Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer
Abstract
:1. Introduction
2. Experimental Methods
2.1. Characterization
2.2. Materials
2.3. Sample Preparation
3. Results and Discussion
3.1. Formula of Alkali Activated Metakaolin-Based Geopolymer
3.2. Influence of Curing Temperature and Time on the Properties of Alkali Activated Metakaolin-Based Geopolymer
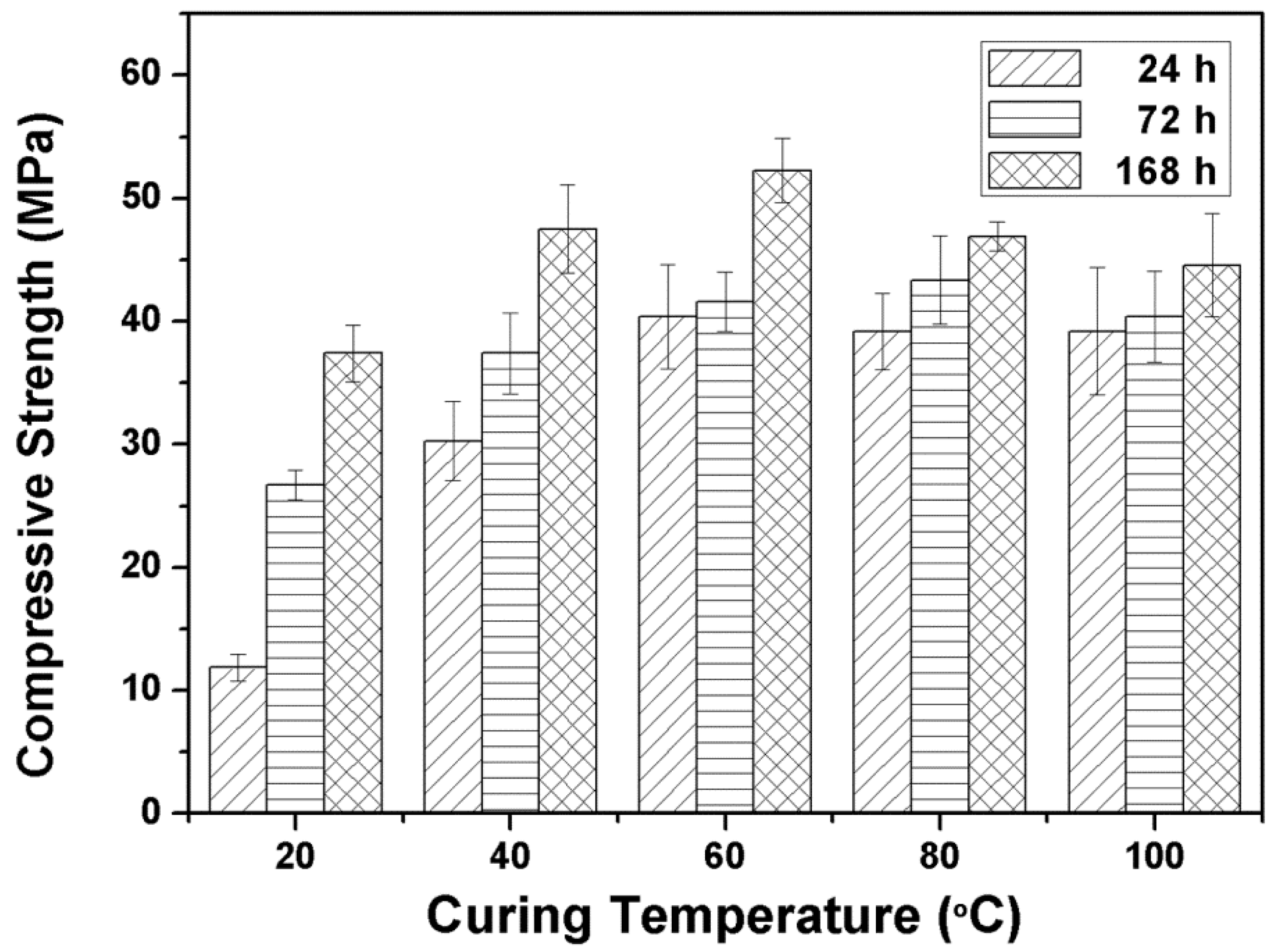
3.2.1. Influence of Curing Temperature and Time on the Compressive Strength of Alkali Activated Metakaolin-Based Geopolymers
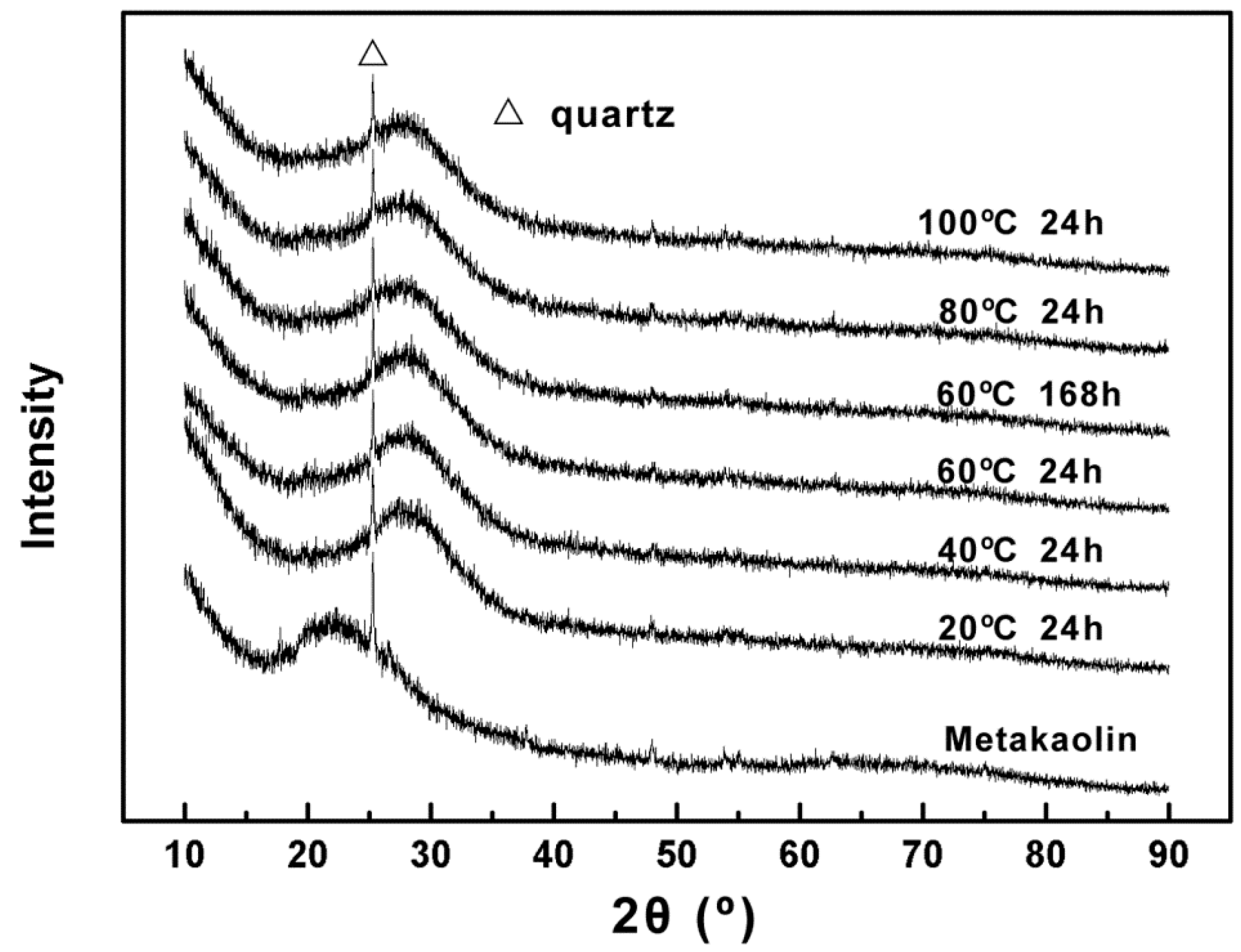
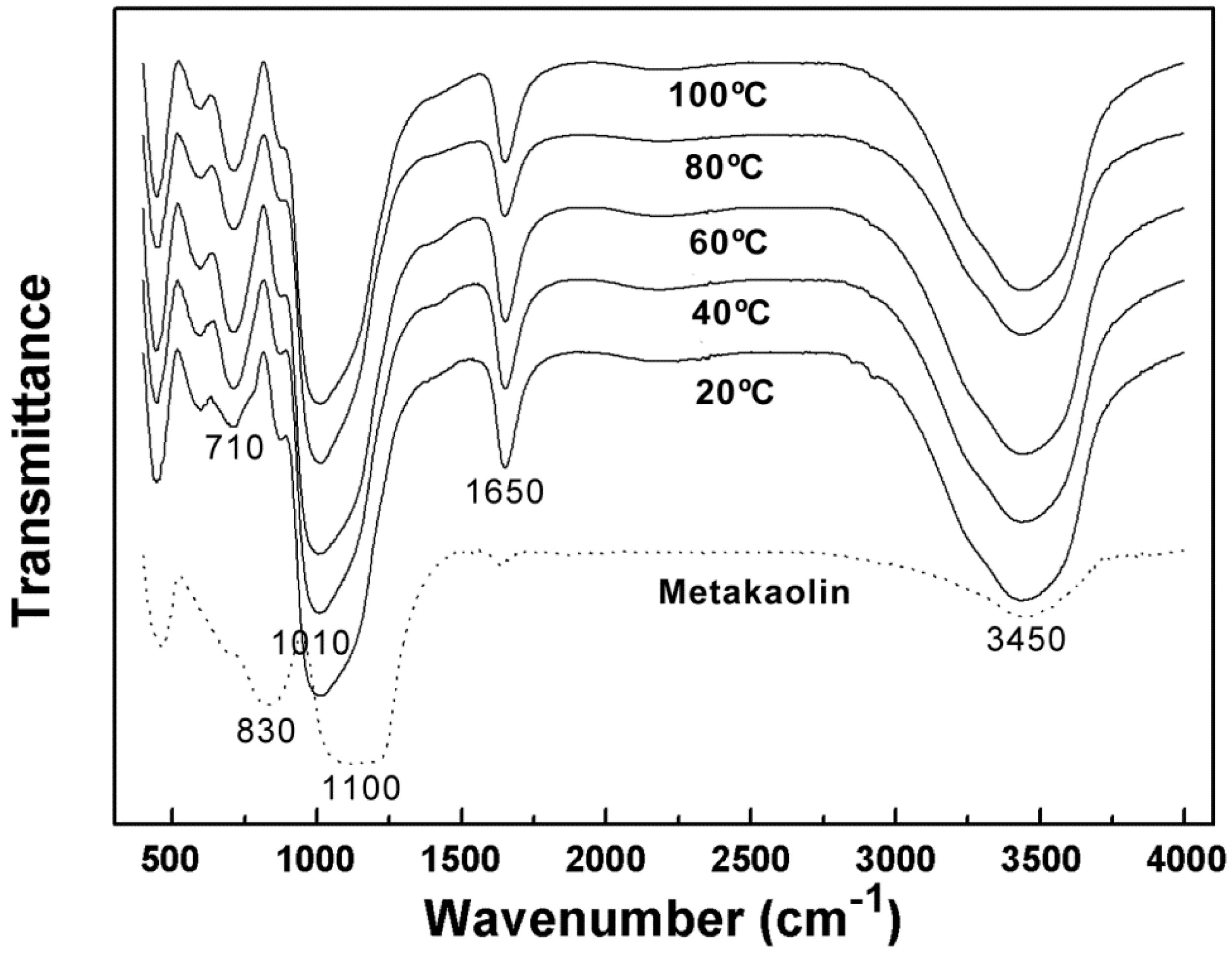
3.2.2. Influence of Curing Temperature and Time on the Microstructure of Alkali Activated Metakaolin-Based Geopolymer
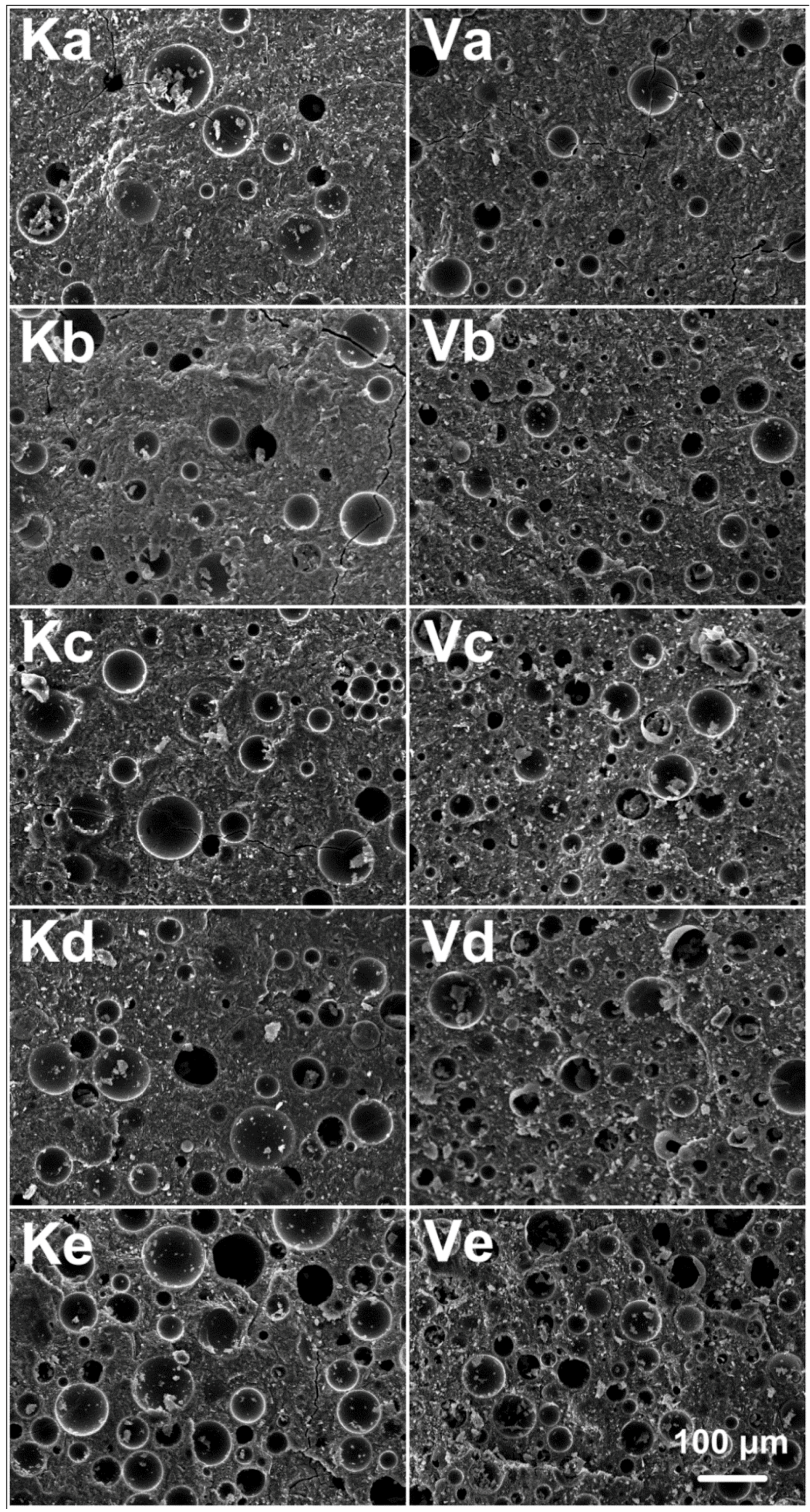
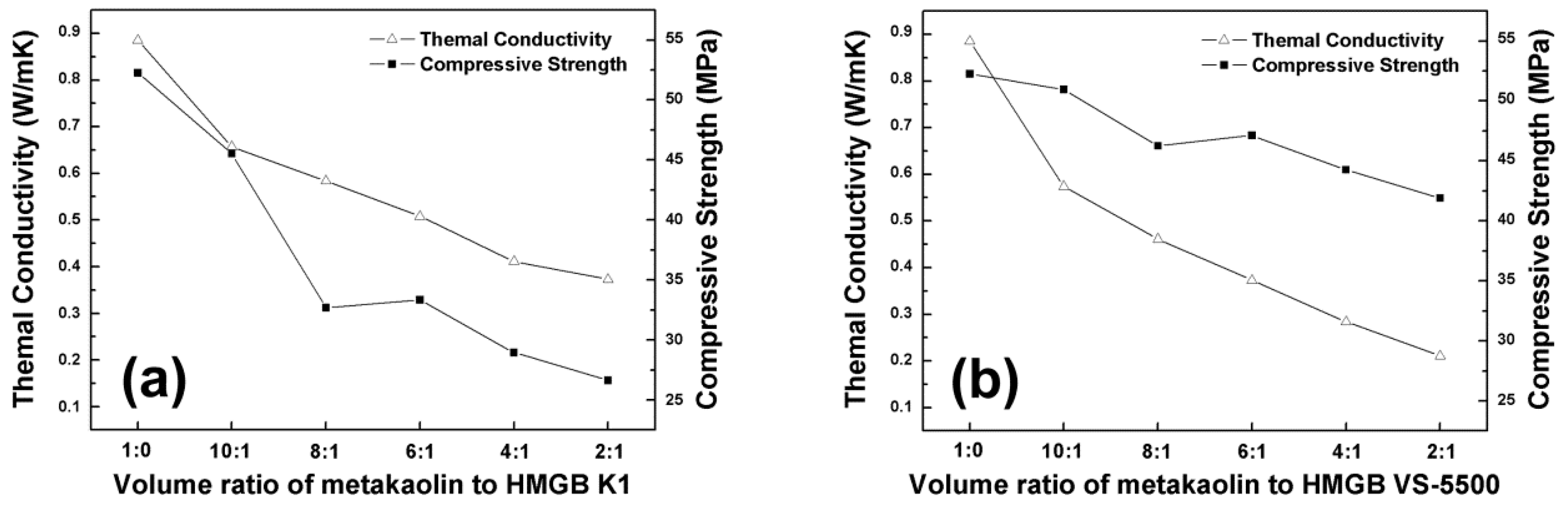
3.3. Alkali Activated Metakaolin-Based Geopolymer for Thermal Insulation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Rashad, A.M. Metakaolin as cementitious material: History, scours, production and composition—A comprehensive overview. Constr. Build. Mater. 2013, 41, 303–318. [Google Scholar] [CrossRef]
- Yan, D.; Chen, S.; Zeng, Q.; Xu, S.; Li, H. Correlating the elastic properties of metakaolin-based geopolymer with its composition. Mater. Des. 2016, 95, 306–318. [Google Scholar] [CrossRef]
