The Synthesis of LiMnxFe1−xPO4/C Cathode Material through Solvothermal Jointed with Solid-State Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of LiMnPO4 and LiFePO4 Nano-Plates
2.2. Synthesis of LiMnxFe1−xPO4/C Composite
2.3. Materials Characterization
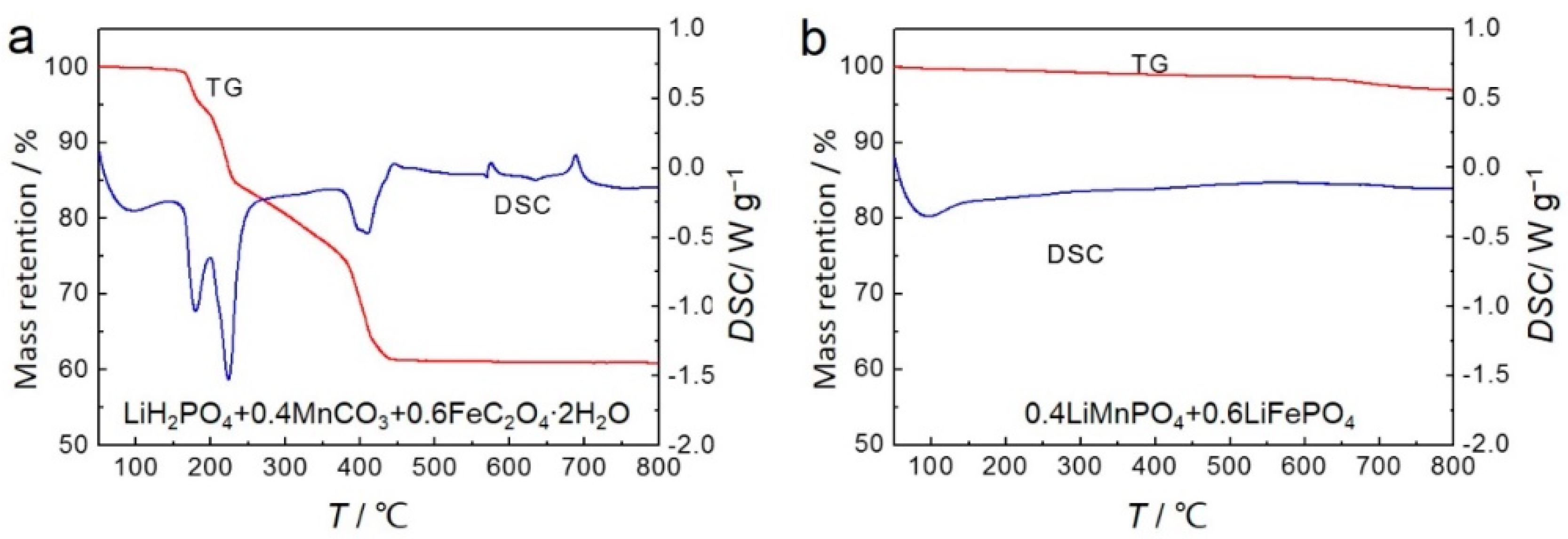
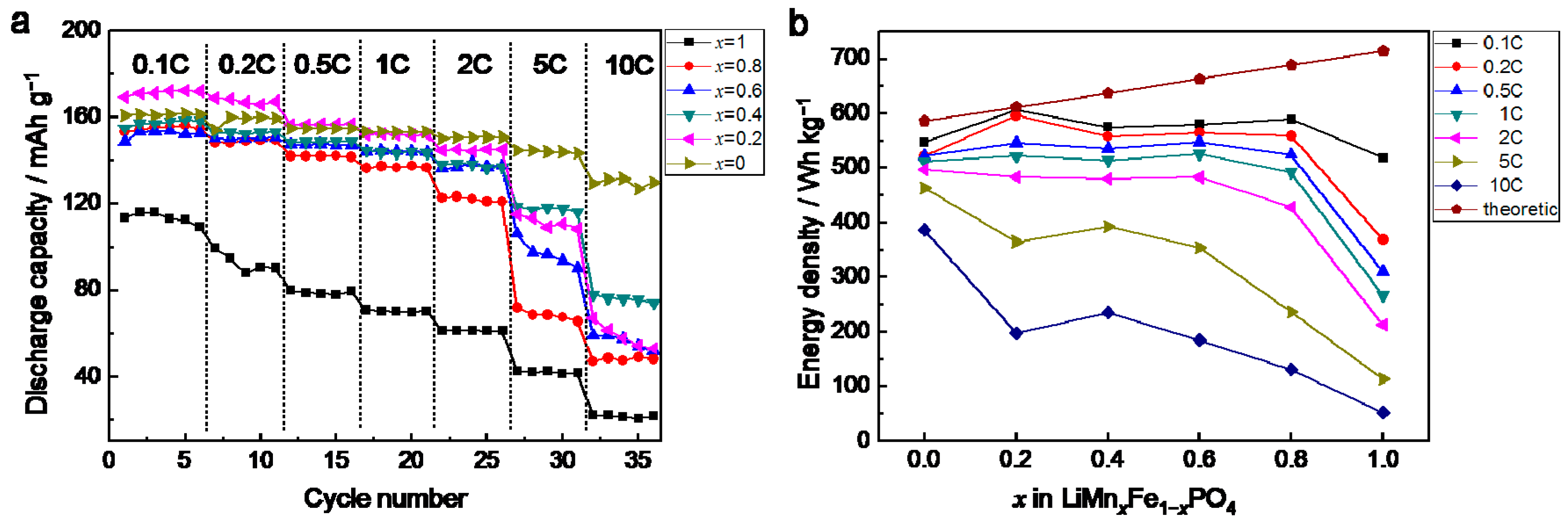
3. Results and Discussion
3.1. Structures and Morphologies Characterization
3.2. Electrochemical Performancesof LiMnxFe1−xPO4/CMaterials
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Delacourt, C.; Laffont, L.; Bouchet, R.; Wurm, C.; Leriche, J.B.; Morcrette, M.; Tarascon, J.M.; Masquelier, C. Toward understanding of electrical limitations (electronic, ionic) in LiMPO4 (M = Fe, Mn) electrode materials. J. Electrochem. Soc. 2005, 152, A913–A921. [Google Scholar] [CrossRef]
- Devaraju, M.K.; Honma, I. Hydrothermal and solvothermal process towards development of LiMPO4 (M = Fe, Mn) nanomaterials for lithium-ion batteries. Adv. Energy Mater. 2012, 2, 284–297. [Google Scholar] [CrossRef]
