Assembly and Rearrangement of Particles Confined at a Surface of a Droplet, and Intruder Motion in Electro-Shaken Particle Films
Abstract
:1. Introduction
2. Results
2.1. Formation of a Particle Film
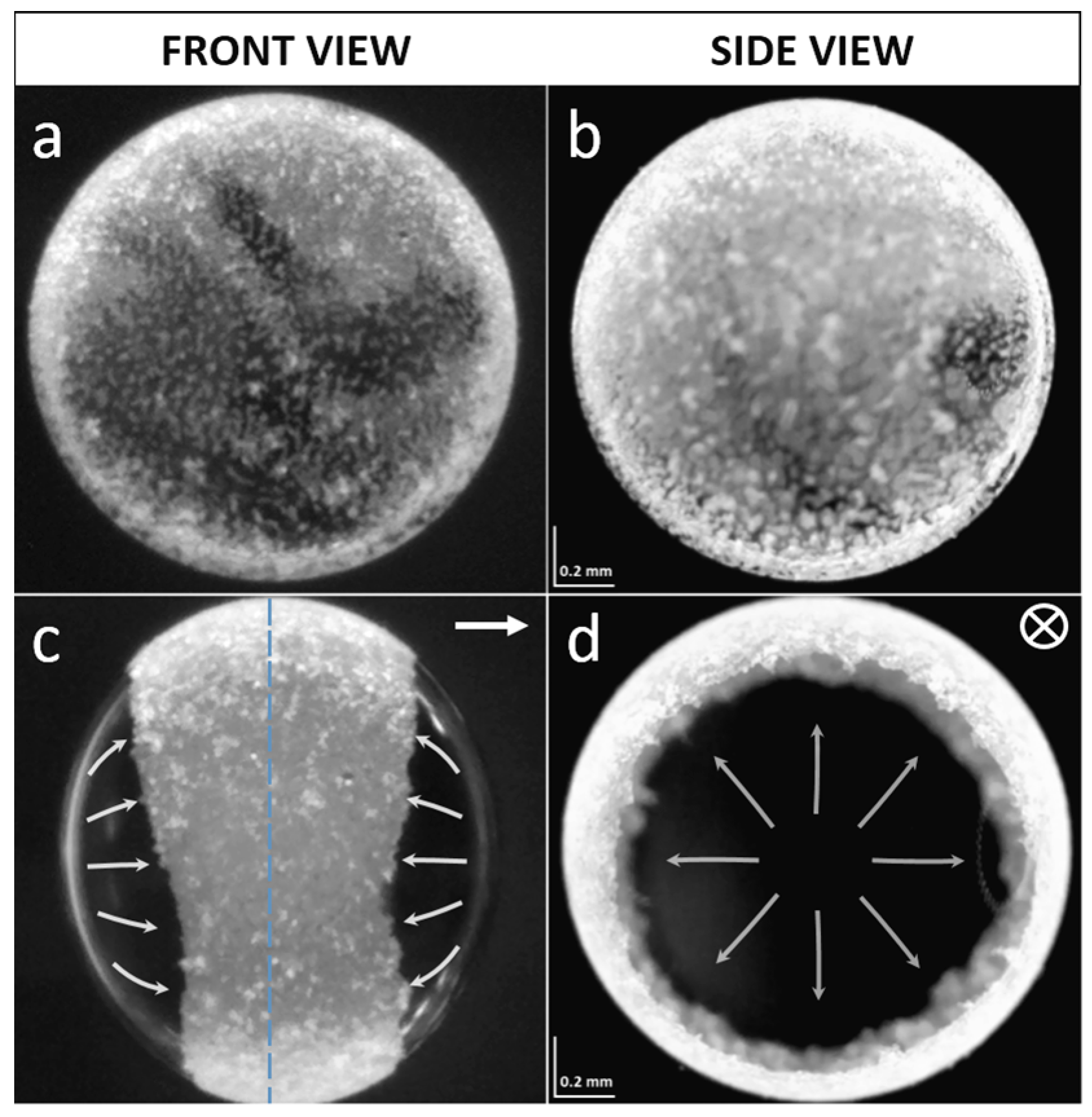
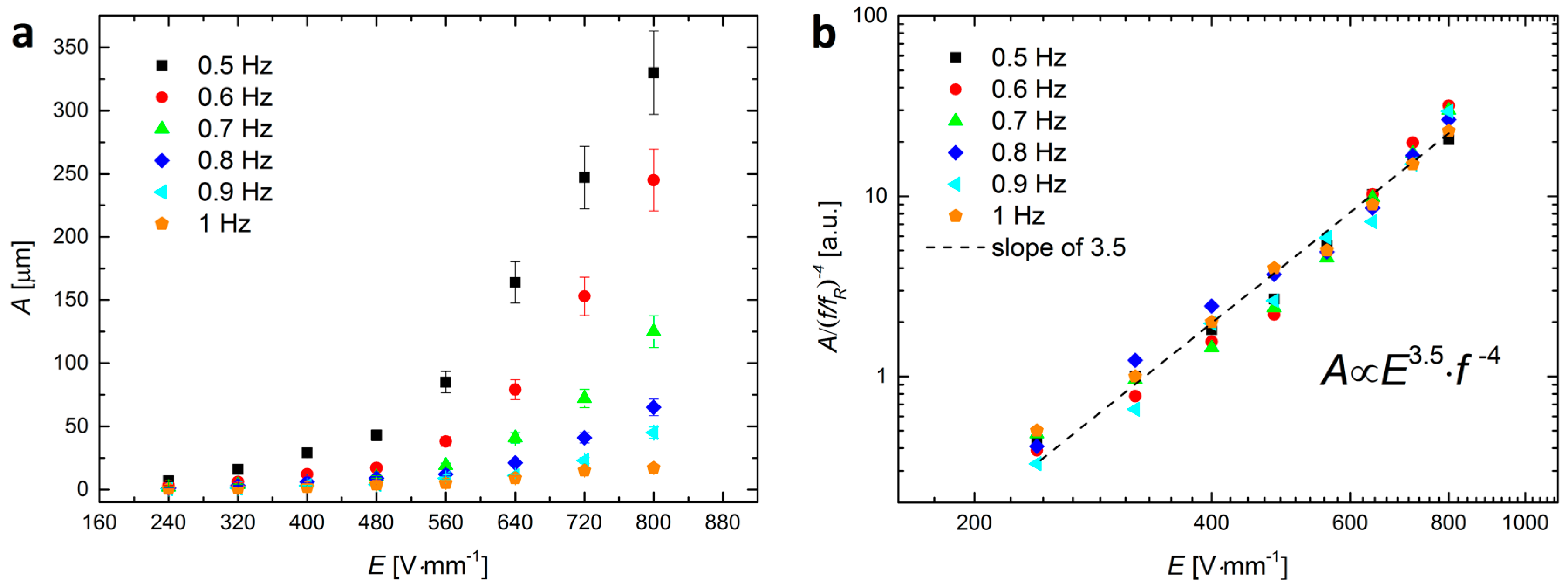
2.2. Electric-Field-Induced ‘Shaking’ of Particles
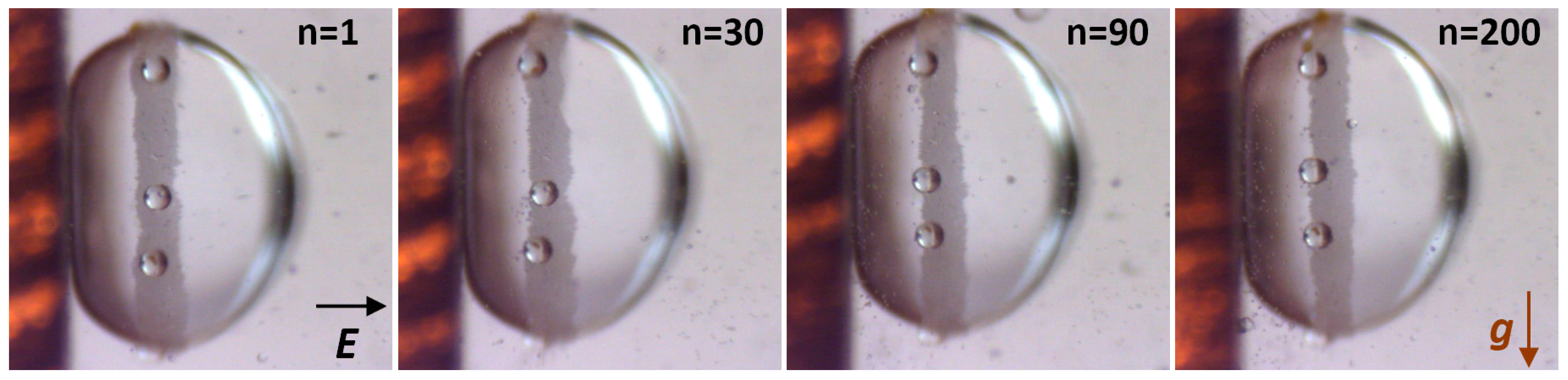
2.3. An Intruder Travelling through the Particle Film
2.4. Size-Segregation of Surface Particles
3. Discussion
4. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Y.; Wang, Y.; Breed, D.R.; Manoharan, V.N.; Feng, L.; Hollingsworth, A.D.; Weck, M.; Pine, D.J. Colloids with valence and specific directional bonding. Nature 2012, 491, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Evers, C.H.J.; Luiken, J.A.; Bolhuis, P.G.; Kegel, W.K. Self-assembly of microcapsules via colloidal bond hybridization and anisotropy. Nature 2016, 534. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, O.A.; Klumov, B.A.; Tkachenko, A.V. Chromatic patchy particles: Effects of specific interactions on liquid structure. Phys. Rev. E 2015, 92. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wu, H.; Luijten, E. Electric double layer of anisotropic dielectric colloids under electric fields. Eur. Phys. J. Spec. Top. 2016, 225, 685–698. [Google Scholar] [CrossRef]
- Sander, J.S.; Studart, A.R. Monodisperse Functional Colloidosomes with Tailored Nanoparticle Shells. Langmuir 2011, 27, 3301–3307. [Google Scholar] [CrossRef] [PubMed]
- Song, P.C.; Wang, Y.F.; Wang, Y.; Hollingsworth, A.D.; Weck, M.; Pine, D.J.; Ward, M.D. Patchy Particle Packing under Electric Fields. J. Am. Chem. Soc. 2015, 137, 3069–3075. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R. Review Article-Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics 2010, 4. [Google Scholar] [CrossRef]