- Zivica, V.; Balkovic, S.; Drabik, M. Properties of metakaolin geopolymer hardened paste prepared by high-pressure compaction. Constr. Build. Mater. 2011, 25, 2206–2213. [Google Scholar] [CrossRef]
- Martin, A.; Pastor, J.Y.; Palomo, A.; Fernandez Jimenez, A. Mechanical behaviour at high temperature of alkali-activated aluminosilicates (geopolymers). Constr. Build. Mater. 2015, 93, 1188–1196. [Google Scholar] [CrossRef] [Green Version]
- Temuujin, J.; Rickard, W.; Lee, M.; van Riessen, A. Preparation and thermal properties of fire resistant metakaolin-based geopolymer-type coatings. J. Non-Cryst. Solids 2011, 357, 1399–1404. [Google Scholar] [CrossRef]
- Azimi, E.A.; Al Bakri Abdullah, M.M.; Liew Yun, M.; Heah Cheng, Y.; Hussin, K.; Aziz, I.H. Review of geopolymer materials for thermal insulating applications. Key Eng. Mater. 2015, 660, 17–22. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Mechanical, thermal insulation, thermal resistance and acoustic absorption properties of geopolymer foam concrete. Cem. Concr. Compos. 2015, 62, 97–105. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Rickard, W.; Lee, M.; Williams, I.; van Riessen, A. Preparation of metakaolin based geopolymer coatings on metal substrates as thermal barriers. Appl. Clay Sci. 2009, 46, 265–270. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; Monzo, M.; Vicent, M.; Barba, A.; Palomo, A. Alkaline activation of metakaolin-fly ash mixtures: Obtain of zeoceramics and zeocements. Microporous Mesoporous Mater. 