a Sierra Nevada mixed conifer forest. For. Ecol. Manag. 2008, 256, 367–374. [Google Scholar] [CrossRef]
- Bárcenas-Moreno, G.; García-Orenes, F.; Mataix-Solera, J.; Mataix-Beneyto, J.; Bååth, E. Soil microbial recolonisation after a fire in a Mediterranean forest. Biol. Fertil. Soils 2011, 47, 261–272. [Google Scholar] [CrossRef]
- Boerner, R.E.J.; Decker, K.L.M.; Sutherland, E.K. Prescribed burning effects on soil enzyme activity in a southern Ohio hardwood forest: A landscape-scale analysis. Soil Biol. Biochem. 2000, 32, 899–908. [Google Scholar] [CrossRef]
- Boerner, R.E.J.; Brinkman, J.A.; Smith, A. Seasonal variations in enzyme activity and organic carbon in soil of a burned and unburned hardwood forest. Soil Biol. Biochem. 2005, 37, 1419–1426. [Google Scholar] [CrossRef]
- Carter, M.C.; Foster, C.D. Prescribed burning and productivity in southern pine forests: A review. For. Ecol. Manag. 2004, 191, 93–109. [Google Scholar]
- Geldenhuys, C.J.; van Wilgen, B.W. Fire effects on the maintenance of biodiversity, soil and nutrients. In Wildland Fire Management Handbook for Sub-Sahara Africa; Goldammer, J.G., de Ronde, C., Eds.; Global Fire Monitoring Center (GFMC): Freiburg, Germany, 2004; pp. 88–113. [Google Scholar]
- Morley, S.; Grant, C.; Hobbs, R.; Cramer, V. Long-term impact of prescribed burning on the nutrient status and fuel loads of rehabilitated bauxite mines in Western Australia. For. Ecol. Manag. 2004, 190, 227–239. [Google Scholar]
- Kashian, D.M.; Romme, W.H.; Tinker, D.B.; Turner, M.G.; Ryan, M.G. Carbon storage on landscapes with stand-replacing fires. Bioscience 2006, 56, 598–606. [Google Scholar] [CrossRef]
- Gough, C.M.; Vogel, C.S.; Harrold, K.H.; George, K.; Curtis, P.S. The legacy of harvest and fire on ecosystem carbon storage in a north temperate forest. Glob. Change Biol. 2007, 13, 1935–1949. [Google Scholar] [CrossRef]
- Irvine, J.; Law, B.E.; Hibbard, K.A. Postfire carbon pools and fluxes in semiarid ponderosa pine in Central Oregon. Glob. Change Biol. 2007, 13, 1748–1760. [Google Scholar] [CrossRef]
- Kurz, W.A.; Stinson, G.; Rampley, G.J.; Dymond, C.C.; Neilson, E.T. Risk of natural disturbances makes future contribution of Canada’s forests to the global carbon cycle highly uncertain. Proc. Natl. Acad. Sci. USA 2008, 105, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Meigs, G.W.; Donato, D.C.; Campbell, J.L.; Martin, J.G.; Law, B.E. Forest Fire Impacts on Carbon Uptake, Storage, and Emission: The Role of Burn Severity in the Eastern Cascades, Oregon. Ecosystem 2009, s12, 1246–1267. [Google Scholar] [CrossRef]
- Ponder, F., Jr.; Tadros, M.; Loewenstein, E.F. Microbial properties and litter and soil nutrients after two prescribed fires in developing savannas in an upland Missouri Ozark Forest. For. Ecol. Manag. 2009, 257, 755–763. [Google Scholar] [CrossRef]
- Xue, L.; Hagihara, A. Growth analysis on the competition-density effect in Chinese fir (Cunninghamia lanceolata) and Masson pine (Pinus massoniana) stands. For. Ecol. Manag. 2001, 150, 331–337. [Google Scholar] [CrossRef]
- Xue, L.; Lie, G.W.; Lu, G.C.; Shao, Y.R. Allometric scaling among tree components in Pinus massoniana stands with different sites. Ecol. Res. 2013, 28, 327–333. [Google Scholar]
- Fölster, H.; Dezzeo, N.; Priess, J.A. Soil-vegetation relationship in base-deficient premontane moist forest-savanna mosaics of the Venezuelan Guayana. Geoderma 2001, 104, 95–113. [Google Scholar]