- Martha, S.K.; Grinblat, J.; Haik, O.; Zinigrad, E.; Drezen, T.; Miners, J.H.; Exnar, I.; Kay, A.; Markovsky, B.; Aurbach, D. LiMn0.8Fe0.2PO4: An advanced cathode material for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2009, 48, 8559–8563. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Xu, W.; Choi, D.; Zhang, J.-G. Synthesis and characterization of lithium manganese phosphate by a precipitation method. J. Electrochem. Soc. 2010, 157, A142–A147. [Google Scholar] [CrossRef]
- Oh, S.-M.; Myung, S.-T.; Choi, Y.S.; Oh, K.H.; Sun, Y.-K. Co-precipitation synthesis of micro-sized spherical LiMn0.5Fe0.5PO4 cathode material for lithium batteries. J. Mater. Chem. 2011, 21, 19368–19374. [Google Scholar] [CrossRef]
- Arnold, G.; Garche, J.; Hemmer, R.; Ströbele, S.; Vogler, C.; Wohlfahrt-Mehrens, M. Fine-particle lithium iron phosphate LiFePO4 synthesized by a new low-cost aqueous precipitation technique. J. Power Sources 2003, 119, 247–251. [Google Scholar] [CrossRef]
- Dai, Z.; Wang, L.; Ye, F.; Huang, C.; Wang, J.; Huang, X.; Wang, J.; Tian, G.; He, X.; Ouyang, M. Influence of anion species on the morphology of solvothermal synthesized LiMn0.9Fe0.1PO4. Electrochim. Acta 2014, 134, 13–17. [Google Scholar] [CrossRef]
- Yang, S.-L.; Ma, R.-G.; Hu, M.-J.; Xi, L.-J.; Lu, Z.-G.; Chung, C.Y. Solvothermal synthesis of nano-LiMnPO4 from Li3PO4 rod-like precursor: Reaction mechanism and electrochemical properties. J. Mater. Chem. 2012, 22, 25402–25408. [Google Scholar] [CrossRef]
- Kim, T.-H.; Park, H.-S.; Lee, M.-H.; Lee, S.-Y.; Song, H.-K. Restricted growth of LiMnPO4 nanoparticles evolved from a precursor seed. J. Power Sources 2012, 210, 1–6. [Google Scholar] [CrossRef]
- Kim, S.-W.; Kim, J.; Gwon, H.; Kang, K. Phase Stability Study of Li1−x MnPO4 (0 ≤ x ≤ 1 ) Cathode for Li Rechargeable Battery. J. Electrochem. Soc. 2009, 156, A635–A638. [Google Scholar] [CrossRef]
- Yamada, A.; Yonemura, M.; Takei, Y.; Sonoyama, N.; Kanno, R. Fast charging LiFePO4. Electrochem. Solid State Lett. 2005, 8, A55–A58. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Xiang, K.; Xing, W.; Borkiewicz, O.J.; Wiaderek, K.M.; Gionet, P.; Chapman, K.W.; Chupas, P.J.; Chiang, Y.M. Extended solid solutions and coherent transformations in nanoscale olivine cathodes. Nano Lett. 2014, 14, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Qiu, B.; Xia, Y.; Qin, Z.; Qin, L.; Zhou, X.; Liu, Z. Solvothermal synthesis of Fe-doping LiMnPO4 nanomaterials for Li-ion batteries. J. Power Sources 2014, 248, 246–252. [Google Scholar] [CrossRef]
- Fisher, C.A.J.; Hart Prieto, V.M.; Islam, M.S. Lithium battery materials LiMPO4 (M = Mn, Fe, Co, and Ni): Insights into defect association, transport mechanisms, and doping behavior. Chem. Mater. 2008, 20, 5907–5915. [Google Scholar] [CrossRef]
- Yang, G.; Ni, H.; Liu, H.; Gao, P.; Ji, H.; Roy, S.; Pinto, J.; Jiang, X. The doping effect on the crystal structure and electrochemical properties of LiMnxM1−xPO4 (M = Mg, V, Fe, Co, Gd). J. Power Sources 2011, 196, 4747–4755. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Sun, W.; Wang, J.; Li, Y.; Fan, S. Crystal Orientation Tuning of LiFePO4 nanoplates for high rate lithium battery cathode materials. Nano Lett. 2012, 12, 5632–5636. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wang, L.; He, X.; Ye, F.; Huang, C.; Li, J.; Gao, J.; Wang, J.; Tian, G.; Ouyang, M. Morphology regulation of nano LiMn0.9Fe0.1PO4 by solvothermal synthesis for lithium ion batteries. Electrochim. Acta 2013, 112, 144–148. [Google Scholar] [CrossRef]
- Nan, C.; Lu, J.; Li, L.; Li, L.; Peng, Q.; Li, Y. Size and shape control of LiFePO4 nanocrystals for better lithium ion battery cathode materials. Nano Res. 2013, 6, 469–477. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.-Y.; Park, I.; Yoo, J.-K.; Hong, J.; Kang, K. Thermal stability of Fe-Mn binary olivine cathodes for Li rechargeable batteries. J. Mater. Chem. 2012, 22, 11964–11970. [Google Scholar] [CrossRef]
- Hu, C.; Yi, H.; Fang, H.; Yang, B.; Yao, Y.; Ma, W.; Dai, Y. Improving the electrochemical activity of LiMnPO4 via Mn-site co-substitution with Fe and Mg. Electrochem. Commun. 2010, 12, 1784–1787. [Google Scholar] [CrossRef]