2008, 108, 41–49. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Physical evolution of na-geopolymer derived from metakaolin up to 1000 degrees c. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- Cheng, T.W.; Lee, M.L.; Ko, M.S.; Ueng, T.H.; Yang, S.F. The heavy metal adsorption characteristics on metakaolin-based geopolymer. Appl. Clay Sci. 2012, 56, 90–96. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Factors affecting the performance of metakaolin geopolymers exposed to elevated temperatures. J. Mater. Sci. 2008, 43, 824–831. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Roviello, G.; Menna, C.; Tarallo, O.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Asprone, D.; di Maggio, R.; Cappelletto, E.; Prota, A.; et al. Preparation, structure and properties of hybrid materials based on geopolymers and polysiloxanes. Mater. Des. 2015, 87, 82–94. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, C.; Duan, P.; Liu, Y.; Zhang, Z.; Qiu, X.; Li, D. A comparative study of high- and low-Al2O3 fly ash based-geopolymers: The role of mix proportion factors and curing temperature. Mater. Des. 2016, 95, 63–74. [Google Scholar] [CrossRef]
- Rieger, D.; Kovarik, T.; Riha, J.; Medlin, R.; Novotny, P.; Belsky, P.; Kadlec, J.; Holba, P. Effect of thermal treatment on reactivity and mechanical properties of alkali activated shale-slag binder. Constr. Build. Mater. 2015, 83, 26–33. [Google Scholar] [CrossRef]