- Priess, J.A.; Fölster, H. Microbial properties and soil respiration in submontane forests of Venezuelan Guayana: Characteristics and response to fertilizer treatments. Soil Biol. Biochem. 2001, 33, 503–509. [Google Scholar] [CrossRef]
- Baird, M.; Zabowski, D.; Everett, R.L. Wildfire effects on carbon and nitrogen in inland coniferous forests. Plant Soil 1999, 209, 233–243. [Google Scholar] [CrossRef]
- Johnson, D.W.; Murphy, J.D.; Susfalk, R.B.; Caldwell, T.G.; Miller, W.W.; Walker, R.F.; Powers, R.F. The effects of wildfire, salvage logging, and post-fire N fixation on the nutrient budgets of a Sierran forest. For. Ecol. Manag. 2005, 220, 155–165. [Google Scholar]
- Johnson, D.; Murphy, J.D.; Walker, R.F.; Glass, D.W.; Miller, W.W. Wildfire effects on forest carbon and nutrient budgets. Ecol. Eng. 2007, 31, 183–192. [Google Scholar] [CrossRef]
- Bartoli, F.; Boivin, A.; Schiavon, M. Water and herbicide transient flow transport in field dried topsoils during controlled infiltration I. Water capillary and gravity-driven transient flows: A preliminary examination. Geoderma 2006, 135, 269–283. [Google Scholar] [CrossRef]
- Yi, X.S.; Li, G.S.; Yin, Y.Y. Comparison of three methods to develop pedotransfer functions for the saturated water content and field water capacity in permafrost region. Cold Reg. Sci. Technol. 2013, 88, 10–16. [Google Scholar] [CrossRef]
- Zhang, W.R.; Yang, G.Y.; Tu, X.Y.; Zhang, P. Determination of forest soil water-physical properties. China Criterion of Forest Technique, 1999. No. LY/T 1215. (In Chinese) [Google Scholar]
- Cheng, S.; Yang, G.; Yu, H.; Li, J.Y.; Zhang, L. Impacts of Wenchuan Earthquake-induced landslides on soil physical properties and tree growth. Ecol. Indic. 2012, 15, 263–270. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods of Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis, Part 2, 2nd ed.; Page, A.L., Miller, R.H., Keeney, R.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Guan, S.Y. Soil Enzyme and Its Research Methods; Chinese Agricultural Press: Beijing, China, 1986; pp. 295–323. (In Chinese) [Google Scholar]
- Powers, R.F.; Alban, D.H.; Miller, R.E.; Tiarks, A.E.; Wells, C.G.; Avers, P.E.; Cline, R.G.; Fitzgerald, R.O.; Loftus, N.S. Sustaining site productivity in North American forests: Problems and prospects. In Sustained Productivity of Forest Soils, Proceedings of the 7th North American Forest Soils Conference, University of British Columbia, Vancouver, BC, Canada, July 1988; Gessel, S.A., Lacate, D.S., Weetman, G.F., Powers, R.F., Eds.; pp. 49–79.
- Yildiz, O.; Esen, D.; Karaoz, O.M.; Sarginci, M.; Toprak, B.; Soysal, Y. Effects of different site preparation methods on soil carbon and nutrient removal from Eastern beech regeneration sites in Turkey’s Black Sea region. Appl. Soil Ecol. 2010, 45, 49–55. [Google Scholar] [CrossRef]
- Neary, D.G.; Jokela, E.J.; Comerford, N.B.; Colbert, S.R.; Cooksey, T.E. Understanding competition for soil nutrients-the key to site productivity on southeastern Coastal Plain spodosols. In Sustained Productivity of Forest Soils, Proceedings of the 7th North American Forest Soils Conference, University of British Columbia, Forestry Publications, Faculty of Forestry, Vancouver, BC, Canada, 24–28 July 1988; Gessel, S.P., Lacate, D.S., Weetman, G.F., Powers, R.F., Eds.; pp. 432–450.