- Baraban, L.; Streubel, R.; Makarov, D.; Han, L.; Karnaushenko, D.; Schmidt, O.G.; Cuniberti, G. Fuel-Free Locomotion of Janus Motors: Magnetically Induced Thermophoresis. ACS Nano 2013, 7, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Bickel, T.; Zecua, G.; Wuerger, A. Polarization of active Janus particles. Phys. Rev. E 2014, 89. [Google Scholar] [CrossRef] [PubMed]
- Rozynek, Z.; Józefczak, A. Patchy colloidosomes—An emerging class of structures. Eur. Phys. J. Spec. Top. 2016, 225, 741–756. [Google Scholar] [CrossRef]
- Loget, G.; Kuhn, A. Bulk synthesis of Janus objects and asymmetric patchy particles. J. Mater. Chem. 2012, 22, 15457–15474. [Google Scholar] [CrossRef]
- Pawar, A.B.; Kretzschmar, I. Fabrication, assembly, and application of patchy particles. Macromol. Rapid Commun. 2010, 31, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fernandez, D.; Liz-Marzan, L.M. Metallic Janus and Patchy Particles. Part. Part. Syst. Charact. 2013, 30, 46–60. [Google Scholar] [CrossRef]
- Shah, A.A.; Schultz, B.; Kohlstedt, K.L.; Glotzer, S.C.; Solomon, M.J. Synthesis, assembly, and image analysis of spheroidal patchy particles. Langmuir 2013, 29, 4688–4696. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Muller, A.H. Janus particles: Synthesis, self-assembly, physical properties, and applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A.B.; Abkarian, M.; Stone, H.A. Controlled assembly of jammed colloidal shells on fluid droplets. Nat. Mater. 2005, 4, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Rozynek, Z.; Castberg, R.; Kalicka, A.; Jankowski, P.; Garstecki, P. Electric field manipulation of particles in leaky dielectric liquids. Arch. Mech. 2015, 67, 385–399. [Google Scholar]