- Muniz-Villarreal, M.S.; Manzano-Ramirez, A.; Sampieri-Bulbarela, S.; Ramon Gasca-Tirado, J.; Reyes-Araiza, J.L.; Rubio-Avalos, J.C.; Perez-Bueno, J.J.; Apatiga, L.M.; Zaldivar-Cadena, A.; Amigo-Borras, V. The effect of temperature on the geopolymerization process of a metakaolin-based geopolymer. Mater. Lett. 2011, 65, 995–998. [Google Scholar] [CrossRef]
- Yahya, Z.; Bakri, A.M.M.A.; Kamarudin, H.; Nizar, K.; Rafiza, A.R. Reviews on the Geopolymer Materials for Coating Application. Adv. Mater. Res. 2013, 626, 958–962. [Google Scholar]
- Wiyono, D.; Antoni; Hardjito, D. Improving the Durability of Pozzolan Concrete Using Alkaline Solution and Geopolymer Coating. Procedia Eng. 2015, 125, 747–753. [Google Scholar] [CrossRef]
- Rovnanik, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Turker, H.T.; Balcikanli, M.; Durmus, I.H.; Ozbay, E.; Erdemir, M. Microstructural alteration of alkali activated slag mortars depend on exposed high temperature level. Constr. Build. Mater. 2016, 104, 169–180. [Google Scholar] [CrossRef]
- Bakri, A.M.M.A.; Kamarudin, H.; BinHussain, M.; Nizar, I.K.; Zarina, Y.; Rafiza, A.R. The Effect of Curing Temperature on Physical and Chemical Properties of Geopolymers. Phys. Procedia 2011, 22, 286–291. [Google Scholar] [CrossRef]