- Ketterings, Q.M.; Bigham, J.M.; Laperche, V. Changes in soil mineralogy and texture caused by slash and burn fires in Sumatra, Indonesia. Soil. Sci. Soc. Am. J. 2000, 64, 1108–1117. [Google Scholar] [CrossRef]
- Marcos, E.; Tarrega, R.; Luis-Calabuig, E. Comparative analysis of runoff and sediment yield with a rainfall simulator after experimental fire. Arid Soil Res. Rehab. 2000, 14, 293–307. [Google Scholar]
- Giovannini, G.; Lucchesi, S.; Giachetti, M. Effects of heating on some physical and chemical parameters related to soil aggregation and erodibility. Soil Sci. 1988, 146, 255–261. [Google Scholar]
- Durgin, P.B.; Vogelsang, P.J. Dispersion of kaolinite by water extracts of Douglas-fir ash. Can. J. Soil Sci. 1984, 64, 439–443. [Google Scholar] [CrossRef]
- McIntosh, P.D.; Laffan, M.D.; Hewitt, A.E. The role of fire and nutrient loss in the genesis of the forest soils of Tasmania and southern New Zealand. For. Ecol. Manag. 2005, 220, 185–215. [Google Scholar] [CrossRef]
- Granged, A.J.P.; Jordán, A.; Zavala, L.M.; Muñoz-Rojas, M.; Mataix-Solera, J. Short-term effects of experimental fire for a soil under eucalyptus forest (SE Australia). Geoderma 2011, 167–168, 125–134. [Google Scholar] [CrossRef]
- Rhoades, C.C.; Meier, A.J.; Rebertus, A.J. Soil properties in fire-consumed log burnout openings in a Missouri oak savanna. For. Ecol. Manag. 2004, 192, 277–284. [Google Scholar]
- Úbeda, X.; Lorca, M.; Outeiro, L.R.; Bernia, S.; Castellnou, M. The effects of prescribed fire on soil quality (Prades Mountains, North East Spain). Int. J. Wildland Fire 2005, 14, 379–384. [Google Scholar] [CrossRef]
- Jordán, A.; González, F.A.; Zavala, L.M. Re-establishment of soil water repellency after destruction by intense burning in a Mediterranean heathland (SW Spain). Hydrol. Process 2010, 24, 736–748. [Google Scholar] [CrossRef]
- Pereira, P.; Bodí, M.; Úbeda, X.; Cerdà, A.; Mataix-Solera, J.; Balfour, V.; Woods, S. Las cenizas en el ecosistema suelo. In Actualización en métodos y técnicas para el estudio de los suelos afectados por incendios forestales; Cerdà, A., Jordán, A., Eds.; Càtedra deDivulgació de la Ciència, Universitat de València: Valencia, Spain, 2010; pp. 349–402. [Google Scholar]
- Zhang, X.; Zhu, J.; Cui, Y.C.; Huo, D.; Wang, L.L.; Wu, P.; Chen, J.; Pan, D.Q.; Yang, C.H. Influence of fire on a Pinus massoniana soil in a karst mountain area at the center of Guizhou Province, China. Acta Ecol. Sin. 2011, 31, 5809–5817, (In Chinese with English abstract). [Google Scholar]
- Johnson, D.L.; Curtis, P.S. Effects of forest management on soil C and N storage: Meta analysis. For. Ecol. Manag. 2001, 140, 227–238. [Google Scholar] [CrossRef]
- Doerr, S.H.; Shakesby, R.A.; Walsh, R.P.D. Soil water repellency: Its causes, characteristics and hydrogeomorphological significance. Earth Sci. Rev. 2000, 51, 33–65. [Google Scholar] [CrossRef]
- Kovacic, D.A.; Swift, D.M.; Ellis, J.E.; Hakonson, T.E. Immediate effects of prescribed burning on mineral soil nitrogen in ponderosa pine of New Mexico. Soil Sci. 1986, 141, 71–75. [Google Scholar]
- Schoch, P.; Binkley, D. Prescribed burning increased nitrogen availability in a mature loblolly pine stand. For. Ecol. Manag. 1986, 14, 13–22. [Google Scholar] [CrossRef]
- Covington, W.W.; Sackett, S.S. Soil mineral nitrogen changes following prescribed burning in ponderosa pine. For. Ecol. Manag. 1992, 54, 175–191. [Google Scholar] [CrossRef]
- Raison, R.J.; Khanna, P.K.; Woods, P.V. Mechanisms of element transfer to the atmosphere during vegetation fires. Can. J. For. Res. 1985, 15, 132–140. [Google Scholar] [CrossRef]
- Bell, R.L.; Binkley, D. Soil nitrogen mineralization and immobilization in response to periodic prescribed fire in a loblolly pine plantation. Can. J. For. Res. 1989, 19, 816–820. [Google Scholar] [CrossRef]