- Rozynek, Z.; Mikkelsen, A.; Dommersnes, P.; Fossum, J.O. Electroformation of Janus and patchy capsules. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Dommersnes, P.; Rozynek, Z.; Mikkelsen, A.; Castberg, R.; Kjerstad, K.; Hersvik, K.; Otto Fossum, J. Active structuring of colloidal armour on liquid drops. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Nudurupati, S.; Janjua, M.; Aubry, N.; Singh, P. Concentrating particles on drop surfaces using external electric fields. Electrophoresis 2008, 29, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, D. Redistribution of charged aluminum nanoparticles on oil droplets in water in response to applied electrical field. J. Nanopart. Res. 2016, 18. [Google Scholar] [CrossRef]
- Nudurupati, S.; Janjua, M.; Singh, P.; Aubry, N. Effect of parameters on redistribution and removal of particles from drop surfaces. Soft Matter 2010, 6, 1157–1169. [Google Scholar] [CrossRef]
- Amah, E.; Shah, K.; Fischer, I.; Singh, P. Electrohydrodynamic manipulation of particles adsorbed on the surface of a drop. Soft Matter 2016, 12, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Rozynek, Z.; Dommersnes, P.; Mikkelsen, A.; Michels, L.; Fossum, J.O. Electrohydrodynamic controlled assembly and fracturing of thin colloidal particle films confined at drop interfaces. Eur. Phys. J. Spec. Top. 2014, 223, 1859–1867. [Google Scholar] [CrossRef]
- Rozynek, Z.; Zacher, T.; Janek, M.; Caplovicova, M.; Fossum, J.O. Electric-field-induced structuring and rheological properties of kaolinite and halloysite. Appl. Clay Sci. 2013, 77–78, 1–9. [Google Scholar] [CrossRef]
- Rozynek, Z.; Wang, B.; Fossum, J.O.; Knudsen, K.D. Dipolar structuring of organically modified fluorohectorite clay particles. Eur. Phys. J. E Soft Matter 2012, 35. [Google Scholar] [CrossRef] [PubMed]