- Aredes, F.G.M.; Campos, T.M.B.; Machado, J.P.B.; Sakane, K.K.; Thim, G.P.; Brunell, D.D. Effect of cure temperature on the formation of metakaolinite-based geopolymer. Ceram. Int. 2015, 41, 7302–7311. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Sun, W.; Li, Z.; Liu, Z. Preparation of metakaolin based geopolymer and its three-dimensional pore structural characterization. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2015, 30, 550–555. [Google Scholar] [CrossRef]
- Palomo, A.; Glasser, F.P. Chemically-bonded cementitious materials based on metakaolin. Br. Ceram. Trans. J. 1992, 91, 107–112. [Google Scholar]
- Rahier, H.; Mele, B.B.; Biesemans, M.; Wastiels, J.; Wu, X. Low-temperature synthesized aluminosilicate glasses. J. Mater. Sci. 1996, 31, 71–79. [Google Scholar] [CrossRef]
- Choquette, M.; Berube, M.A.; Locat, L. Behaviour of common rock-forming minerals in a strongly basic NaOH solution. Can. Mineral. 1991, 29, 163–173. [Google Scholar]
- Palmero, P.; Formia, A.; Antonaci, P.; Brini, S.; Tulliani, J.-M. Geopolymer technology for application-oriented dense and lightened materials. Elaboration and characterization. Ceram. Int. 2015, 41, 12967–12979. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Gong, K.C. Geopolymer. Mater. Sci. Eng. 2003, 3, 430–436. [Google Scholar]