- Knoepp, J.D.; Swank, W.T. Comparison of available soil nitrogen assays in control and burned forest sites. Soil. Sci. Soc. Am. J. 1995, 59, 1750–1754. [Google Scholar] [CrossRef]
- Moghaddas, E.E.Y.; Stephens, S.L. Thinning, burning, and thin-burn fuel treatment effects on soil properties in a Sierra Nevada mixed conifer forests. For. Ecol. Manag. 2007, 250, 156–166. [Google Scholar] [CrossRef]
- Sands, R. Physical changes to sandy soils planted to radiate pine. In Proceedings of the IUFRO Symposium on Forest Site and Continuous Productivity, Seattle, Washington, 22–28 August 1982; Ballard, R., Gessel, S.P., Eds.;
- Busse, M.D.; DeBano, L.F. Soil biology. In Wildland Fire in Ecosystems: Effects of Fire on Soil and Water; General Technical Report RMRS-GTR-42-vol. 4; Neary, D.G., Ryan, K.C., DeBano, L.F., Eds.; USDA Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2005; pp. 73–91. [Google Scholar]
- Zhang, Y.M.; Wu, N.; Zhou, G.Y.; Bao, W.K. Changes in enzyme activities of spruce (Picea balfouriana) forest soil as related to burning in the eastern Qinghai-Tibetan Plateau. Appl. Soil Ecol. 2005, 30, 215–225. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Sui, Y.Y.; Zhang, X.D.; Meng, K.; Herbert, S.J. Spatial Variability of Nutrient Properties in Black Soil of Northeast China. Pedosphere 2007, 17, 19–29. [Google Scholar] [CrossRef]
- Gundale, M.J.; DeLuca, T.H.; Fiedler, C.E.; Ramsey, P.W.; Harrington, M.G.; Gannon, J.E. Restoration management in a Montana ponderosa pine forest: Effects on soil physical, chemical, and biological properties. For. Ecol. Manag. 2005, 213, 25–38. [Google Scholar] [CrossRef]
- Kaye, J.; Hart, S.C. Ecological restoration alters nitrogen transformations in a ponderosa pine-bunchgrass ecosystem. Ecol. Appl. 1998, 8, 1052–1060. [Google Scholar]
- Grogan, P.; Bruns, T.D.; Chapin, F.S., III. Fire effects on ecosystem nitrogen cycling in a Californian bishop pine forest. Oecologia 2000, 122, 537–544. [Google Scholar] [CrossRef]
- MacKenzie, M.D.; DeLuca, T.H.; Sala, A. Forest structure and organic matter analysis along a fire chronosequence in the low elevation forests of western Montana. For. Ecol. Manag. 2004, 203, 331–343. [Google Scholar] [CrossRef]
- Weston, C.J.; Attiwill, P.M. Effects of fire and harvesting on nitrogen transformations and ionic mobility in soils of Eucalyptus regnans forests of south-eastern Australia. Oecologia 1990, 83, 20–26. [Google Scholar] [CrossRef]
- Miesel, J.R.; Skinner, C.M.; Boerner, R.E.J. Impact of Fire on Soil Resource Patterns in a Northern California Montane Ecosystem. In Proceedings of the 23rd Tall Timbers Fire Ecology Conference: Fire in Grassland and Shrubland Ecosystems, Tall Timbers Research Station, Tallahassee, FL, USA, 17–20 October 2005; Masters, R.E., Galley, K.E.M., Eds.; pp. 94–102.
- Wallbrink, P.; Blake, W.; Doerr, S.; Shakesby, R.; Humphreys, G.; English, P. Using Tracer Based Sediment Budgets to Assess Redistribution of Soil and Organic Material after Severe Bush Fires; Walling, D.E., Horowitz, A.J., Eds.; Sediment Budgets, IAHS Publication: Wallingford, UK, 2005; pp. 223–230. [Google Scholar]
- Giardina, C.P.; Sanford, R.L.; Døckersmith, I.C. Changes in soil phosphorus and nitrogen during slash and burn clearing of a dry tropical forest. Soil Sci. Soc. Am. J. 2000, 64, 399–405. [Google Scholar] [CrossRef]
- Klopatek, J.M. Nitrogen mineralization and nitrification in mineral soils of pinyon-juniper ecosystems. Soil Sci. Soc. Am. J. 1987, 51, 453–457. [Google Scholar] [CrossRef]
- Huffman, E.L.; MacDonald, L.H.; Stednick, J.D. Strength and persistence of fire-induced soil hydrophobicity under ponderosa and lodgepole pine, Colorado Front Range. Hydrol. Process 2001, 15, 2877–2892. [Google Scholar] [CrossRef]