- Zheng, J.; Li, X.; Wang, Z.; Guo, H.; Zhou, S. LiFePO4 with enhanced performance synthesized by a novel synthetic route. J. Power Sources 2008, 184, 574–577. [Google Scholar] [CrossRef]
- Lepage, D.; Michot, C.; Liang, G.; Gauthier, M.; Schougaard, S.B. A soft chemistry approach to coating of LiFePO4 with a conducting polymer. Angew. Chem. Int. Ed. 2011, 50, 6884–6887. [Google Scholar] [CrossRef] [PubMed]
- Damen, L.; De Giorgio, F.; Monaco, S.; Veronesi, F.; Mastragostino, M. Synthesis and characterization of carbon-coated LiMnPO4 and LiMn1−xFexPO4 (x = 0.2, 0.3) materials for lithium-ion batteries. J. Power Sources 2012, 218, 250–253. [Google Scholar] [CrossRef]
- Saravanan, K.; Ramar, V.; Balaya, P.; Vittal, J.J. Li(MnxFe1−x)PO4/C (x = 0.5, 0.75 and 1) nanoplates for lithium storage application. J. Mater. Chem. 2011, 21, 14925–14935. [Google Scholar] [CrossRef]
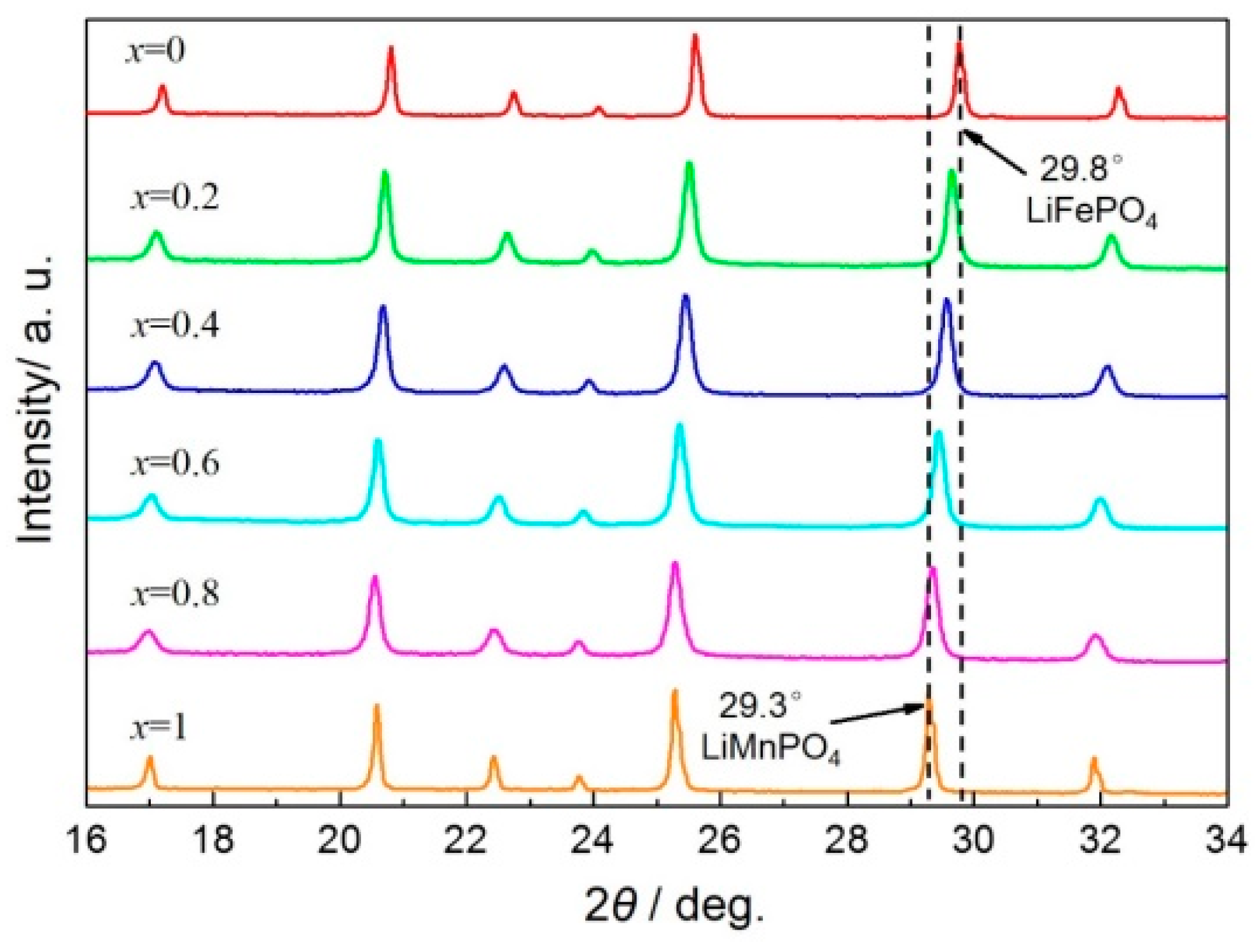


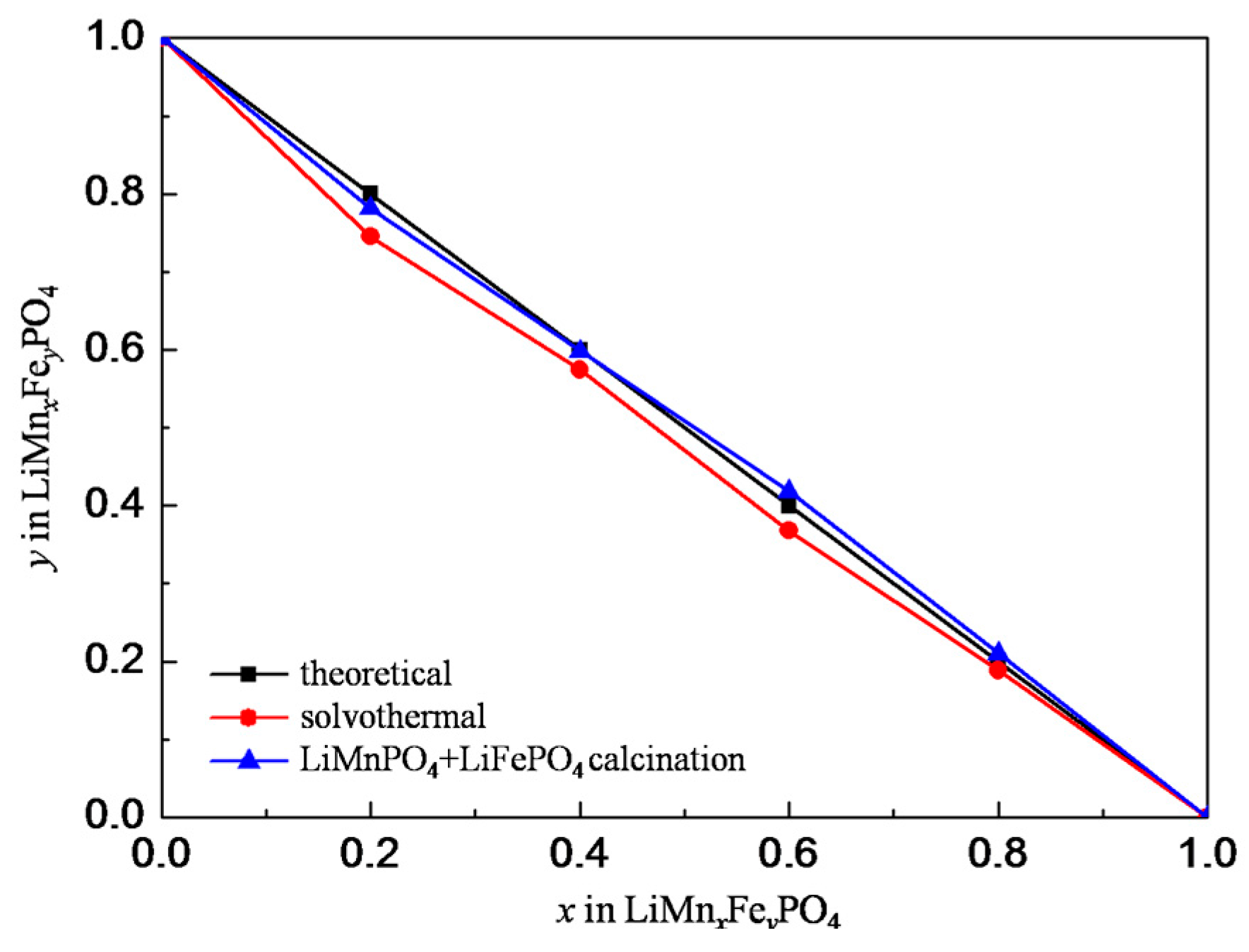
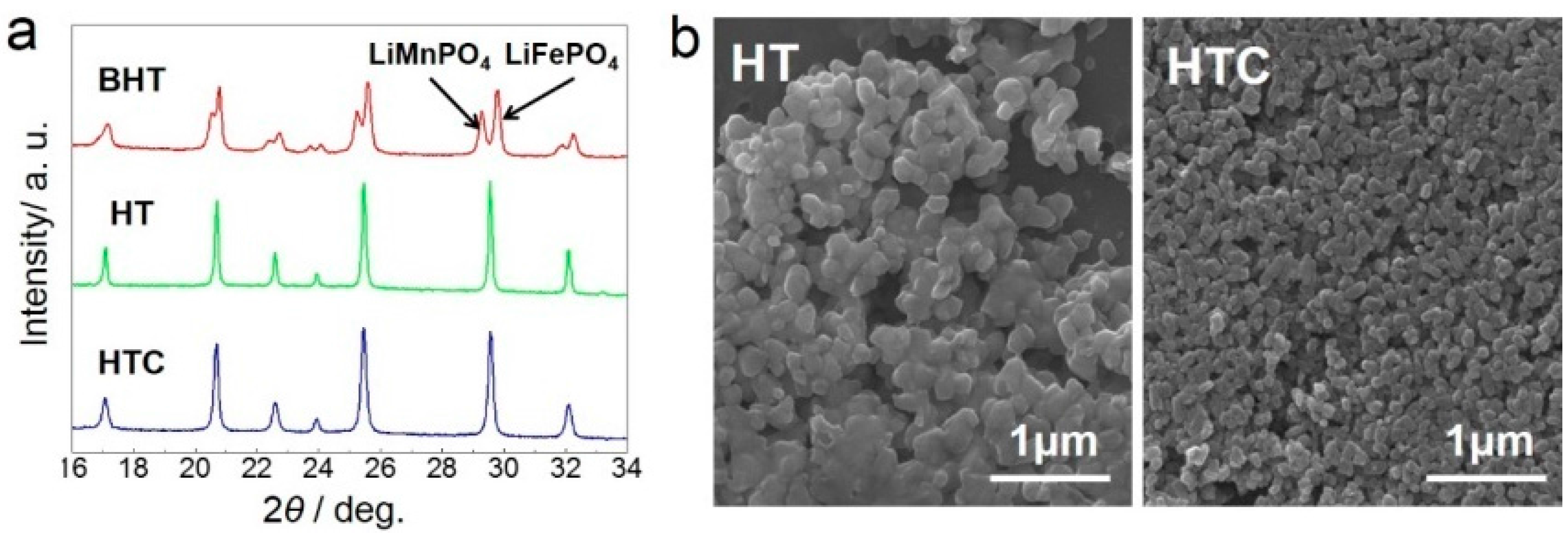