- Tippayasam, C.; Balyore, P.; Thavorniti, P.; Kamseu, E.; Leonelli, C.; Chindaprasirt, P.; Chaysuwan, D. Potassium alkali concentration and heat treatment affected metakaolin-based geopolymer. Constr. Build. Mater. 2016, 104, 293–297. [Google Scholar] [CrossRef]
- Forst, R.L.; Fredericks, P.M.; Shurvell, H.F. Raman microscopy of some kaolinite clay minerals. Can. J. Appl. Spectrosc. 1996, 41, 10–14. [Google Scholar]
- Parker, R.W.; Forst, R.L. The application of drift spectroscopy to the multicomponent analysis of organic chemicals adsorbed on montmorillonite. Clays Clay Miner. 1996, 44, 32–40. [Google Scholar] [CrossRef]
- Sitarz, M.; Handke, M.; Mozgawa, W. Identification of silicooxygen rings in SiO2 based on IR spectra. Spectrochim. Acta A 2000, 56, 1819–1823. [Google Scholar] [CrossRef]
- Sitarz, M.; Handke, M.; Mozgawa, W.; Galuskin, E.; Galuskina, I. The non-ring cations influence on silicooxygen ring vibrations. J. Mol. Struct. 2000, 555, 357–362. [Google Scholar] [CrossRef]
- Lancellotti, I.; Catauro, M.; Ponzoni, C.; Bollino, F.; Leonelli, C. Inorganic polymers from alkali activation of metakaolin: Effect of setting and curing on structure. J. Solid State Chem. 2013, 200, 341–348. [Google Scholar] [CrossRef]
- Aziz, I.H.; Al Bakri Abdullah, M.M.; Heah Cheng, Y.; Liew Yun, M.; Hussin, K.; Azimi, E.A. A review on mechanical properties of geopolymer composites for high temperature application. Key Eng. Mater. 2015, 660, 34–38. [Google Scholar] [CrossRef]