- Cade-Menun, B.J.; Berch, S.M.; Preston, C.M.; Lavkulich, L.M. Phosphorus forms and related soil chemistry of Podzolic soils on northern Vancouver Island. II. The effects of clear-cutting and burning. Can. J. For. Res. 2000, 30, 1726–1741. [Google Scholar]
- Serrasolsas, I.; Khanna, P.K. Changes in heated and autoclaved forest soils of S.E. Australia. II. Phosphorus and phosphatase activity. Biogeochemistry 1995, 29, 25–41. [Google Scholar]
- Romanya, J.; Khanna, P.K.; Raison, R.J. Effects of slash burning on soil phosphorus fractions and sorption and desorption of phosphorus. For. Ecol. Manag. 1994, 65, 89–103. [Google Scholar] [CrossRef]
- Macadam, A.M. Effects of broadcast slash burning on fuels and soil chemical properties in the sub-boreal spruce zone of central British Columbia. Can. J. For. Res. 1987, 17, 1577–1584. [Google Scholar] [CrossRef]
- Badìa, D.; Martí, C. Plant ash and heat intensity effects on chemical and physical properties of two contrasting soils. Arid Land Res. Manag. 2003, 17, 23–41. [Google Scholar] [CrossRef]
- Adams, P.W.; Boyle, J.R. Effects of fire on soil nutrients in clearcut and whole-tree harvest sites in Central Michigan. Soil Sci. Soc. Am. J. 1980, 44, 847–850. [Google Scholar] [CrossRef]
- Tomkins, I.B.; Kellas, J.D.; Tolhurst, K.G.; Oswin, D.A. Effects of fire intensity on soil chemistry in a eucalypt (Eucalyptus sp.) forest. Aust. J. Soil Res. 1991, 29, 25–47. [Google Scholar] [CrossRef]
- Liu, F.L.; Zhang, S.Y.; Zeng, S.Q.; Wang, W. Effects of fire disturbance on soil chemical properties of Pinus Massoniana forest. Chin. J. Soil Sci. 2009, 40, 1270–1275, (In Chinese with English abstract). [Google Scholar]
- Kong, J.J.; Yang, J. Effects of fire on soil properties and nutrient availability in a Dahurian larch forest in Great Xing’an Mountains of Northeast China. Chin. J. Ecol. 2013, 32, 2837–2843, (In Chinese with English abstract). [Google Scholar]
- Gu, H.Y.; Jin, J.B.; Chen, X.W.; Wang, E.H.; Zou, Y.Y.; Chai, Y.F. The Long-term impacts on chemical properties of Larix gmelini forest on the northern slope of Greater Xing’an Mountains from a forest fire of varying fire intensity. J. Nat. Resour. 2010, 25, 1114–1121, (In Chinese with English abstract). [Google Scholar]
- Hernández, T.; García, C.; Reinhardt, I. Short-term effect of wildfire on the chemical, biochemical and microbiological properties of Mediterranean pine forest soils. Biol. Fer. Soils 1997, 25, 109–116. [Google Scholar] [CrossRef]
- Nannipieri, P.; Nuccini, L.; Ciardi, C. Microbial biomass and enzyme activities: Production and persistence. Soil Biol. Biochem. 1983, 15, 679–685. [Google Scholar] [CrossRef]
- Kandeler, E.; Eder, G. Effect of cattle slurry in grasslands on microbial biomass and on activities of various enzymes. Soil Biol. Biochem. 1993, 16, 249–254. [Google Scholar]
- Waldrop, M.P.; Zak, D.R.; Sinsabaugh, R.L.; Gallo, M.; Lauber, C. Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol. Appl. 2004, 14, 1172–1177. [Google Scholar] [CrossRef]
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J.; Simberloff, D.; Swanson, F.J.; Stocks, B.J.; Wotton, B.M. Climate change and forest disturbances. BioScience 2001, 51, 723–734. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, L.; Li, Q.; Chen, H. Effects of a Wildfire on Selected Physical, Chemical and Biochemical Soil Properties in a Pinus massoniana Forest in South China. Forests 2014, 5, 2947-2966. https://doi.org/10.3390/f5122947
Xue L, Li Q, Chen H. Effects of a Wildfire on Selected Physical, Chemical and Biochemical Soil Properties in a Pinus massoniana Forest in South China. Forests. 2014; 5(12):2947-2966. https://doi.org/10.3390/f5122947
Chicago/Turabian StyleXue, Li, Qiujing Li, and Hongyue Chen. 2014. "Effects of a Wildfire on Selected Physical, Chemical and Biochemical Soil Properties in a Pinus massoniana Forest in South China" Forests 5, no. 12: 2947-2966. https://doi.org/10.3390/f5122947