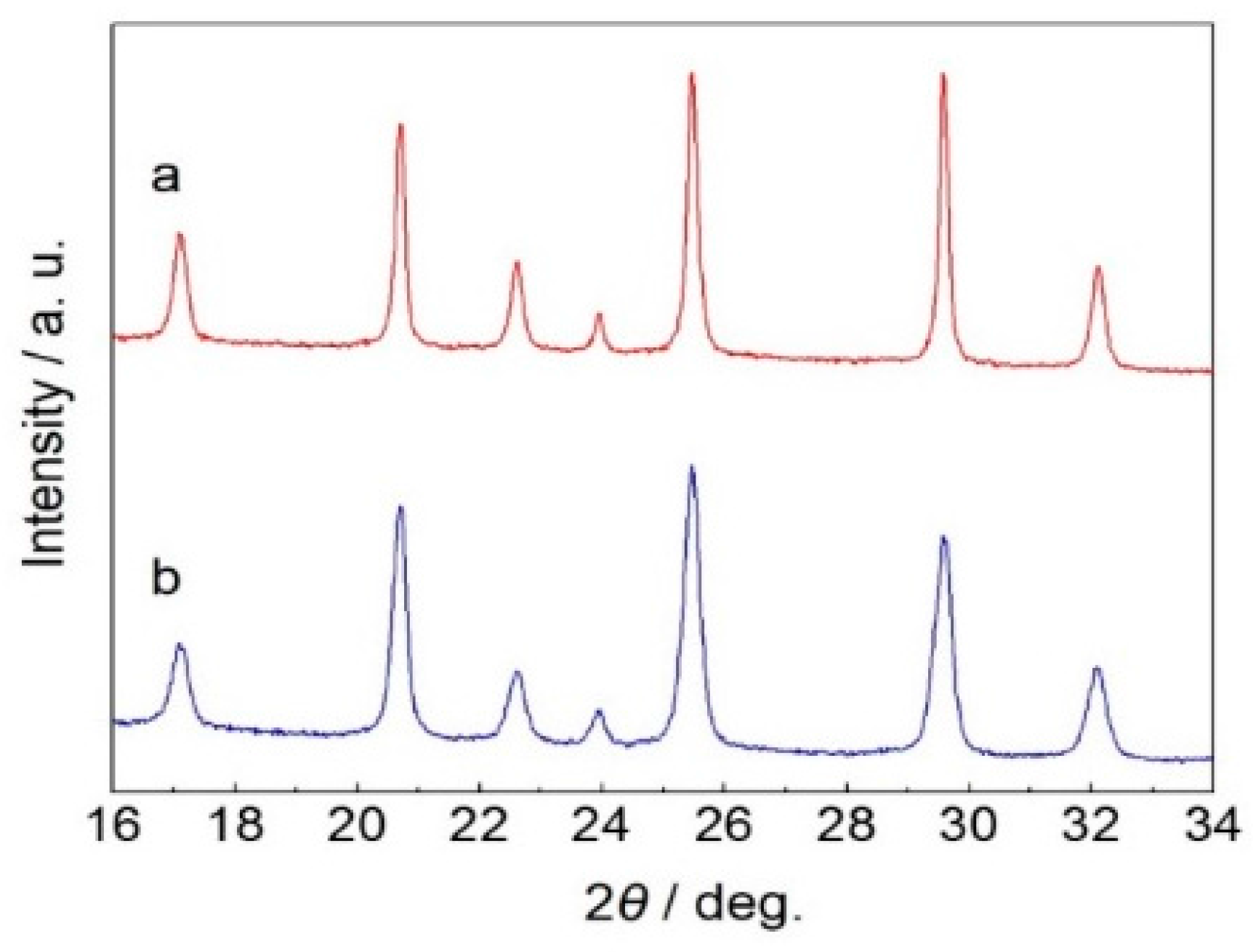

| Peak Position/° | 17.1 | 20.7 | 22.6 | 23.9 | 25.5 | 29.5 | 32.1 |
|---|---|---|---|---|---|---|---|
| fwhm of HT/° | 0.138 | 0.139 | 0.143 | 0.140 | 0.149 | 0.132 | 0.149 |
| fwhm of HTC/° | 0.216 | 0.182 | 0.241 | 0.187 | 0.209 | 0.193 | 0.233 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Wang, J.; Dai, Z.; Wang, L.; Tian, G. The Synthesis of LiMnxFe1−xPO4/C Cathode Material through Solvothermal Jointed with Solid-State Reaction. Materials 2016, 9, 766. https://doi.org/10.3390/ma9090766
He X, Wang J, Dai Z, Wang L, Tian G. The Synthesis of LiMnxFe1−xPO4/C Cathode Material through Solvothermal Jointed with Solid-State Reaction. Materials. 2016; 9(9):766. https://doi.org/10.3390/ma9090766
Chicago/Turabian StyleHe, Xiangming, Jixian Wang, Zhongjia Dai, Li Wang, and Guangyu Tian. 2016. "The Synthesis of LiMnxFe1−xPO4/C Cathode Material through Solvothermal Jointed with Solid-State Reaction" Materials 9, no. 9: 766. https://doi.org/10.3390/ma9090766