- Salahuddin, M.B.M.; Norkhairunnisa, M.; Mustapha, F. A review on thermophysical evaluation of alkali-activated geopolymers. Ceram. Int. 2015, 41, 4273–4281. [Google Scholar] [CrossRef]

| SiO2 | Al2O3 | Fe2O3 | CaO | K2O | Na2O | SO3 | P2O5 | TiO2 | ZrO2 |
|---|---|---|---|---|---|---|---|---|---|
| 53.75% | 43.82% | 0.45% | 0.16% | 0.18% | 0.26% | 0.02% | 0.43% | 0.86% | 0.029% |
| HMGB | Compressive Strength (MPa) | Density (g/cm3) | Size Distribution (μm, Volume Ratio) | Color | |||
|---|---|---|---|---|---|---|---|
| 10th% | 50th% | 90th% | Max | ||||
| K1 | 1.72 | 0.125 | 30 | 65 | 110 | 120 | white |
| VS-5500 | 37.9 | 0.38 | 15 | 40 | 75 | 85 | white |
| Factor Level | Metakaolin (g) | NaOH (g) | Sodium Silicate (g) | Water (g) |
|---|---|---|---|---|
| 1 | 24 | 3 | 25 | 4 |
| 2 | 25 | 4 | 26 | 5 |
| 3 | 26 | 5 | 27 | 6 |
| Sample | Metakaolin | NaOH | Sodium Silicate | Water | Compressive Strength | Standard Deviation |
|---|---|---|---|---|---|---|
| (g) | (g) | (g) | (g) | (MPa) | (MPa) | |
| #1 | 24.0 | 3.0 | 25.0 | 4.0 | 32.59 | 2.34 |
| #2 | 24.0 | 4.0 | 26.0 | 5.0 | 30.24 | 3.69 |
| #3 | 24.0 | 5.0 | 27.0 | 6.0 | 26.42 | 3.29 |
| #4 | 25.0 | 3.0 | 26.0 | 6.0 | 34.40 | 3.59 |
| #5 | 25.0 | 4.0 | 27.0 | 4.0 | 37.40 | 2.01 |
| #6 | 25.0 | 5.0 | 25.0 | 5.0 | 29.32 | 3.72 |
| #7 | 26.0 | 3.0 | 27.0 | 5.0 | 31.80 | 2.81 |