- Uñac, R.O.; Benito, J.G.; Vidales, A.M.; Pugnaloni, L.A. Arching during the segregation of two-dimensional tapped granular systems: Mixtures versus intruders. Eur. Phys. J. E 2014, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Zhou, K.; Qiu, K.; Zhao, Y.M. Segregation of large granules from close-packed cluster of small granules due to buoyancy. Phys. Rev. E 2006, 73. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.H.; Hsiau, S.S.; Kruelle, C.A. Density segregation in a vertically vibrated granular bed. Powder Tech. 2010, 204, 255–262. [Google Scholar] [CrossRef]
- Goetzendorfer, A.; Tai, C.-H.; Kruelle, C.A.; Rehberg, I.; Hsiau, S.-S. Fluidization of a vertically vibrated two-dimensional hard sphere packing: A granular meltdown. Phys. Rev. E 2006, 74. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozynek, Z.; Kaczmarek-Klinowska, M.; Magdziarz, A. Assembly and Rearrangement of Particles Confined at a Surface of a Droplet, and Intruder Motion in Electro-Shaken Particle Films. Materials 2016, 9, 679. https://doi.org/10.3390/ma9080679
Rozynek Z, Kaczmarek-Klinowska M, Magdziarz A. Assembly and Rearrangement of Particles Confined at a Surface of a Droplet, and Intruder Motion in Electro-Shaken Particle Films. Materials. 2016; 9(8):679. https://doi.org/10.3390/ma9080679
Chicago/Turabian StyleRozynek, Zbigniew, Milena Kaczmarek-Klinowska, and Agnieszka Magdziarz. 2016. "Assembly and Rearrangement of Particles Confined at a Surface of a Droplet, and Intruder Motion in Electro-Shaken Particle Films" Materials 9, no. 8: 679. https://doi.org/10.3390/ma9080679
APA StyleRozynek, Z., Kaczmarek-Klinowska, M., & Magdziarz, A. (2016). Assembly and Rearrangement of Particles Confined at a Surface of a Droplet, and Intruder Motion in Electro-Shaken Particle Films. Materials, 9(8), 679. https://doi.org/10.3390/ma9080679