| #8 | 26.0 | 4.0 | 25.0 | 6.0 | 32.43 | 3.77 |
| #9 | 26.0 | 5.0 | 26.0 | 4.0 | 29.59 | 2.91 |
| K1 a | 89.25 | 98.78 | 94.33 | 99.58 | ||
| K2 | 101.12 | 100.07 | 94.24 | 91.36 | ||
| K3 | 93.82 | 85.34 | 95.62 | 93.25 | ||
| k1 b | 29.75 | 32.93 | 31.44 | 33.19 | ||
| k2 | 33.71 | 33.36 | 31.41 | 30.45 | ||
| k3 | 31.27 | 28.45 | 31.87 | 31.08 | ||
| R c | 3.96 | 4.91 | 0.46 | 2.74 |
| Curing Temperature (°C) | Transmittance Peak at Approximately 1010 cm−1 | Transmittance Peak at Approximately 710 cm−1 | ||
|---|---|---|---|---|
| Wavenumber (cm−1) | Transmittance | Wavenumber (cm−1) | Transmittance | |
| None | 1110 | 0.415 | 831 | 0.578 |
| 20 | 1018 | 0.053 | 715 | 0.796 |
| 40 | 1014 | 0.082 | 713 | 0.702 |
| 60 | 1008 | 0.045 | 709 | 0.656 |
| 80 | 1014 | 0.095 | 717 | 0.742 |
| 100 | 1012 | 0.059 | 715 | 0.702 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Wang, Z.; Wang, Y.; Feng, J. Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer. Materials 2016, 9, 767. https://doi.org/10.3390/ma9090767
Chen L, Wang Z, Wang Y, Feng J. Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer. Materials. 2016; 9(9):767. https://doi.org/10.3390/ma9090767
Chicago/Turabian StyleChen, Liang, Zaiqin Wang, Yuanyi Wang, and Jing Feng. 2016. "Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer" Materials 9, no. 9: 767. https://doi.org/10.3390/ma9090767






